Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and preclinical evaluation of novel L-cystine-based polyamide nanocapsules loaded with a fixed-dose combination of thymoquinone and doxorubicin for targeted pulmonary anticancer drug delivery
Hadeel Fayez
Banat
a,
Dalia Khalil
Ali
b,
Qais
Jarrar
a,
Esra’a
Alomary
a and
Eman Zmaily
Dahmash
 *ac
*ac
aDepartment of Applied Pharmaceutical Sciences and Clinical Pharmacy, Faculty of Pharmacy, Isra University, Amman, Jordan
bDepartment of Physiotherapy Department, Faculty of Allied Medical Sciences, Isra University, Amman, Jordan
cDepartment of Chemical and Pharmaceutical Sciences, School of Life Sciences, Pharmacy and Chemistry, Kingston University London, Kingston Upon Thames KT1 2EE, London, UK. E-mail: eman.dahmash@kingston.ac.uk
First published on 27th January 2025
Abstract
Innovative synthetic biodegradable polymers containing amino acid moieties are used as pulmonary anticancer drug delivery systems to efficiently administer drugs in a controlled manner while also altering the physical and chemical characteristics of therapeutic molecules and the way they are delivered to the lungs. In this study, the aim was to prepare a new polyamide based on L-cystine amino acid loaded with a combination of thymoquinone (TQ) and doxorubicin (DOX) nanocapsules (TQ-DOX/Cys-Py/PA NCs) to be delivered directly to the lungs. TQ-DOX/Cys-Py/PA NCs were created using a single-step interfacial polycondensation method. The aerodynamic performance assessment shows that the prepared TQ-DOX/Cys-Py/PA NCs were able to deliver 98.7% and 97.1% of the TQ and DOX nominated dose, respectively. TQ and DOX with emitted doses of 2008.2 and 110.2 μg can reach the lower parts of the respiratory system and have an aerodynamic particle size between 1 and 5 μm, which revealed that the optimum formulation would produce a small particle size (19.89 nm) with high entrapment efficiency (TQ: 85.4%, DOX: 99.49%) and loading efficiency (TQ: 52.2%, DOX: 15.03). The targeted release of TQ and DOX in 0.1 M GSH-containing buffer solution demonstrated a faster onset of action, with 50% released within the first 2 hours. In vivo studies were conducted to demonstrate the efficacy of TQ-DOX/Cys-Py/PA NCs in enabling targeted drug delivery to the lungs for the treatment of lung cancer. The results demonstrate exceptional lung targeting and sustained lung retention for at least 24 hours. Furthermore, the toxicity of the TQ-DOX/Cys-Py/PA NCs was assessed by quantifying the protein carbonyl content. The results showed that the TQ-DOX/Cys-Py/PA NCs exhibited reduced toxicity to the heart, liver, and kidney compared to free DOX and DOX/Cys-Py/PA NCs.
1. Introduction
Development of novel polymer-based drug delivery systems is required to effectively deliver drugs in a regulated manner while also modifying the physicochemical and pharmacokinetic properties of therapeutic molecules.1–5 Polymer nanotechnology focuses on reducing the toxicity of anticancer drugs and improving their bioavailability.6–8Pulmonary drug delivery is a favourable alternative method of administering drugs, offering numerous advantages compared to conventional routes.9,10 It involves minimal enzymatic exposure, has fewer systemic side effects, avoids the first-pass metabolism impact and delivers a higher and more concentrated amount of medication to the site of the disease.11,12 Pulmonary drug delivery allows for direct drug access to the lungs with minimal side effects and a rapid pharmacological response and is considered a promising alternative strategy for the treatment of lung cancer.13,14 Pulmonary drug delivery formulations can be composed of either the drug alone or the drug combined with a compatible carrier. Different types of carriers, including lipids, sugars, and polymers, are utilized for transporting pulmonary drugs to the lungs.15–19
Amino acids are precious building blocks that can be used to design biodegradable or biocompatible polyamides with many physical features.20–22 Creating novel synthetic biodegradable polymers with amino acid moieties for use as drug delivery systems is highly desirable.23,24 Among the several categories of polymers, polyamides prepared using amino acids possess notable characteristics like excellent biocompatibility, gradual degradability, and versatile physicochemical modification capabilities.25,26 The hydrophilicity of the constituent amino acids in the polymer affects the degradation rates of the polymer chain.27–29 Polyamides are employed in the composition of chemotherapeutics to achieve targeted administration over a specific period, thereby reducing drug-related side effects and enhancing effectiveness.30,31
A targeted drug delivery system utilising cystine amino acid as a diamine monomer to create a nonpeptidic polyamide with a biodegradable component due to the reduction of disulfide bonds would be appealing. Polymers with disulfide bonds can react with the elevated levels of glutathione inside cells and release the drugs they carry by undergoing disulfide–thiol exchange reactions with GSH. These polyamides were used to regulate the release of anticancer medications due to the increased concentration of GSH in some tumour cells compared to normal cells.32–35
Doxorubicin (DOX) is a highly effective drug for the treatment of various hematopoietic malignancies and solid tumours. Its use has been limited because of the low bioavailability and severe side effects, such as irreversible cardiotoxicity, ROS production, targeting topoisomerase IIβ in cardiomyocytes, DNA damage, therapy-related malignancy, and gonadotoxicity.36,37 Due to these limitations, new formulations containing DOX have been developed.38–42
Thymoquinone (TQ) is obtained from the black seed, a plant that has shown effectiveness in the treatment of cancer. Studies have shown that the effectiveness of the treatment is improved when it is enclosed in a liposome that is specifically designed to target cancer cells. Multiple studies have demonstrated that TQ possesses the capacity to impede the progression of the tumour cell cycle. Pharmacokinetic studies have demonstrated that TQ exhibits a low bioavailability, a sluggish absorption rate, and a rapid elimination rate.43–45
Co-administering TQ with other chemotherapeutic drugs can synergistically enhance their therapeutic efficacy while minimising their toxic effects on cells.46 The combination of anticancer drugs TQ and DOX can minimise cytotoxicity and simultaneously maximise efficacy. Encapsulated TQ into lipid-polymer nanoparticles was reported to enhance its anticancer and oral delivery efficiency. TQ nanoparticles with free DOX were prepared to increase DOX efficiency.47 The results demonstrate that the co-delivery of encapsulated TQ through oral administration and free DOX could improve the anticancer efficiency of DOX and result in a higher anticancer activity of the encapsulated TQ nanoparticles than free TQ. In addition, this delivery system enhances the TQ bioavailability and cellular uptake.48–50
Several independent studies have indicated that combining TQ and DOX anticancer drugs can have a substantial effect, reducing the harmful effects on cells while maximising effectiveness. However, the potential of using polymeric nanocapsules derived from cystine amino acid to enhance the delivery of TQ with DOX for lung cancer treatment remains unexplored. These newly synthesized TQ–DOX polyamide based nanocapsules could serve as a promising drug delivery system, potentially revolutionising lung cancer treatment. We hypothesise that pulmonary drug administration of these polymeric nanocapsules of anticancer drugs TQ and DOX could directly target the lungs, bypassing cytotoxicity and offering a new ray of hope in the fight against lung cancer.51–55
2. Materials and methods
2.1 Materials
L-Cystine amino acid, DOX and TQ were purchased from Sigma Chemicals Co. (Pool, UK). Chloroform, acetonitrile, trifluoroacetic acid (TFA), HPLC grade water (H2O), dimethylsulphoxide (DMSO), dimethylformamide (DMF), dithiothreitol (DTT) and phosphate buffered saline (PBS) were supplied by Alpha Chemika (Mumbai, India). Methanol and thionyl chloride were supplied by Tedia high-purity solvents (Fairfield, USA). 2,6-Pyridinedicarbonyl dichloride was obtained from Thermo Fisher Scientific (Karlsruhe, Germany). The protein carbonyl content kit was obtained from Abcam (Cambridge, United Kingdom).2.2 Polyamide and TQ–DOX loaded polyamide
2.3 High performance liquid chromatography (HPLC) for the quantification of TQ and DOX
The Dionex Ultimate 3000 HPLC was used for concurrent quantitative analysis of TQ and DOX, and an appropriate method was developed for each drug. The provided system uses a gradient pump, UV detector set at 250 nm (TQ) and 550 nm (DOX), and Fortis C18 analytical column (Fortis Technologies Ltd C18, 250 × 4.6 mm). The mobile phase consisted of acetonitrile:0.1% TFA in water (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 v/v). The temperature of the sample was set at 27 °C. The sample injection volume was 10 μL with a flow rate of 0.8 mL min−1. The run time was 12 minutes. In terms of specificity, accuracy, precision, and linearity, as well as limits of detection and quantification, the HPLC technique was validated according to ICH guidelines.56 For the calibration curve, samples were prepared in acetonitrile and serial dilutions were made using the same solvent.
15 v/v). The temperature of the sample was set at 27 °C. The sample injection volume was 10 μL with a flow rate of 0.8 mL min−1. The run time was 12 minutes. In terms of specificity, accuracy, precision, and linearity, as well as limits of detection and quantification, the HPLC technique was validated according to ICH guidelines.56 For the calibration curve, samples were prepared in acetonitrile and serial dilutions were made using the same solvent.
2.4 Characterisation of the polyamide and TQ-DOX loaded polyamides
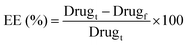 | (1) |
As for DLC, 10 mg of TQ-DOX/Cys-Py/PA NCs was dissolved in 10 mL of DMSO and 10 μL were injected into an HPLC using the same method to calculate the DLC% of TQ and DOX in TQ-DOX/Cys-Py/PA NCs. The DLC was determined using eqn (2).
 | (2) |
2.5 In vitro study
The results obtained from the NGI analysis were used to calculate the main aerodynamic performance parameters of the formula. The cumulative quantity of TQ or DOX collected from the induction tube, the pre-separator, trays 1–7 and the final micro-orifice collector (MOC) were used to calculate the emitted dose (ED%), as can be seen from eqn (3). ED is a true representation of the efficiency of the formulation to be discharged from the capsules. The next parameter is the respirable dosage (RD), which is the representation of the total amount of the formula that can reach the lower parts of the respiratory system, which is within the size range of 1–5 μm and is the sum of drug content from trays 2 to 7. The last aerodynamic parameters are the fine particle fraction of the emitted dose (FPFED) and the fine particle fraction of the theoretical dose (FPFTD). These two parameters were calculated using eqn (4) and (5) respectively.
 | (3) |
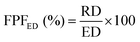 | (4) |
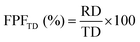 | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–16
000–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da]. The bags were suspended in 30 mL of PBS in beakers and kept in a shaking water bath at 37 °C and 100 rpm covered. 1 mL was withdrawn from the beaker at 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14, 16, 20 and 24 h. The TQ and DOX API content was quantified using the validated HPLC method. Fresh PBS was added after each withdrawal to maintain the sink conditions. Another 10 mg of the TQ-DOX/Cys-Py/PA NCs was also prepared and was dispersed in PBS that contained 0.1 M glutathione (GSH) and the same procedure was carried out. The experiment was performed in triplicate and results were calculated as mean % release ±SD.
000 Da]. The bags were suspended in 30 mL of PBS in beakers and kept in a shaking water bath at 37 °C and 100 rpm covered. 1 mL was withdrawn from the beaker at 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14, 16, 20 and 24 h. The TQ and DOX API content was quantified using the validated HPLC method. Fresh PBS was added after each withdrawal to maintain the sink conditions. Another 10 mg of the TQ-DOX/Cys-Py/PA NCs was also prepared and was dispersed in PBS that contained 0.1 M glutathione (GSH) and the same procedure was carried out. The experiment was performed in triplicate and results were calculated as mean % release ±SD.
2.6 In vivo studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 minutes; the supernatant was kept in the refrigerator at −80 °C for later analysis.
000 rpm for 30 minutes; the supernatant was kept in the refrigerator at −80 °C for later analysis.
For the quantification of DOX and TQ, 75 μL of diluted perchloric acid solution, in a ratio of 1.195 to 100 mL distilled water, was added to 300 μL of the supernatant to destroy any remaining lung tissue and centrifuged for 10 min at a speed of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and then the sample was diluted with 0.5 mL of acetonitrile. The sample was filtered and injected in the HPLC using the same procedure as for TQ and DOX validation.57
000 rpm and then the sample was diluted with 0.5 mL of acetonitrile. The sample was filtered and injected in the HPLC using the same procedure as for TQ and DOX validation.57
| Groups | Description |
|---|---|
| Group 1: control group | Untreated mice |
| Group 2: negative control | Each mouse got 2.5 mg kg−1 DOX intraperitoneally every other day for 12 days (total doses = 6) |
| Group 3: TQ-DOX/Cys-Py/PA NCs | Each mouse got 17 mg of the formula, which contained 2.5 mg of DOX and 9 mg of TQ via the inhalation route every other day for 12 days (total doses = 6) |
| Group 4: DOX/Cys-Py/PA NCs | Each mouse got 19 mg of the DOX polyamide formula, which contained 2.5 mg of DOX, via the inhalation route every other day for 12 days (total doses = 6) |
On day 13, the mice were sacrificed by cervical dislocation, and the hearts, livers, and kidneys were collected, flushed with PSB, weighed, and kept in the refrigerator at – 80 °C. After that, the hearts, livers, and kidneys were thawed in an ice bath before being washed in distilled water, cut into small pieces, and ground with a mortar and pestle with liquid nitrogen; the powder was then stored at −80 °C in Eppendorf tubes for later analysis. Using a homogeniser, 50 mg of each sample was homogenised with 1.5 mL of PSB (pH 7.4) with 1% DTT. The homogenate samples were centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm at 4 °C for 20 minutes; the supernatant was collected and stored at – 80 °C until needed. To determine the amount of extracted soluble proteins present in the samples by the Bradford Assay, 5 μL was taken from each sample and transferred into 96 well plates, and the reading was taken at 595 nm. A bovine serum albumin calibration curve was used.60 This study used a protein carbonyl content kit from Abcam, UK. According to the kit booklet, the samples were treated with streptozocin to remove the nucleic acid and then DNPH and trichloroacetic acid were added and washed with cold acetone twice to get rid of free DNPH, and then the proteins were solubilised using guanidine solution. After that, 100 μl of each sample was transferred to the 96-well plate, and the absorbance was measured at 375 nm in a microplate reader. The carbonyl content was calculated. The data are presented as mean ± SD. Statistical significance was determined using one-way ANOVA.61–64
000 rpm at 4 °C for 20 minutes; the supernatant was collected and stored at – 80 °C until needed. To determine the amount of extracted soluble proteins present in the samples by the Bradford Assay, 5 μL was taken from each sample and transferred into 96 well plates, and the reading was taken at 595 nm. A bovine serum albumin calibration curve was used.60 This study used a protein carbonyl content kit from Abcam, UK. According to the kit booklet, the samples were treated with streptozocin to remove the nucleic acid and then DNPH and trichloroacetic acid were added and washed with cold acetone twice to get rid of free DNPH, and then the proteins were solubilised using guanidine solution. After that, 100 μl of each sample was transferred to the 96-well plate, and the absorbance was measured at 375 nm in a microplate reader. The carbonyl content was calculated. The data are presented as mean ± SD. Statistical significance was determined using one-way ANOVA.61–64
3. Results and discussion
3.1 Synthesis of polyamide and polyamide loaded with anticancer drugs
3.2 Quantification of TQ and DOX using HPLC
According to the ICH requirements for analytical technique validation, the HPLC method for TQ and DOX quantification has been validated.56 Both actives demonstrated a good linearity over the specified concentration range where the retention time for DOX was 2.46 ± 0.17 min at 550 nm, while TQ eluted at 6.05 ± 0.22 min at 250 nm. The linear calibration curves were created at concentrations that ranged from 3.9 to 62.5 μg mL−1 for TQ and from 2.0 to 125 μg mL−1 for DOX. Recovery calculations were conducted with percentage recovery ranging from 98.23 to 99.78%. The RSD for the active (TQ and DOX) for all concentrations over three days (9 samples) was less than 2%, indicating that the technique was accurate and precise. The calculated LOD was 2.774 μg mL−1 for TQ and 0.942 μg mL−1 for DOX, whereas the LOQ was 8.408 μg mL−1 for TQ and 2.854 μg mL−1 for DOX. Overall, the optimised and validated method was demonstrated to be a good and valid method for the quantification of TQ and DOX. The method was specific to DOX and TQ and the polymer did not dissolve in the dissolution or HPLC medium (CH3CN).3.3 Characterisation of Cys-Py/PA and TQ-DOX/Cys-Py/PA NCs
The solubility of the TQ, DOX, Cys-Py/PA, and TQ-DOX/Cys-Py/PA NCs was studied in various common organic solvents; the results are shown in Table 2. Apparently, Cys-Py/PA and TQ-DOX/Cys-Py/PA NCs were soluble in DMSO and slightly soluble in DMF. As both TQ and DOX were soluble in acetonitrile (CH3CN), they were used for the HPLC standard solutions and calibration curve preparation. The change in the solubility of DOX and TQ in the formula due to the polymer chain surrounding the encapsulated sample (DOX and TQ) changes the solubility upon encapsulation through reduced interaction with solvent molecules.
| H2O | MeOH | EtOH | DMSO | Acetone | CHCl3 | DMF | CH3CN | |
|---|---|---|---|---|---|---|---|---|
| TQ | − | + | + | + | + | + | + | + |
| DOX | + | ± | + | + | − | − | + | + |
| Cys-Py/PA | − | − | − | + | − | − | ± | − |
| (TQ-DOX/Cys-P/PA) NCs | − | − | − | + | − | − | ± | − |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group in the ketone, as shown in Fig. 3(a). The FTIR spectrum of DOX revealed a characteristic sharp peak at 1730 cm−1, which is attributed to the carbonyl (C
O) group in the ketone, as shown in Fig. 3(a). The FTIR spectrum of DOX revealed a characteristic sharp peak at 1730 cm−1, which is attributed to the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group. Additionally, IR bands corresponding to the N–H bond in the amine group were detected at 3320 cm−1 and those corresponding to the O–H groups were detected at 3526 cm−1, as shown in Fig. 3(b).55,65
O) group. Additionally, IR bands corresponding to the N–H bond in the amine group were detected at 3320 cm−1 and those corresponding to the O–H groups were detected at 3526 cm−1, as shown in Fig. 3(b).55,65
The FTIR spectra of Cys-Py/PA and TQ-DOX/Cys-Py/PA NCs exhibited identical characteristic features, which could be attributed to the overlapping of the spectra of Cys-Py/PA and drugs (TQ and DOX). The most important bands exhibit two distinct infrared stretching absorptions that are characteristic of carboxylic acid. The O–H group exhibited a broad spectral range between 2384 and 3648 cm−1, while the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group displayed stretching vibrations at 1722 cm−1. Conversely, in the amide group, a distinct stretching vibration band for the C
O group displayed stretching vibrations at 1722 cm−1. Conversely, in the amide group, a distinct stretching vibration band for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the formed amide group was observed at approximately 1655 cm−1. Additionally, IR bands corresponding to the N–H bond were detected at 3363 cm−1 for stretching vibrations and at 1622 cm−1 for bending vibrations, as depicted in Fig. 3(c) and (d).25
O bond of the formed amide group was observed at approximately 1655 cm−1. Additionally, IR bands corresponding to the N–H bond were detected at 3363 cm−1 for stretching vibrations and at 1622 cm−1 for bending vibrations, as depicted in Fig. 3(c) and (d).25
The 13C-NMR spectrum of Cys-Py/PA: the carbonyl carbon of the amide bond is observed at a chemical shift of 163.85 ppm, while the carbon of the carboxylic acid was observed at a chemical shift of 172.8 ppm. The chemical shifts of the carbons of the pyridine ring unit (CHs) were observed at 139.97 and 125.44, while the quaternary carbon of aromatic pyridine ring carbon was observed at 148.62 ppm. The carbon chemical shift of the CH group attached to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the carboxylic acid in Cys-Py/PA was determined at 52.27 ppm. The chemical shift of the carbon in CH2 was determined at 39.57 ppm, as presented in Fig. 4(b) and (c).
O group of the carboxylic acid in Cys-Py/PA was determined at 52.27 ppm. The chemical shift of the carbon in CH2 was determined at 39.57 ppm, as presented in Fig. 4(b) and (c).
The 2D-NMR spectrum of Cys-Py/PA: the 1H–1H COSY spectrum indicates the presence of an AMX splitting pattern in the protons of the CH–CH2 group within the cystine unit. The A, M, and X signals are observed as a pattern consisting of two sets of distinct peaks. Correlations can be observed between the signals of protons labelled as H9 and H9′′ and the signal of protons labelled as H6.66 as shown in the 1H–1H COSY NMR spectrum in Fig. 4(d).
 | ||
| Fig. 5 DSC thermograms of (a) TQ, (b) DOX, (c) Cys-Py/PA, and (d) TQ-DOX/Cys-Py/PA NCs, and (e) compiled thermograms of all the materials. | ||
The TGA of Cys-Py/PA and the TQ-DOX/Cys-Py/PA NCs is presented in Fig. 6(a) and (b) respectively. The two materials had a moisture content of 3% and the degradation of the polymer started at around 200 °C. From the figures, it could be concluded that the polymer is thermally stable and the addition of the two APIs (TQ and DOX) did not alter the thermal stability of the polymer.
Particle size analysis using a Zetasizer indicates the NC size of the Cys-Py/PA and TQ-DOX/Cys-Py/PA NCs, as depicted in Table 3. From the table, it is noted that the Cys-Py/PA and TQ-DOX/Cys-Py/PA NCs are within the nanosize range. A slight increase in the particle size was noted with the addition of the drug due to the encapsulation effect and the increase in size with the encapsulation of the drug within the polymeric chains.57,71 The particles demonstrated a PDI that ranges between 0.41 and 0.65, indicating the aggregated nature of the Cys-Py/PA and TQ-DOX/Cys-Py/PA NCs.
| Material | Particle size (nm) | Polydispersity index (PDI) | Zeta potential (mV) |
|---|---|---|---|
| TQ | 676.5 ± 121.62 | 0.64 ± 0.21 | −4.21 ± 1.57 |
| DOX | 518.7 ± 80.76 | 0.55 ± 0.1 | 4.94 ± 2.05 |
| Cys-Py/PA | 114.9 ± 47.09 | 0.41 ± 0.03 | −30.27 ± 1.01 |
| (TQ/Cys-Py/PA) NCs | 125.7 ± 30.76 | 0.58 ± 0.1 | −26.81 ± 0.67 |
| (DOX/Cys-Py/PA) NCs | 130.9 ± 57.09 | 0.48 ± 0.13 | −21.83 ± 1.69 |
| (TQ-DOX/Cys-Py/PA) NCs | 145.99 ± 56.12 | 0.65 ± 0.08 | −28.30 ± 0.78 |
The TEM micrographs of the TQ-DOX/Cys-Py/PA NCs are presented in Fig. 7. As can be seen, the particles were within the nanosized range. Using Image J software, the average particle size was 19.89 ± 6.5 nm (n = 25). Particles were spherical in shape. However, the average particle size, as obtained from laser diffraction, was 145.99 ± 56.12 nm. This difference in size using the two methods could be attributed to the nature of the polymeric material. The variance between the particle sizes observed by these two methods was because the TQ-DOX/Cys-Py/PA NCs were swelled with water during DLS. When the TQ-DOX/Cys-Py/PA NCs are subjected to DLS, they come into contact with water, which leads to significant swelling. This polymeric type exhibits a remarkable swelling capacity of approximately 200% within 20 minutes. As the NCs absorb water, their dimensions expand, resulting in a larger apparent size when measured by DLS. This swelling highlights the influence of hydration on the physical characteristics of nanoparticles, necessitating careful interpretation of DLS results.25,71 In contrast, TEM showed the definite size of the NCs. Such phenomena were previously reported, where using laser diffraction analysis, it was found that the presence of a hydrodynamic layer around the polymeric material could affect the size, and, hence, a larger particle size was observed.5 Furthermore, the NCs aggregated with a PDI exceeding 0.41 during the laser diffraction analysis; therefore, the presence of aggregates added to the discrepancy in the particle size. The zeta potential of the produced polymeric NCs (CYS-PA) was on the negative scale (−30.27 ± 1.01 mV). Such results are promising as they can aid colloidal de-aggregation of the particles due to repulsive forces between particles. However, the charge was reduced upon encapsulation of the two actives due to the neutral charge of the actives.
3.4 In vitro study
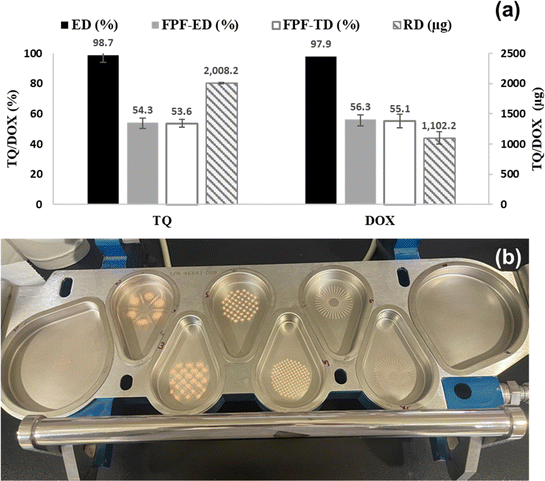
In general, the aerodynamic performance results of the synthesised NC formulations containing TQ and DOX highlighted the nano-aggregates' capability and efficiency in delivering particles to the lungs. The low SD of the aerodynamic parameters indicated the reproducibility of results in Fig. 9(b).
TQ and DOX behaviours were investigated in PBS with a pH of 7.4, at 37 °C, in the presence or absence of GSH. Fig. 10 shows the release patterns of TQ and DOX as time progresses. The release profile of DOX from 0.1 M GSH-containing buffer solution demonstrated a faster onset of action, with 50% released within the first 2 hours. This would be favourable, as once the nanoparticles are taken up by the cancer cell that contains higher GSH levels, the drug will be immediately released. However, when GSH was not added, there was a delay in release for almost 6 hours to start releasing the drug from NCs. Overall, after 24 hours, the release in the two media reached almost 80%. As for TQ, similar to DOX, the 0.1 M GSH containing media promoted the degradation of the polymer and hence faster release of the drug with 50% release within the first 2 hours.55,75
3.5 In vivo study
NCs are promising drug delivery systems with numerous biological benefits in vivo.77 These tiny molecules can quickly penetrate physiological barriers and reach target locations with high drug concentrations.78 Natural NCs are biocompatible and biodegradable and have lower side effects than alternative drug delivery systems.79,80 The use of inhalable natural NCs provides significant advantages in medical treatment by enhancing targeted therapy and reducing systemic toxicity. These NCs are designed to deliver drugs directly to the lung, allowing for more precise targeting of respiratory diseases. This targeted approach ensures that the medication reaches the affected area more efficiently, improving therapeutic outcomes. Additionally, by localising the drug delivery, systemic exposure to the medication is minimised, which significantly reduces the risk of side effects and systemic toxicity. This method not only improves the efficacy of the treatment but also enhances patient safety and comfort.81,82 In this regard, this study was undertaken with the assumption that employing NCs via inhalation can be an effective and safe approach to improving delivery of TQ and DOX drugs. The study found that inhaling NCs delivered TQ and DOX directly to the lung, resulting in sustained increases in TQ and DOX lung concentration after 0.5, 1, 2, 4, 8,16, and 24 hours of therapy (see Fig. 11). These findings suggest that TQ and DOX can directly reach the lung without passing through the gastrointestinal tract or liver. These findings indicate that inhaling NCs can provide a rapid therapeutic response while avoiding the adverse effects of oral DOX treatment. However, additional pharmacological studies are needed to corroborate these findings.
At the liver level, there was no significant increase in the carbonyl content; this indicates that no liver injury happened to the treated mice in the 4 groups (p = 0.336862), Fig. 12(b); this may be due to the low dose of DOX since the previous literature found that the low dose of DOX does not cause liver toxicity.87
In kidneys, a significant increase in carbonyl content in group 4 was observed compared with the other groups (p < 0.00001), and this may indicate that the prepared Cys-Py/PA may cause an increase in oxidative stress in the kidney; on the other hand, the mice in group 3 showed no significant difference compared to those in groups 1 and 2 (p = 0.99743 and 0.79346), respectively, and this may be due to the antioxidant effect of TQ on reducing the nephrotoxicity (Fig. 12(c)). However, additional pharmacological studies are needed to corroborate these findings.
4. Conclusions
Developing dry powder inhalers (DPIs) that have a consistent distribution of ingredients and consistent ability to reach the desired areas of the respiratory system is difficult. Hence, the objective of this project was to create a fixed-dose combination (FDC) of TQ and DOX. Using an interfacial polycondensation method, this combination was loaded onto a new cystine-based polyamide. The process involved polymerisation and encapsulation of TQ and DOX, all done in a single step. The aerodynamic performance assessment showed that the formulated substance could deliver 98.7% and 97.1% of the intended dose to the lungs. The emitted dose could reach the lower regions of the respiratory system effectively. The particle size ranges from 1 to 5 μm, which makes it well-suited for aerodynamic purposes. In addition, when examined in a 0.1 M GSH-containing buffer solution, TQ and DOX were selectively released, demonstrating a swift initiation of activity, with 50% of the compounds being released within the first 2 hours. In vivo studies showed that the formula enables targeted drug delivery to treat lung cancer. Toxicity was assessed by measuring protein carbonyl content. The prepared TQ-DOX/Cys-Py/PA NCs had lower heart, liver, and kidney toxicity. Overall, this study developed and applied novel TQ and DOX NCs to deliver anticancer drugs to the lung through pulmonary administration. This treatment can exert its effects specifically in the localised area of lung cancer.Author contributions
Hadeel Hafez Banat: writing – original draft, methodology, investigation, formal analysis and data curation; Dalia Khalil Ali: writing – original draft, writing – reviewing, editing the final version of the draft, project conceptualization and administration, methodology, formal analysis, funding acquisition; Qais Jarrar: methodology, formal analysis, data curation, writing – original draft, writing – reviewing and editing; Esra’a Alomary: methodology, data curation; and Eman Zmaily Dahmash: writing – original draft, writing – reviewing, editing the final version of the draft, project conceptualization, methodology, formal analysis.Data availability
All the data used in this research project are included in the study.Conflicts of interest
The authors confirm that there are no conflicts to declare.Acknowledgements
The authors acknowledge the support of Isra University (Jordan) for funding Dr Dalia Ali (4-18/2022/2023). Also, the authors further acknowledge Kingston University for supporting Dr Eman Dahmash.References
- R. Wen, A. C. Umeano, P. Chen and A. A. Farooqi, Polymer-based drug delivery systems for cancer, Crit. Rev. Ther. Drug Carrier Syst., 2018, 35(6), 521–554, DOI:10.1615/CritRevTherDrugCarrierSyst.2018021124.
- H. AbdulKarim, D. K. Ali, E. Taybeh, H. S. Alyami, S. M. Assaf and E. Z. Dahmash, Novel poly (ester amide) derived from tyrosine amino acid for targeted pulmonary drug delivery of fluticasone propionate, J. Appl. Polym. Sci., 2023, e53672 CrossRef CAS.
- N. M. Al-baldawi, D. Khalil, Q. Jarrar, R. Abuthawabeh and E. Zmaily, Journal of Drug Delivery Science and Technology Quality by design for the synthesis and optimisation of arginine-poly(ester-amide) nanocapsules as promising carriers for nose-brain delivery of carbamazepine, 89, 2023.
- M. H. Alyami, E. Z. Dahmash, D. K. Ali, H. S. Alyami, H. AbdulKarim and S. A. Alsudir, Novel Fluticasone Propionate and Salmeterol Fixed-Dose Combination Nano-Encapsulated Particles Using Polyamide Based on L-Lysine, Pharmaceuticals, 2022, 15(3), 321 CrossRef CAS PubMed.
- E. Z. Dahmash, L. M. Attiany, D. Ali, S. M. Assaf, J. Alkrad and H. Alyami, Development and Characterization of Transdermal Patches Using Novel Thymoquinone-L-Arginine-Based Polyamide Nanocapsules for Potential Use in the Management of Psoriasis, AAPS PharmSciTech, 2024, 25(4), 1–16, DOI:10.1208/s12249-024-02781-2.
- J. Kaur, et al., A hand-held apparatus for ‘nose-only’ exposure of mice to inhalable microparticles as a dry powder inhalation targeting lung and airway macrophages, Eur. J. Pharm. Sci., 2008, 34(1), 56–65, DOI:10.1016/j.ejps.2008.02.008.
- R. Gobi, P. Ravichandiran, R. S. Babu and D. J. Yoo, Biopolymer and synthetic polymer-based nanocomposites in wound dressing applications: A review, Polymers, 2021, 13(12), 1962, DOI:10.3390/polym13121962.
- M. F. Maitz, Applications of synthetic polymers in clinical medicine, Biosurf. Biotribol., 2015, 1(3), 161–176, DOI:10.1016/j.bsbt.2015.08.002.
- Z. Liang, R. Ni, J. Zhou and S. Mao, Recent advances in controlled pulmonary drug delivery, Drug Discovery Today, 2015, 20(3), 380–389, DOI:10.1016/j.drudis.2014.09.020.
- F. Lavorini, M. Pistolesi and O. S. Usmani, Recent advances in capsule-based dry powder inhaler technology, Multidiscip. Respir. Med., 2017, 12(1), 11, DOI:10.1186/s40248-017-0092-5.
- H. Banat, R. Ambrus and I. Csóka, Drug combinations for inhalation: Current products and future development addressing disease control and patient compliance, Int. J. Pharm., 2023, 643, 123070, DOI:10.1016/j.ijpharm.2023.123070.
- Q. Fei, I. Bentley, S. N. Ghadiali and J. A. Englert, Pulmonary drug delivery for acute respiratory distress syndrome, Pulm. Pharmacol. Ther., 2023, 79, 102196, DOI:10.1016/j.pupt.2023.102196.
- J. S. Patton, C. S. Fishburn and J. G. Weers, The Lungs as a Portal of Entry for Systemic Drug Delivery, Proc. Am. Thorac. Soc., 2004, 1(4), 338–344, DOI:10.1513/pats.200409-049TA.
- P. Sharma, et al., Emerging trends in the novel drug delivery approaches for the treatment of lung cancer, Chem. – Biol. Interact., 2019, 309, 108720, DOI:10.1016/j.cbi.2019.06.033.
- J. S. Patil and S. Sarasija, Pulmonary drug delivery strategies: A concise, systematic review, Lung India, 2012, 29(1), 44–49, DOI:10.4103/0970-2113.92361.
- N. Politakos, V. G. Gregoriou and C. L. Chochos, Pulmonary Drug Delivery through Responsive Materials, pp. 490–508, 2024.
- W. Lee, E. Lee, D. Jang, Y. Cho, J. Cha and H. Lee, Polymeric Carriers for Pulmonary Drug Delivery, Yakhak Hoeji, 2016, 60(4), 173–179, DOI:10.17480/psk.2016.60.4.173.
- Y. Javadzadeh and S. Yaqoubi, Therapeutic nanostructures for pulmonary drug delivery, Nanostructures for Drug Delivery, Elsevier, 2017, pp. 619–638 Search PubMed.
- M. Kumar, A. R. Hilles, S. Hamed, A. Bhatia and S. Mahmood, Micro and nano-carriers-based pulmonary drug delivery system: Their current updates, challenges, and limitations – A review, JCIS Open, 2023, 12, 100095, DOI:10.1016/j.jciso.2023.100095.
- S. S. Gupta, V. Mishra, M. Das Mukherjee, P. Saini and K. R. Ranjan, Amino acid derived biopolymers: Recent advances and biomedical applications, Int. J. Biol. Macromol., 2021, 188, 542–567 CrossRef CAS PubMed.
- W. Lu, X. Wang, R. Cheng, C. Deng, F. Meng and Z. Zhong, Biocompatible and bioreducible micelles fabricated from novel α-amino acid-based poly(disulfide urethane)s: Design, synthesis and triggered doxorubicin release, Polym. Chem., 2015, 6(33), 6001–6010, 10.1039/c5py00828j.
- M. Pechar, A. Braunová, K. Ulbrich, M. Jelínková and B. Říhová, Poly(ethylene glycol)-doxorubicin conjugates with pH-controlled activation, J. Bioact. Compat. Polym., 2005, 20(4), 319–341, DOI:10.1177/0883911505055161.
- A. C. Fonseca, M. H. Gil and P. N. Simões, Biodegradable poly(ester amide)s – A remarkable opportunity for the biomedical area: Review on the synthesis, characterization and applications, Prog. Polym. Sci., 2014, 39(7), 1291–1311, DOI:10.1016/j.progpolymsci.2013.11.007.
- B. Al-tayyem and B. Sweileh, Synthesis, characterization and hydrolytic degradation of novel biodegradable poly(ester amide) s derived from Isosorbide and α-amino acids, J. Polym. Res., 2020, 27(5), 1–14 Search PubMed.
- D. K. Ali, A. M. Al-Zuheiri and B. A. Sweileh, pH and reduction sensitive bio-based polyamides derived from renewable dicarboxylic acid monomers and cystine amino acid, Int. J. Polym. Anal. Charact., 2017, 22(4), 361–373, DOI:10.1080/1023666X.2017.1298012.
- S. H. S. Boddu, et al., Polyamide/Poly(Amino Acid) Polymers for Drug Delivery, 2021 Search PubMed.
- M. Miyamoto, et al., Improved nasal absorption of drugs using poly-l-arginine: effects of concentration and molecular weight of poly-l-arginine on the nasal absorption of fluorescein isothiocyanate–dextran in rats, Eur. J. Pharm. Biopharm., 2001, 52(1), 21–30, DOI:10.1016/S0939-6411(01)00149-7.
- E. Stiegelmaier, T. C. Costa, G. Pakuszewski, S. M. A. Guelli Ulson de Souza, A. A. Ulson de Souza and A. P. Serafini Immich, Enhancing polyamide 6: Acid hydrolysis for functionalization and amino group quantification, Polymer, 2024, 298, 126905, DOI:10.1016/J.POLYMER.2024.126905.
- Y. Tao, X. Tan and T. Zhang, Enhancing biocompatibility and degradation control of biodegradable polymers through amino acid grafting: A study on 4arm-PLGA-Amino acid copolymers, Appl. Mater. Today, 2024, 41, 102497, DOI:10.1016/J.APMT.2024.102497.
- J. V. González-Aramundiz, M. V. Lozano, A. Sousa-Herves, E. Fernandez-Megia and N. Csaba, Polypeptides and polyaminoacids in drug delivery, Expert Opin. Drug Delivery, 2012, 9(2), 183–201, DOI:10.1517/17425247.2012.647906.
- É. A. de Souza, et al., Incorporation of the chemotherapy medication cisplatin into polyamide membrane, J. Inorg. Biochem., 2018, 180, 171–178, DOI:10.1016/J.JINORGBIO.2017.12.013.
- J. Wu, et al., Hydrophobic Cysteine Poly(disulfide)-based Redox-Hypersensitive Nanoparticle Platform for Cancer Theranostics, Angew. Chem., Int. Ed., 54, 32, 9218–9223, 2015 DOI:10.1002/anie.201503863.
- L. Yu, L. Kong, J. Xie, W. Wang, C. Chang and H. Che, Reduction-sensitive N,N′-Bis(acryloyl) cystinamide-polymerized Nanohydrogel as a Potential Nanocarrier for Paclitaxel Delivery, Des. Monomers Polym., 2021, 24(1), 98–105, DOI:10.1080/15685551.2021.1914398.
- N. Traverso, et al., Role of glutathione in cancer progression and chemoresistance, Oxid. Med. Cell. Longevity, 2013, 2013, 972913, DOI:10.1155/2013/972913.
- S. P. Hadipour Moghaddam, J. Saikia, M. Yazdimamaghani and H. Ghandehari, Redox-Responsive Polysulfide-Based Biodegradable Organosilica Nanoparticles for Delivery of Bioactive Agents, ACS Appl. Mater. Interfaces, 2017, 9(25), 21133–21146, DOI:10.1021/acsami.7b04351.
- J. J. C. Neefjes, et al., New insights into the activities and toxicities of the old anti-cancer drug doxorubicin. 2020 Search PubMed.
- H. S. Al-malky, S. E. Al Harthi and A. M. M. Osman, Major obstacles to doxorubicin therapy: Cardiotoxicity and drug resistance, J. Oncol. Pharm. Pract., 2020, 26(2), 434–444, DOI:10.1177/1078155219877931.
- S. Sritharan and N. Sivalingam, Review article A comprehensive review on time-tested anticancer drug doxorubicin, Life Sci., 2021, 278, 119527, DOI:10.1016/j.lfs.2021.119527.
- M. Xu, et al., PEG-Detachable Polymeric Micelles Self-Assembled from Amphiphilic Copolymers for Tumor-Acidity-Triggered Drug Delivery and Controlled Release, ACS Appl. Mater. Interfaces, 2019, 11(6), 5701–5713, DOI:10.1021/acsami.8b13059.
- Q. Guo, et al., Doxorubicin-loaded natural daptomycin micelles with enhanced targeting and anti-tumor effect in vivo, Eur. J. Med. Chem., 2021, 222, 113582, DOI:10.1016/j.ejmech.2021.113582.
- I. Micallef and B. Baron, Annals of Clinical Toxicology Doxorubicin: An Overview of the Anti-Cancer and Chemoresistance Mechanisms, 2020.
- O. Tacar, P. Sriamornsak and C. R. Dass, Doxorubicin: an update on anticancer molecular action, J. Pharm. Pharmacol., 2013, 157–170, DOI:10.1111/j.2042-7158.2012.01567.x.
- A. H. Rahmani and S. M. Aly, Nigella Sativa and its active constituents thymoquinone shows pivotal role in the diseases prevention and treatment NIGELLA SATIVA AND ITS ACTIVE CONSTITUENTS THYMOQUINONE SHOWS PIVOTAL, no. January, 2015.
- A. Ahmad, M. Raish and K. M. Alkharfy, The potential role of thymoquinone in preventing the cardiovascular complications of COVID-19, Vasc. Pharmacol., 2021, 106899, DOI:10.1016/j.vph.2021.106899.
- M. A. Khan, M. Tania, S. Fu and J. Fu, Thymoquinone, as an anticancer molecule: From basic research to clinical investigation, Oncotarget, 2017, 8(31), 51907–51919, DOI:10.18632/oncotarget.17206.
- S. Slezakova and J. Ruda-Kucerova, Anticancer activity of artemisinin and its derivatives, Anticancer Res., 2017, 37(11), 5995–6003, DOI:10.21873/anticanres.12046.
- K. E. R. Schobert, Combinatorial effects of thymoquinone on the anti-cancer activity of doxorubicin, Cancer Chemother. Pharmacol., 2011, 867–874, DOI:10.1007/s00280-010-1386-x.
- F. Abbaspour, M. Mahboubeh, E. Fatemeh and R. Y. Robati, Effect of thymoquinone – loaded lipid – polymer nanoparticles as an oral delivery system on anticancer efficiency of doxorubicin, J. Nanostruct. Chem., 2021, 0123456789, DOI:10.1007/s40097-021-00398-6.
- M. Abukhader, Thymoquinone in The clinical Treatment of cancer: Fact or fiction-, Pharmacogn. Rev., 2013, 7(14), 117–120, DOI:10.4103/0973-7847.120509.
- G. M. Ganea, S. O. Fakayode, J. N. Losso, C. F. Van Nostrum, C. M. Sabliov and I. M. Warner, Delivery of phytochemical thymoquinone using molecular micelle modified poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles, Nanotechnology, 2010, 21(28), 285104, DOI:10.1088/0957-4484/21/28/285104.
- O. Shadyro, A. Sosnovskaya, I. Edimecheva, L. Kireicikova, S. Samovich, B. Dubovik, S. Krasny and D. Tzerkovsk, Anticancer activity of thymoquinone and its combinations with doxorubicin and linseed oil in the treatment of xenograft tumors Oleg, Adv. Tradit. Med., 2024 DOI:10.1007/s13596-024-00785-8.
- K. M. Ibiyeye, N. Nordin, M. Ajat and A. B. Zuki, Ultrastructural Changes and Antitumor Effects of Doxorubicin/Nanoparticles on Breast Cancer, Front. Oncol., 2019, 9, 1–14, DOI:10.3389/fonc.2019.00599.
- N. E. El-ashmawy, E. G. Khedr, E. M. Ebeid, M. L. Salem, E. M. Mosalam and A. A. Zidan, Loading ofdoxorubicin and thymoquinone with F2 gel nanofibers improves the antitumor activity and ameliorates doxorubicin- associated nephrotoxicity Nahla, Life Sci., 2017, 109, 525–532, DOI:10.1016/j.lfs.2018.06.008.
- E. Rahmani, S. A. Ghorbanian, H. Rashedi and M. Navaee, Preparation of a pH-responsive chitosan-montmorillonite- nitrogen-doped carbon quantum dots nanocarrier for attenuating doxorubicin limitations in cancer therapy, Eng. Life Sci., 2022, 634–649, DOI:10.1002/elsc.202200016.
- D. K. Ali, S. H. Al-Ali, E. Z. Dahmash, G. Edris and H. S. Alyami, Reduction and pH Dualresponsive Biobased Poly(disulfide-amide) Nanoparticles Using Cystine Amino Acid for Targeting Release of Doxorubicin Anticancer Drug, J. Polym. Environ., 2022, 30, 4809–4820, DOI:10.1007/s10924-022-02552-9.
- ICH, Validation of analytical procedures: Text and methodology Q2(R1), Int. Conf. Harmon. Tech. Requir. Regist. Pharm. Hum. Use, vol. 4, 2005.
- E. Z. Dahmash, et al., Preclinical evaluation of novel synthesised nanoparticles based on tyrosine poly(ester amide) for improved targeted pulmonary delivery, Sci. Rep., 2024, 14(1), 1–16, DOI:10.1038/s41598-024-59588-1.
- E. Y. Podyacheva, E. A. Kushnareva, A. A. Karpov and Y. G. Toropova, Analysis of models of doxorubicin-induced cardiomyopathy in rats and mice. A modern view from the perspective of the pathophysiologist and the clinician, Front. Pharmacol., 2021, 12, 1–12, DOI:10.3389/fphar.2021.670479.
- X. Liu, X. Wang, X. Zhang, Y. Xie, R. Chen and H. Chen, C57BL/6 mice are more appropriate than BALB/C mice in inducing dilated cardiomyopathy with short-term doxorubicin treatment, Acta Cardiol. Sin., 2012, 28(3), 236–240 Search PubMed.
- S. Bruno, L. Ronda, G. Paredi, S. Bettati and A. Mozzarelli, Protein carbonylation detection methods: A comparison, Data Brief, 2018, 19, 2215–2220, DOI:10.1016/j.dib.2018.06.088.
- N. M. Alkurdi, S. H. Hussein-al-ali, A. Albalwi, M. K. Haddad, Y. Aldalahmed and D. K. Ali, Development and Evaluation of a Novel Polymer Drug Delivery System Using Cromolyn-Polyamides-Disulfide using Response Surface Design, vol. 2022, 2022.
- Z. A. G. Ahmed, et al., Development and Evaluation of Amlodipine-Polymer Nanocomposites Using Response Surface Methodology, Int. J. Polym. Sci., 2022, 2022, 427400, DOI:10.1155/2022/3427400.
- A. D. Mytara, K. Chronaki, V. Nikitakos, C. D. Papaspyrides, K. Beltsios and S. Vouyiouka, Synthesis of Polyamide-Based Microcapsules via Interfacial Polymerization: Effect of Key Process Parameters, 2021.
- L. Ripoll and Y. Clement, Polyamide microparticles containing vitamin C by interfacial polymerization: an approach by design of experimentation, Cosmetics, 2016, 3(4), 38 CrossRef.
- E. Z. Dahmash, D. K. Ali, H. S. Alyami, H. Abdulkarim, M. H. Alyami and A. H. Aodah, Novel Thymoquinone Nanoparticles Using Poly(ester amide) Based on L-Arginine-Targeting Pulmonary Drug Delivery, 2022.
- D. K. Ali, Synthesis and Characterization of Novel Biobased Ion – Exchange Bisfuran Polyamides Prepared by Interfacial Polycondensation of Bisfuran Diamine Monomer and Sustainable Dicarboxylic Acid Derivatives, J. Polym. Environ., 2022, 30, 4102–4113, DOI:10.1007/s10924-022-02496-0.
- E. Z. Dahmash, D. K. Ali, H. S. Alyami, H. Abdulkarim, M. H. Alyami and A. H. Aodah, Novel Thymoquinone Nanoparticles Using Poly(Ester Amide) Based on L-Arginine-Targeting Pulmonary Drug Delivery, Polymers, 2022, 14(6), 1082, DOI:10.3390/polym14061082.
- K. Songsurang, N. Praphairaksit, K. Siraleartmukul and N. Muangsin, Electrospray fabrication of doxorubicin-chitosan-tripolyphosphate nanoparticles for delivery of doxorubicin, Arch. Pharmacal Res., 2011, 34(4), 583–592 CrossRef CAS PubMed.
- A. Neacşu, Physicochemical investigation of the complexation between γ-cyclodextrin and doxorubicin in solution and in solid state, Thermochim. Acta, 2018, 661, 51–58, DOI:10.1016/j.tca.2018.01.012.
- X. Zhao, et al., Codelivery of doxorubicin and curcumin with lipid nanoparticles results in improved efficacy of chemotherapy in liver cancer, Int. J. Nanomed., 2014, 10, 257–270, DOI:10.2147/IJN.S73322.
- F. Alregeb, F. Khalili, B. Sweileh and D. K. Ali, Synthesis and Characterization of Chelating Hyperbranched Polyester Nanoparticles for Cd(II) Ion Removal from Water, Molecules, 2022, 27(12), 3656, DOI:10.3390/molecules27123656.
- S. F. A. Tarawneh, et al., Mechanistic Modelling of Targeted Pulmonary Delivery of Dactinomycin Iron Oxide Loaded Nanoparticles for Lung Cancer Therapy, Pharm. Dev. Technol., 2022, 1–35 Search PubMed.
- R. Iyer, et al., Glutathione-responsive biodegradable polyurethane nanoparticles for lung cancer treatment, J. Controlled Release, 2020, 321, 363–371 CrossRef CAS PubMed.
- J. F. Quinn, M. R. Whittaker and T. P. Davis, Glutathione responsive polymers and their application in drug delivery systems, Polym. Chem., 2017, 8(1), 97–126 RSC.
- S. Yu, J. Ding, C. He, Y. Cao, W. Xu and X. Chen, Disulfide cross-linked polyurethane micelles as a reduction-triggered drug delivery system for cancer therapy, Adv. Healthcare Mater., 2014, 3(5), 752–760, DOI:10.1002/adhm.201300308.
- F. Yamashita and M. Hashida, Pharmacokinetic considerations for targeted drug delivery, Adv. Drug Delivery Rev., 2013, 65(1), 139–147, DOI:10.1016/j.addr.2012.11.006.
- S. Deng, M. R. Gigliobianco, R. Censi and P. Di Martino, Polymeric nanocapsules as nanotechnological alternative for drug delivery system: current status, challenges and opportunities, Nanomaterials, 2020, 10(5), 847 CrossRef CAS PubMed.
- U. Bazylińska, D. Wawr, J. Kulbacka, R. Frąckowiak, B. Cichy and A. Bednarkiewicz, Polymeric nanocapsules with up- converting nanocrystals cargo make ideal fluorescent bioprobes, Sci. Rep., 2016, 6(1), 29746, DOI:10.1038/srep29746.
- R. T. Branquinho, et al., Biodegradable polymeric nanocapsules prevent cardiotoxicity of anti-trypanosomal lychnopholide, Sci. Rep., 2017, 7(1), 1–13 CrossRef PubMed.
- Y. Yao, et al., Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance, Front. Mol. Biosci., 2020, 7, 1–14, DOI:10.3389/fmolb.2020.00193.
- W. H. Lee, C. Y. Loo, D. Traini and P. M. Young, Inhalation of nanoparticle-based drug for lung cancer treatment: Advantages and challenges, Asian J. Pharm. Sci., 2015, 10(6), 481–489, DOI:10.1016/j.ajps.2015.08.009.
- P. Muralidharan, M. Malapit, E. Mallory, D. Hayes and H. M. Mansour, Inhalable nanoparticulate powders for respiratory delivery, Nanomedicine, 2015, 11(5), 1189–1199, DOI:10.1016/j.nano.2015.01.007.
- Y. R. Song, J. Kim, H. Lee, S. G. Kim and E. Choi, Serum levels of protein carbonyl, a marker of oxidative stress, are associated with overhydration, sarcopenia and mortality in hemodialysis patients, pp. 1–11, 2020.
- A. Mitsugu, Protein carbonylation: molecular mechanisms, biological implications, and analytical approaches, Free Radical Res., 2021, 55(4), 307–320, DOI:10.1080/10715762.2020.1851027.
- Y. K. Kim, The use of polyolefins in industrial and medical applications, Elsevier Ltd, 2017 Search PubMed.
- D. Karabulut, E. Ozturk, E. Kaymak, A. T. Akin and B. Yakan, Thymoquinone attenuates doxorubicin-cardiotoxicity in rats, J. Biochem. Mol. Toxicol., 2021, 35(1), 1–9, DOI:10.1002/jbt.22618.
- K. N. Timm, et al., Metabolic Effects of Doxorubicin on the Rat Liver Assessed With Hyperpolarized MRI and Metabolomics, Front. Physiol., 2022, 12, 1–8, DOI:10.3389/fphys.2021.782745.
| This journal is © The Royal Society of Chemistry 2025 |