Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Multicore silica microcapsules containing α-tocopherol for potential consumer product applications
Mohammed
Al-Sharabi
 a,
Benjamin T.
Lobel
a,
Benjamin T.
Lobel
 bc,
Daniele
Baiocco
bc,
Daniele
Baiocco
 d,
Olivier J.
Cayre
d,
Olivier J.
Cayre
 b,
Zhibing
Zhang
b,
Zhibing
Zhang
 d and
Alexander F.
Routh
d and
Alexander F.
Routh
 *a
*a
aDepartment of Chemical Engineering and Biotechnology, University of Cambridge, Cambridge CB3 0AS, UK. E-mail: afr10@cam.ac.uk; Tel: +44 01223 334789
bSchool of Chemical and Process Engineering, University of Leeds, Leeds LS2 9JT, UK
cSchool of Mathematics, Statistics, Chemistry and Physics, Murdoch University, Murdoch 6150, Australia
dSchool of Chemical Engineering, University of Birmingham, Birmingham B15 2TT, UK
First published on 23rd January 2025
Abstract
Microencapsulation is an advanced technique for protecting and enhancing the processing, delivery and performance of sensitive active ingredients, such as lipid-soluble vitamins. The fabrication of microcapsules containing such materials in an efficient, cost-effective and environmentally-friendly manner remains an ongoing challenge. Multicore silica microcapsules containing α-tocopherol in their cores were fabricated through salt-induced destabilisation and subsequent agglomeration of silica nanoparticles in an oil-in-water-in-oil double emulsion template at room temperature. The primary emulsion was prepared using three different concentrations (5, 10 and 15 wt%) of the internal oil phase, i.e. a mixture of α-tocopherol and sunflower oil. The external oil phase for the secondary emulsion consisted of different concentrations of Span 80 (0, 0.5 and 1 wt%) in sunflower oil. The capsule core size does not change during storage, confirming the stability of cores within the microcapsules. Mechanical testing provides that the microcapsules containing the lowest concentration of internal oil (5 wt%) have the highest rupture force and nominal rupture stress due to the higher silica content of these microcapsules. The incorporation of Span 80 does not significantly change the adhesion of microcapsules to a Lorica Soft leather substrate, mimicking human skin. The microcapsules are designed to release their contents upon mechanical rupture induced by rubbing against skin. This work shows the potential of such microcapsules to be applied in a range of consumer products, such as cosmetics.
1 Introduction
Vitamins are essential nutrients, needed by the human body in small amounts for its normal functioning, growth and development.1,2 Such substances are classified, based on their solubility, into water-soluble (B and C) or fat-soluble (A, D, E and K) vitamins.3 Vitamin E is a group of eight lipid-soluble naturally occurring compounds that includes four tocopherols (α, β, γ, δ) and four tocotrienols (α, β, γ, δ).4,5 α-Tocopherol is the most naturally abundant, biologically active and bioavailable form of vitamin E, which is commonly incorporated in food, pharmaceutical and cosmetic products, due to its strong antioxidant activities.6–8 For instance, using α-tocopherol as an antioxidant in cosmetic products can prevent photoaging by minimising skin damage from UV radiation.9 Although α-tocopherol is important to the body as a strong antioxidant, the material has some drawbacks, such as hydrophobicity, poor bioavailability and high chemical sensitivity to heat, light and oxidants.10 These factors make developing efficient delivery systems of α-tocopherol in the pharmaceutical, food and cosmetic sectors challenging. Such challenges can be overcome using microencapsulation to protect α-tocopherol from environmental factors and enhance its performance and end-use applications.11Microencapsulation has been extensively used for the protection, preservation and delivery of a wide range of active ingredients in many applications, such as pharmaceutical products,12,13 food products,14,15 cosmetics,16,17 fragrances,18 textiles,19 coatings20 and pesticides.21 Such technology is an effective tool for protecting sensitive bioactive compounds against harsh environmental conditions, such as humidity, chemicals, light and oxygen, and for enhancing their delivery and bioavailability.22,23 Several researchers have conducted work on the encapsulation of α-tocopherol using a number of techniques, such as spray drying,24,25 freeze drying,26 complex coacervation,23 solvent evaporation27 as well as ionic gelation and extrusion.28 Although these techniques are capable of encapsulating hydrophobic active ingredients, a number of limitations are associated with such methods, such as the application of high temperatures for solvent evaporation during spray drying, the high cost of freeze drying as well as the long duration, complexity and generation of chemical residue during the coacervation process.29,30 Therefore, it is important to explore alternative microencapsulation methods that can be used for the fabrication of α-tocopherol microcapsules in a simple, efficient and environmentally-friendly manner, without altering its structure during the encapsulation process.
Colloidosomes, also known as Pickering emulsion microcapsules, are microcapsules with shells consisting of densely-packed colloidal particles.31 Such systems are typically formed via the self-assembly of colloidal particles at the interface between aqueous and oil phases of an emulsion followed by shell reinforcement through thermal annealing, covalent cross-linking, polyelectrolyte complexation or polymerisation.32 Single-core colloidosomes have been used for the encapsulation of a range of materials, such as drugs,33,34 catalysts35 and antibiotics.36 In addition to single-core capsules, multicore colloidosomes have been successfully produced for the encapsulation of hydrophilic and hydrophobic active ingredients. Lee and Weitz37 prepared non-spherical colloidosomes with multiple compartments using water-in-oil-in-water (W/O/W) double emulsions as templates. A glass capillary microfluidic device was utilised to prepare the double emulsions with different morphologies through controlling the number of internal water droplets within the oil droplets. Brossault et al.38 reported a simple, fast and environmentally-friendly method for the production of multicore silica microcapsules with adjustable internal structure and size. This was achieved through the salt-driven assembly of hydrophilic nanoparticles dispersed within an oil-in-water-in-oil (O/W/O) double emulsion template at room temperature. These capsules had an overall diameter of 7 to 35 μm and contained oil cores with a size of 0.9 to 4.2 μm.
The current work aims to extend the methodology reported by Brossault et al.38 to encapsulate α-tocopherol in multicore silica microcapsules and to study the capsule mechanical and substrate adhesion properties. This is a straightforward, environmentally-friendly and promising method for room temperature microencapsulation of fat-soluble vitamins, such as vitamin E. In this work, the morphology of the microcapsules prepared at different formulation conditions, i.e. different concentrations of the internal oil phase and different concentrations of Span 80 in the external oil phase, was investigated using optical and electron microscopy. In addition, the mechanical properties of these microcapsules were measured by a macromanipulation technique and their adhesion to a Lorica Soft substrate mimicking the human skin was measured by atomic force microscopy (AFM). The effect of formulation conditions on the adhesion and mechanical responses of the microcapsules upon application on the human skin was thereby investigated.
2 Materials and methods
2.1 Materials
Silica particles (Ludox HS-40 colloidal silica, 40 wt% suspension in water, diameter of 20 ± 1 nm39), polysorbate 80 (Tween 80), sorbitan monooleate (Span 80), vitamin E (α-tocopherol, ≥96% purity) and Nile red (dry powder) were obtained from Sigma-Aldrich. Sunflower oil was purchased from a local supermarket (Sainsbury's UK). Calcium chloride dihydrate, CaCl2·2H2O (>99%) and absolute ethanol (99.8% purity) were bought from Acros Organics. All materials were used as received without further purification or modification.2.2 Preparation of α-tocopherol containing multicore silica microcapsules
Multicore silica microcapsules containing α-tocopherol were prepared via the self-assembly of silica nanoparticles using an O/W/O double emulsion template at room temperature, as illustrated in Fig. 1. The aqueous phase used for the preparation of the primary emulsion was composed of Ludox (35 wt%) and Tween 80 (10 wt%), whereas the internal oil phase consisted of a mixture of α-tocopherol (10 wt%) in sunflower oil. Sunflower oil naturally contains α-tocopherol40 and is a safe and effective emollient for nourishing and moisturising the skin,41 which makes it a good oil candidate for blending. The primary emulsion was prepared by mixing the aqueous phase (9.5, 9, 8.5 g) with 5, 10 and 15 wt% of the internal oil phase (0.5, 1, 1.5 g, respectively) using an Ultra Turrax mixer (T18 digital, IKA) at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 60 s. In the next step, the external oil phase was composed of pure sunflower oil or a mixture of sunflower oil and Span 80 (0.5 and 1 wt%). The secondary emulsion was prepared by mixing 1 g of the primary emulsion with 9 g of the external oil phase using a vortex mixer (TopMix FB15024, Fisherbrand) at 3000 rpm for 60 s. The mixing speed of the vortex mixer was reduced to 2000 rpm and 1 mL of an aqueous CaCl2 solution (1 M) was added dropwise over 30 s to cause agglomeration of the silica nanoparticles and subsequent formation of the multicore silica microcapsules. The mixture was centrifuged (Multifuge 1 S-R, Heraeus) at 3000 rpm for 5 min to separate the microcapsules from the continuous phase supernatant. The formed microcapsules then underwent two washing cycles (washed with 10 mL of deionised water and centrifuged at 3000 rpm for 5 min) before being dispersed in 10 mL of deionsed water.
000 rpm for 60 s. In the next step, the external oil phase was composed of pure sunflower oil or a mixture of sunflower oil and Span 80 (0.5 and 1 wt%). The secondary emulsion was prepared by mixing 1 g of the primary emulsion with 9 g of the external oil phase using a vortex mixer (TopMix FB15024, Fisherbrand) at 3000 rpm for 60 s. The mixing speed of the vortex mixer was reduced to 2000 rpm and 1 mL of an aqueous CaCl2 solution (1 M) was added dropwise over 30 s to cause agglomeration of the silica nanoparticles and subsequent formation of the multicore silica microcapsules. The mixture was centrifuged (Multifuge 1 S-R, Heraeus) at 3000 rpm for 5 min to separate the microcapsules from the continuous phase supernatant. The formed microcapsules then underwent two washing cycles (washed with 10 mL of deionised water and centrifuged at 3000 rpm for 5 min) before being dispersed in 10 mL of deionsed water.
2.3 Characterisation of emulsions and microcapsules using microscopy
The primary emulsion droplets and the multicore silica microcapsules were imaged using a bright-field optical microscope (Leica DME) equipped with 20×, 40× and 63× objective lenses. Scanning electron microscopy (SEM) using a Tescan Mira3 FEG-SEM instrument operating at 5 kV was used to visualise the morphology of the microcapsules. Scanning transmission electron microscopy (STEM) images of the microcapsules were obtained using a Thermo Scientific (FEI Company) Talos F200X G2 microscope equipped with a Ceta 4k CMOS camera and a Fischione HAADF detector, operating at an acceleration voltage of 200 kV. This was used to investigate the effect of varying the internal oil concentration on the internal structure of the microcapsules. A confocal microscope (Leica TCS SP8, excitation wavelength: 552 nm, emission window: 558 to 661 nm) equipped with a 63 × 1.4 oil immersion objective was used to image Nile red (100 mg L−1) containing microcapsules to confirm the presence of the internal oil in the microcapsules on the day of preparation and after eight months. ImageJ software (version 1.54f, National Institutes of Health, USA) was used to quantify the size (mean diameter ± standard deviation) of the primary emulsion droplets as well as the size of microcapsules and their inner cores using 100 cores and 200 capsules within a field of view for each sample that was randomly selected by eye from the bright-field optical micrographs, considering all capsules within the selected field of view.2.4 Mechanical properties of microcapsules
The extrinsic mechanical properties of microcapsules were quantified using a micromanipulation technique, established at the University of Birmingham.42 This entails the controlled compression of each individual microcapsule between two parallel plates. Two droplets of a silica microcapsule suspension (∼50 μL per droplet) were placed on a specially designed glass substrate featuring a smooth, even flat surface (25 mm by 10 mm; thickness 3 mm), which were allowed to air dry. The glass slide was then affixed to a metal stage and positioned beneath a borosilicate glass probe (1 mm, Harvard Apparatus Ltd, UK) with a flat-end tip (width of ∼80 μm) that was attached to the appropriate force transducer head (FTH, Load Cell, GSC010; sensitivity 8.674 mN V−1; Transducer Techniques, Temecula, USA). The FTH was connected to an electronically powered control box and mounted on a three-dimensional fine micromanipulator operated by a servo motor (DC Servocontroller, CONEX-C, USA), providing a resolution of ±0.2 μm. The glass was moved vertically at a descent speed of 10 μm s−1 to compress each individual microcapsule upon contact. The compression process was monitored through a side-view camera (magnification 10×–140×; 30 frames per second; AM4023CT, DinoEye C-Mount Camera, Dino-Lite, UK). A semi-automatic mode was operated to retract the probe to its initial position once compression was completed. A total of thirty randomly selected microcapsules were compressed to ensure statistically significant results, with the force–displacement data for each compression recorded automatically. Prior to microcapsule compression, the compliance of the force transducer probe was measured thrice, and the average value was used to correct the displacement data. The micromanipulation setup utilised in this study is described in detail elsewhere.432.5 Adhesion measurements
A Bioscope II AFM (Bruker, USA) was used to perform force measurements. The capsules were first washed with propanol to remove oil through three cycles of centrifugation and redispersion steps at 2000 rcf for 10 min. Colloid probe force measurements were performed with a tipless cantilever (AIO-TL-10, Apex Probes, UK) with spring constant 2.7 N m−1, onto which a capsule with either 5 or 15 wt% of internal oil for each concentration of Span 80 was attached using a two-part epoxy adhesive (Selleys Araldite). The spring constant of each cantilever was measured using the native thermal tuning procedure in the instrument software (Nanoscope), with an average of five discrete measurements used for subsequent data analysis. Size and position of the probe once attached were determined using optical microscopy (BX51 Olympus Reflected light microscope). A substrate sample, i.e. Lorica Soft leather (Ehrlich Leder, Germany), which is reported to mimic mechanical properties of human skin,44,45 was prepared using double sided tape to affix it to a glass slide. One hundred force ramp measurements were performed, then the sample was moved 20 μm in each axial direction from the starting position and the ramp measurements repeated at each location. Cantilever deflection (nm) as a function of distance travelled normal to the sample (nm) was converted to geometry-normalised normal force (mN m−1) as a function of sample-tip separation (nm) using established methods in order to obtain the magnitude of the probe-substrate pull-off force.46–483 Results and discussion
3.1 Morphology of microcapsules
The microcapsules were characterised using optical and electron microscopy to investigate the impact of the internal oil concentration and Span 80 on the morphology of the microcapsules. Fig. 2 shows that spherical multicore silica microcapsules containing α-tocopherol in their cores were successfully fabricated through salt-induced destabilisation and agglomeration of silica nanoparticles in an O/W/O double emulsion template at room temperature. This is the case for all the different concentrations of internal oil phase and concentrations of Span 80 (0, 0.5 and 1 wt%) incorporated in the external oil phase of the secondary emulsions. It can be seen from Fig. 2 that the number of oil cores within the microcapsules increases with increasing concentration of the internal oil phase at the same concentration of Span 80. Fig. 2 also shows that the primary emulsion droplets, as well as the cores within the microcapsules, appear to be uniform with similar sizes across all formulations.The size of cores and microcapsules was determined for all the different formulations to examine the impact of internal oil concentration and Span 80 concentration on the core and capsule size. Fig. 3 shows the size of the multicore silica microcapsules and the corresponding core size, on the day of preparation and after six weeks. The microcapsules have an average diameter of 14.4 to 15.1 μm (Fig. 3(a)). The core size (Fig. 3(b)) is similar for the three different concentrations of internal oil phase at the same concentration of Span 80. The core size is also similar to the size of the primary emulsion droplets, suggesting that the primary emulsion droplets do not coalesce during the formation of capsules. Furthermore, the core size does not change upon the addition of Span 80, for a fixed concentration of the internal oil phase. The similarity in size for all the formulations can be attributed to the fabrication of all the microcapsules using a constant mixing speed for preparing the primary emulsions. Brossault et al.38 demonstrated that the mixing speed of the primary emulsification has an impact on the core size at the same concentrations. It was found that the core size increases with a decrease in the mixing speed of the primary emulsification. The core size in Fig. 3(b) and (c) is similar to that of the cores within the multicore microcapsules containing pure sunflower oil that were previously reported at the same emulsification conditions (i.e. mixing speed of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for the primary emulsion and 3000 rpm for the secondary emulsion).38 This indicates that the core size is governed by the mixing speed during preparation of the primary emulsion. Fig. 3(c) shows that the core size does not change over a period of six weeks post preparation, confirming the stability of such cores within the microcapsules during storage and the absence of coalescence over time. It is worth mentioning that the microcapsules were kept in an aqueous suspension at room temperature during storage. The error bars in Fig. 3(b) and (c) indicate that the Ultra Turrax mixer is suitable for the production of uniform oil droplets/cores containing α-tocopherol.
000 rpm for the primary emulsion and 3000 rpm for the secondary emulsion).38 This indicates that the core size is governed by the mixing speed during preparation of the primary emulsion. Fig. 3(c) shows that the core size does not change over a period of six weeks post preparation, confirming the stability of such cores within the microcapsules during storage and the absence of coalescence over time. It is worth mentioning that the microcapsules were kept in an aqueous suspension at room temperature during storage. The error bars in Fig. 3(b) and (c) indicate that the Ultra Turrax mixer is suitable for the production of uniform oil droplets/cores containing α-tocopherol.
The effect of varying the concentration of Span 80 in the external oil phase on the microcapsule external structure was investigated using SEM. Fig. 4 shows SEM images of the microcapsules prepared at different internal oil concentrations (5, 10 and 15 wt%) and different Span 80 concentrations (0.5 and 1 wt%). It can be observed that the microcapsules prepared with 1 wt% of Span 80 have a slightly rougher and more porous structure compared to the microcapsules prepared with 0.5 wt% of Span 80 at the same internal oil concentration. This is consistent with the findings reported in literature,39,49,50 which show that an increase in the concentration of Span 80 results in an increase of porosity. The impact of Span 80 is more pronounced in composite silica microbeads39 in comparison with multicore silica microcapsules at the same surfactant concentration of 1 wt%. This is likely due to a lower silica content near the surface of the microcapsules as compared to that of the microbeads, which do not contain oil near the surface.
The internal structure of the microcapsules was investigated using STEM (Fig. 5), which confirms that the internal structure consists of multiple oil cores. It can be seen that the number of cores within the capsules increases with increasing internal oil concentration. This is consistent with the results obtained by the bright-field optical microscopy (Fig. 2). In addition, the oil cores within the capsules with the highest internal oil concentration of 15 wt% can be seen more easily than for the lower concentrations of 5 and 10 wt%. This can be explained by the decrease in silica content with an increase in internal oil concentration, resulting in more light being transmitted through the capsule structure. For the STEM experiments the capsules were not washed with any solvent prior to imaging the samples to avoid the removal of oil from within the capsules.
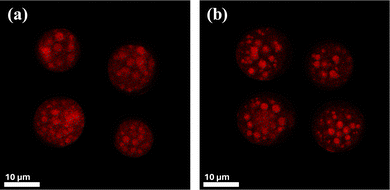
Confocal microscopy was used to confirm the presence of α-tocopherol in the internal oil mixture within the microcapsules. The technique also allowed the investigation of α-tocopherol retention over time. Fig. 6 shows the confocal microscopy images of microcapsules prepared with an internal oil concentration of 10 wt% and a Span 80 concentration of 1 wt%, on the day of preparation and eight months post preparation. The images confirm the presence of α-tocopherol in the cores of the microcapsules as evident from the red colour, corresponding to the Nile red which is only soluble in the internal oil. Fig. 6(b) also demonstrates the long retention of the internal oil within the microcapsules, as it was still present within the microcapsules after eight months storage. The results also show that no coalescence occurs after eight months indicating the stability of the cores within these microcapsules. These cores are held apart by the silica particles and therefore governed by the silica particle mobility. In a close-packed solid system, this mobility is very slow, which explains the long-term stability of cores. It is unlikely that humidity or oil type affect the core stability dramatically. It is also anticipated that the temperature might have an impact but this is likely to be small since these cores are held apart by the rigid silica particles.
3.2 Mechanical testing
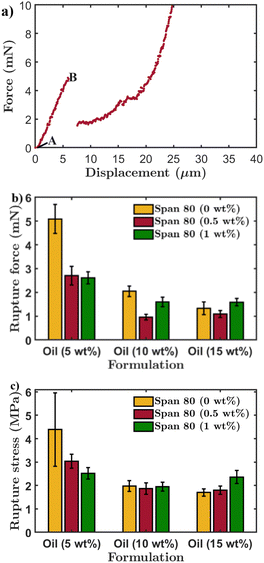
The effect of varying the internal oil concentration in the primary emulsion and Span 80 concentration in the secondary emulsion on the mechanical properties of the microcapsules was investigated using the micromanipulation technique. Fig. 7(a) shows typical force–displacement data obtained from the compression of a microcapsule that was prepared with 5 wt% of the internal oil phase in the absence of Span 80. Point A in Fig. 7(a) corresponds to the initial contact point between the probe and microcapsule. The force increases as the probe proceeds until the microcapsule experiences brittle fracture at point B. Such brittle fracture of silica microcapsules has also been observed in previous studies.51,52Fig. 7(b) and (c) show the average rupture force and nominal rupture stress of microcapsules prepared at different internal oil concentrations and different concentrations of Span 80. It can be seen that the microcapsules containing the lowest concentration of internal oil have the highest rupture force and nominal rupture stress. This can be attributed to the higher silica content within the microcapsules with the lowest concentration of internal oil (5 wt%). O'Sullivan et al.51 investigated the compressive breaking force of silica microcapsules containing silicon oil cores using the micromanipulation technique. It was found that the rupture force increases linearly with an increase in the shell thickness, i.e. higher silica content. This qualitatively shows that incorporating a larger amount of silica into the structure of a microcapsule results in a stronger microcapsule. Fig. 7(b) and (c) also show that the addition of Span 80 during preparation has a more pronounced effect on the mechanical properties of the microcapsules with the lowest concentration of internal oil. The rupture force and nominal rupture stress of the capsules prepared in the absence of Span 80 are larger. This is due to the lager porosity of the capsules upon the addition of Span 80, as reported in previous studies.39,49,50 As intuitively expected, the porosity and lower connectivity of the network results in lower mechanical properties.53 The increase in porosity makes the capsules weaker, which explains the lower values of rupture force and nominal rupture stress of the capsules prepared with Span 80. The rupture force and nominal rupture stress do not change significantly upon increasing the concentration of Span 80 from 0.5 to 1 wt%, at the same concentration of internal oil phase. This is likely because the Span 80 concentration needed to cause a significant change in the surface porosity and hence the rupture force and nominal rupture stress of the microcapsules is very small.
3.3 Adhesion analysis
A Lorica Soft substrate was used for measuring the adhesion of microcapsules using AFM. Hurtado et al.54 have shown that Lorica has a similar tribological performance to that of ex vivo human skin.Fig. 8 shows the adhesion force of microcapsules containing different concentrations of internal oil and Span 80. It can be seen that the adhesion force is similar for the different concentrations of internal oil. This is expected as the AFM measurements are influenced by the interaction between the external surface of the microcapsule and the substrate. It can also be observed that the addition of Span 80 does not cause a significant change in the adhesion of microcapsules to the Lorica Soft substrate. This is likely because addition of a small amount of Span 80 to the external phase of the secondary emulsion does not cause a significant modification of the external porosity of microcapsule, in agreement with the SEM observations (Fig. 4).
This work demonstrates the straightforward and environmentally-friendly approach for the encapsulation of a lipid soluble vitamin, i.e. vitamin E in the form of α-tocopherol, within multicore silica microcapsules that were produced via salt-induced destabilisation of silica nanoparticles at room temperature. This method can potentially be used for the encapsulation of a range of hydrophobic active ingredients in a number of products, such as cosmetics, pharmaceuticals and detergents. Further work can be conducted to investigate the encapsulation efficiency of different active ingredients, core size and release in different environments, including physiological-pH media (e.g. phosphate-buffered saline).
4 Conclusions
Multicore silica microcapsules containing α-tocopherol were successfully prepared through the self-assembly and agglomeration of nanoparticles within an O/W/O double emulsion template. The results show that the internal oil within the microcapsules is retained with no coalescence of the oil cores over a period of eight months. Mechanical testing shows that the microcapsules prepared with the lowest concentration of the internal oil (5 wt%), in the absence of Span 80, are stronger than the microcapsules prepared with higher amounts of internal oil. This is due to the amount of silica in the microcapsules. In addition, the adhesion of the microcapsules to a Lorica Soft substrate does not change significantly upon the addition of Span 80 to the external oil phase of the secondary emulsion. This work shows the potential of using these microcapsules for the protection and delivery of hydrophobic active ingredients in a number of products, such as cosmetics, detergents and drugs. For example, the microcapsules fabricated in this work allow for the delivery of fat-soluble vitamins within oil cores with release that can potentially be triggered by shear. The capsules can potentially be incorporated in a range of cosmetic products, such as lotions and creams.Data availability
Data will be available upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the U.K. Engineering and Physical Sciences Research Council (EPSRC) for their funding (EP/V027727/1, EP/V027646/1, and EP/V027654/1, EP/P030467/1). The authors would also like to thank Dr Heather Greer from the Department of Chemistry at the University of Cambridge for assisting with the SEM and STEM imaging and Dr Martin Lenz from the Cambridge Advanced Imaging Centre for helping with the confocal microscopy imaging.References
- D. O. Kennedy, B vitamins and the brain: Mechanisms, dose and efficacy – a review, Nutrients, 2016, 8, 68 CrossRef PubMed.
- M. Michalak, M. Pierzak, B. Kręcisz and E. Suliga, Bioactive compounds for skin health: A review, Nutrients, 2021, 13, 203 CrossRef CAS PubMed.
- S. P. Dhakal and J. He, Microencapsulation of vitamins in food applications to prevent losses in processing and storage: A review, Food Res. Int., 2020, 137, 109326 CrossRef CAS PubMed.
- F. Galli, A. Azzi, M. Birringer, J. M. Cook-Mills, M. Eggersdorfer, J. Frank, G. Cruciani, S. Lorkowski and N. K. Özer, Vitamin e: Emerging aspects and new directions, Free Radicals Biol. Med., 2017, 102, 16–36 CrossRef CAS PubMed.
- H. Y. Peh, W. S. D. Tan, W. Liao and W. S. F. Wong, Vitamin e therapy beyond cancer: Tocopherol versus tocotrienol, Pharmacol. Ther., 2016, 162, 152–169 CrossRef CAS PubMed.
- S. R. Tintino, C. D. Morais-Tintino, F. F. Campina, R. L. Pereira, M. d S. Costa, M. F. B. M. Braga, P. W. Limaverde, J. C. Andrade, J. P. Siqueira-Junior, H. D. M. Coutinho, V. Q. Balbino, T. C. Leal-Balbino, J. Ribeiro-Filho and L. J. Quintans Jr, Action of cholecalciferol and alpha-tocopherol on staphylococcus aureus efflux pumps, EXCLI J., 2016, 15, 315–322 Search PubMed.
- L. Liang, V. Tremblay-Hébert and M. Subirade, Characterisation of the β-lactoglobulin/α-tocopherol complex and its impact on α-tocopherol stability, Food Chem., 2011, 126, 821–826 CrossRef CAS.
- Y. Qi, Z. Zhang, Y. Wang, Z. Wu, Z. Qin, Y. Zhou and X. Yang, Preparation and characterization of vitamin e microcapsules stabilized by zein with different polysaccharides, Int. J. Biol. Macromol., 2024, 268, 131975 CrossRef CAS PubMed.
- G. Niculae, I. Lacatusu, A. Bors and R. Stan, Photostability enhancement by encapsulation of α-tocopherol into lipid-based nanoparticles loaded with a UV filter, C. R. Chim, 2014, 17, 1028–1033 CrossRef CAS.
- F. Edilino de Lima, R. Giordano Viegas and O. Vital de Oliveira, In silico study of the encapsulation of α-tocopherol and α-tocotrienol vitamins e into cucurbit[7]uril, Chem. Phys. Lett., 2023, 826, 140647 CrossRef CAS.
- Z. A. Raza, S. Khalil, A. Ayub and I. M. Banat, Recent developments in chitosan encapsulation of various active ingredients for multifunctional applications, Carbohydr. Res., 2020, 492, 108004 CrossRef CAS PubMed.
- P. Li, Z. Yang, Y. Wang, Z. Peng, S. Li, L. Kong and Q. Wang, Microencapsulation of coupled folate and chitosan nanoparticles for targeted delivery of combination drugs to colon, J. Microencapsulation, 2015, 32, 40–45 CrossRef CAS PubMed.
- W.-C. Liao, Y. S. Sohn, M. Riutin, A. Cecconello, W. J. Parak, R. Nechushtai and I. Willner, The application of stimuli-responsive vegf- and atp-aptamer-based microcapsules for the controlled release of an anticancer drug, and the selective targeted cytotoxicity toward cancer cells, Adv. Funct. Mater., 2016, 4262–4273 CrossRef CAS.
- M. Calderón-Oliver and E. Ponce-Alquicira, The role of microencapsulation in food application, Molecules, 2022, 27, 1499 CrossRef PubMed.
- R. Hu, D. Dong, J. Hu and H. Liu, Improved viability of probiotics encapsulated in soybean protein isolate matrix microcapsules by coacervation and cross-linking modification, Food Hydrocolloids, 2023, 138, 108457 CrossRef CAS.
- F. Casanova and L. Santos, Encapsulation of cosmetic active ingredients for topical application – a review, J. Microencapsulation, 2016, 33, 1–17 CrossRef CAS PubMed.
- A. Zhang, X. Wu, X. Ouyang, H. Lou, D. Yang, Y. Qian and X. Qiu, Preparation of light-colored lignosulfonate sunscreen microcapsules with strengthened UV-blocking and adhesion performance, ACS Sustainable Chem. Eng., 2022, 10, 9381–9388 CrossRef CAS.
- H. Lee, C.-H. Choi, A. Abbaspourrad, C. Wesner, M. Caggioni, T. Zhu and D. A. Weitz, Encapsulation and enhanced retention of fragrance in polymer microcapsules, ACS Appl. Mater. Interfaces, 2016, 8, 4007–4013 CrossRef CAS PubMed.
- Z. Chen, W. Zhang, Z. Yuan, Z. Wang, R. Ma and K. Chen, Preparation of strawberry chitosan composite microcapsules and their application in textiles, Colloids Surf., A, 2022, 652, 129845 CrossRef CAS.
- C. Zotiadis, I. Patrikalos, V. Loukaidou, D. M. Korres, A. Karantonis and S. Vouyiouka, Self-healing coatings based on poly(urea-formaldehyde) microcapsules: In situ polymerization, capsule properties and application, Prog. Org. Coat., 2021, 161, 106475 CrossRef CAS.
- M. Zhao, Z. Chen, L. Hao, H. Chen, X. Zhou and H. Zhou, Cmc based microcapsules for smart delivery of pesticides with reduced risks to the environment, Carbohydr. Polym., 2023, 300, 120260 CrossRef CAS PubMed.
- N. Choudhury, M. Meghwal and K. Das, Microencapsulation: An overview on concepts, methods, properties and applications in foods, Food Front., 2021, 2, 426–442 CrossRef CAS.
- J. Carpentier, E. Conforto, C. Chaigneau, J.-E. Vendeville and T. Maugard, Microencapsulation and controlled release of Î ± -tocopherol by complex coacervation between pea protein and tragacanth gum: A comparative study with arabic and tara gums, Innovative Food Sci. Emerging Technol., 2022, 77, 102951 CrossRef CAS.
- C. Saldanha do Carmo, C. Maia, J. Poejo, I. Lychko, P. Gamito, I. Nogueira, M. R. Bronze, A. T. Serra and C. M. M. Duarte, Microencapsulation of α-tocopherol with zein and β-cyclodextrin using spray drying for colour stability and shelf-life improvement of fruit beverages, RSC Adv., 2017, 7, 32065–32075 RSC.
- A. M. Ribeiro, B. N. Estevinho and F. Rocha, Improvement of vitamin e microencapsulation and release using different biopolymers as encapsulating agents, Food Bioprod. Process., 2021, 130, 23–33 CrossRef CAS.
- P. Laine, A.-M. Lampi, M. Peura, J. Kansikas, K. Mikkonen, S. Willför, M. Tenkanen and K. Jouppila, Comparison of microencapsulation properties of spruce galactoglucomannans and arabic gum using a model hydrophobic core compound, J. Agric. Food Chem., 2010, 58, 981–989 CrossRef CAS PubMed.
- P. Scarfato, E. Avallone, M. R. Galdi, L. Di Maio and L. Incarnato, Preparation, characterization, and oxygen scavenging capacity of biodegradable α-tocopherol/pla microparticles for active food packaging applications, Polym. Compos., 2017, 38, 981–986 CrossRef CAS.
- S.-H. Yoo, Y.-B. Song, P.-S. Chang and H. G. Lee, Microencapsulation of α-tocopherol using sodium alginate and its controlled release properties, Int. J. Biol. Macromol., 2006, 38, 25–30 CrossRef CAS PubMed.
- A. Cittadini, P. E. S. Munekata, M. Pateiro, M. V. Sarriés, R. Domínguez and J. M. Lorenzo, Chapter 17 – encapsulation techniques to increase lipid stability, in Food Lipids, ed. J. M. Lorenzo, P. E. S. Munekata, M. Pateiro, F. J. Barba and R. Domínguez, Academic Press, 2022, pp. 413–459 Search PubMed.
- N. Mehta, P. Kumar, A. K. Verma, P. Umaraw, Y. Kumar, O. P. Malav, A. Q. Sazili, R. Domínguez and J. M. Lorenzo, Microencapsulation as a noble technique for the application of bioactive compounds in the food industry: A comprehensive review, Appl. Sci., 2022, 12, 1424 CrossRef CAS.
- D. Lee and D. A. Weitz, Double emulsion-templated nanoparticle colloidosomes with selective permeability, Adv. Mater., 2008, 20, 3498–3503 CrossRef CAS.
- K. L. Thompson, M. Williams and S. P. Armes, Colloidosomes: Synthesis, properties and applications, J. Colloid Interface Sci., 2015, 447, 217–228 CrossRef CAS PubMed.
- Q. Sun, H. Gao, G. B. Sukhorukov and A. F. Routh, Silver-coated colloidosomes as carriers for an anticancer drug, ACS Appl. Mater. Interfaces, 2017, 9, 32599–32606 CrossRef CAS PubMed.
- A. Singh, S. S. Das, J. Ruokolainen, K. K. Kesari and S. K. Singh, Biopolymer-capped pyrazinamide-loaded colloidosomes: In vitro characterization and bioavailability studies, ACS Omega, 2023, 8, 25515–25524 CrossRef CAS PubMed.
- X. Yang, B. Ouyang, P. Shen, Y. Sun, Y. Yang, Y. Gao, E. Kan, C. Li, K. Xu and Y. Xie, Ru colloidosome catalysts for the hydrogen oxidation reaction in alkaline media, J. Am. Chem. Soc., 2022, 144, 11138–11147 CrossRef CAS PubMed.
- Q. Sun, Z. Zhao, E. A. H. Hall and A. F. Routh, Metal coated colloidosomes as carriers for an antibiotic, Front. Chem., 2018, 6, 196 CrossRef PubMed.
- D. Lee and D. A. Weitz, Nonspherical colloidosomes with multiple compartments from double emulsions, Small, 2009, 5, 1932–1935 CrossRef CAS PubMed.
- D. F. F. Brossault, T. M. McCoy and A. F. Routh, Preparation of Multicore Colloidosomes: Nanoparticle-Assembled Capsules with Adjustable Size, Internal Structure, and Functionalities for Oil Encapsulation, ACS Appl. Mater. Interfaces, 2021, 13, 51495–51503 CrossRef CAS PubMed.
- M. Al-Sharabi, D. Baiocco, B. T. Lobel, O. J. Cayre, Z. Zhang and A. F. Routh, Magnetic zinc oxide/silica microbeads for the photocatalytic degradation of azo dyes, Colloids Surf., A, 2024, 695, 134169 CrossRef CAS.
- S. M. Bakre, D. K. Gadmale, R. B. Toche and V. B. Gaikwad, Rapid determination of alpha tocopherol in olive oil adulterated with sunflower oil by reversed phase high-performance liquid chromatography, J. Food Sci. Technol., 2015, 52, 3093 CrossRef CAS PubMed.
- R. Sarkar, I. Podder, N. Gokhale, S. Jagadeesan and V. K. Garg, Use of vegetable oils in dermatology: an overview, Int. J. Dermatol., 2017, 56, 1080–1086 CrossRef PubMed.
- D. Baiocco, J. A. Preece and Z. Zhang, Encapsulation of hexylsalicylate in an animal-free chitosan-gum arabic shell by complex coacervation, Colloids Surf., A, 2021, 625, 126861 CrossRef CAS.
- D. Baiocco and Z. Zhang, Microplastic-free microcapsules to encapsulate health-promoting limonene oil, Molecules, 2022, 27, 7215 CrossRef CAS PubMed.
- L. C. Gerhardt, A. Schiller, B. Müller, N. D. Spencer and S. Derler, Fabrication, Characterisation and Tribological Investigation of Artificial Skin Surface Lipid Films, Tribol. Lett., 2009, 34, 81–93 CrossRef CAS.
- D. J. Cottenden and A. M. Cottenden, A study of friction mechanisms between a surrogate skin (lorica soft) and nonwoven fabrics, J. Mech. Behav. Biomed. Mater., 2013, 28, 410–426 CrossRef PubMed.
- H. J. Butt, B. Cappella and M. Kappl, Force measurements with the atomic force microscope: Technique, interpretation and applications, Surf. Sci. Rep., 2005, 59, 1–152 CrossRef CAS.
- J. Ralston, I. Larson, M. W. Rutland, A. A. Feiler and M. Kleijn, Atomic force microscopy and direct surface force measurements (iupac technical report), Pure Appl. Chem., 2005, 77, 2149–2170 CrossRef CAS.
- B. T. Lobel, H. Robertson, G. B. Webber, P. M. Ireland and E. J. Wanless, Impact of surface free energy on electrostatic extraction of particles from a bed, J. Colloid Interface Sci., 2022, 611, 617–628 CrossRef CAS PubMed.
- D. F. F. Brossault and A. F. Routh, Salt-driven assembly of magnetic silica microbeads with tunable porosity, J. Colloid Interface Sci., 2020, 562, 381–390 CrossRef CAS PubMed.
- D. F. F. Brossault, T. M. McCoy and A. F. Routh, Self-assembly of TiO2/Fe3O4/SiO2 microbeads: A green approach to produce magnetic photocatalysts, J. Colloid Interface Sci., 2021, 584, 779–788 CrossRef CAS PubMed.
- M. OSullivan, Z. Zhang and B. Vincent, Silica-shell/oil-core microcapsules with controlled shell thickness and their breakage stress, Langmuir, 2009, 25, 7962–7966 CrossRef CAS PubMed.
- P. Iqbal, T. Lu, Z. Zhang and Y. Li, Preparation of multilayer microcapsules encapsulating aqueous lithium bromide and their mechanical stability, Ind. Eng. Chem. Res., 2019, 58, 6364–6374 CrossRef CAS.
- T. Woignier, J. Primera, A. Alaoui, P. Etienne, F. Despestis and S. Calas-Etienne, Mechanical properties and brittle behavior of silica aerogels, Gels, 2015, 1, 256–275 CrossRef PubMed.
- M. M. Hurtado, M. Peppelman, X. Zeng, P. van Erp and E. Van Der Heide, Tribological behaviour of skin equivalents and ex vivo human skin against the material components of artificial turf in sliding contact, Tribol. Int., 2016, 102, 103–113 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |