Enhancing electrocatalytic CO2 reduction via engineering substrate–cluster interaction†
Qian
Sun
a,
Huiru
Yang
*a,
Chunmei
Zhang
 *ac and
Aijun
Du
*ac and
Aijun
Du
 b
b
aSchool of Physics, Northwest University, Xi’an 710127, China. E-mail: y1573349346@163.com; chunmeizhang@nwu.edu.cn
bSchool of Chemistry and Physics and QUT Center for Materials Science, Queensland University of Technology (QUT), Brisbane, QLD 4000, Australia
cShaanxi Key Laboratory for Theoretical Physics Frontiers, Xi’an 710127, China
First published on 16th May 2025
Abstract
Cu clusters exhibit exceptional performance in the electrocatalytic carbon dioxide reduction reaction (CO2RR) due to their tunable size. Using first-principles calculations, we systematically investigate the CO2RR on small Cun clusters (n = 3, 8, 13) anchored on a T′-WTe2 substrate, denoted as Cun@T′-WTe2. Given that the hydrogen evolution reaction (HER) often competes with the CO2RR, we further investigated the competition between the CO2RR and HER. Our results show that Cun@T′-WTe2 outperforms pure Cun clusters as catalysts, with enhanced CO2RR activity. The CO2RR performance of Cun@T′-WTe2 enhances with increasing cluster size, and surpasses the HER activity in Cu13@T′-WTe2. This enhancement stems from the substrate–cluster interactions, where the buckled “non-uniform surface” of T′-WTe2 deforms the larger Cu13 cluster, optimizing the CO2RR efficiency. We propose a potential strategy for WTe2-supported Cu clusters to boost CO2RR while suppressing HER by leveraging substrate-supported Cu clusters.
1. Introduction
The accumulation of greenhouse gases, particularly carbon dioxide (CO2), drives global warming and poses significant environmental and energy challenges.1,2 To address rising energy demands and mitigate CO2 emissions, researchers have explored various strategies to convert CO2 into valuable chemicals and feedstocks,3 including chemical reforming,4,5 bioconversion,6 photocatalysis,7,8 and electrocatalysis.9,10 Among them, the electrochemical CO2 reduction reaction (CO2RR) stands out as a promising approach, transforming CO2 into valuable products8,10–12 like methane (CH4),6,13 formic acid (HCOOH),14 and methanol (CH3OH).15,16 However, the CO2RR faces challenges such as low efficiency and poor product selectivity4,9,17 exacerbated by the competing hydrogen evolution reaction (HER), which significantly reduces the performance of the CO2RR.18To overcome these limitations, significant efforts have focused on designing efficient electrocatalysts that activate inert CO2 molecules while suppressing the competitive HER. Various systems, including metals,19,20 metal oxides,21,22 clusters,23 carbon-based materials,24,25 and metal–organic frameworks,26,27 have been explored. Among them, clusters, in particular Cu clusters, demonstrate excellent CO2RR catalytic performance.28,29 When supported on substrates, the synergistic interaction between the substrate and cluster significantly influences the CO2RR efficiency.30 Thus, optimizing the substrate–cluster pairing is critical to maximizing the CO2RR activity and minimizing HER interference.
In this study, we employ first-principles calculations to examine the CO2RR performance of Cun clusters (n = 3, 8, 13) anchored on a T′-WTe2 substrate denoted as Cun@WTe2.31–33 Compared to unsupported Cun (Cu3, Cu8, and Cu13),28,34–36 our findings reveal that the T′-WTe2 substrate reduces the absolute limiting potential for the CO2RR process (|UL|),37 while inhibiting the competitive HER. This improvement is attributed to the strong distortion of larger Cu13 clusters by the buckled T′-WTe2 surface, facilitating rapid charge transfer between the cluster and substrate. Our work provides an effective approach for enhancing CO2RR efficiency via engineering substrate–cluster interactions.
2. Computational methods
Spin-polarized density functional theory (DFT) calculations were carried out using the Vienna ab initio simulation package (VASP).38,39 The projector-augmented-wave (PAW) method was employed to treat the core wave functions.40 The generalized gradient approximation (GGA) in the Perdew–Burke–Ernzerhof (PBE) form is adopted.41,42 Long-range van der Waals (vdW) interaction was corrected using the DFT-D3 scheme,43 and a vacuum space exceeding 20 Å was used to minimize periodic interactions. The 3 × 3 × 1 Monkhorst–Pack k-points in the Brillouin zone are sampled for structural optimizations, and dense 6 × 6 × 1 k-meshes are set for the density of states (DOS) calculations for T′-WTe2. 15 Å × 15 Å × 15 Å cubic cells are used to optimize the isolated Cu3, Cu8 and Cu13 clusters before loading on the WTe2 surfaces. The cutoff energy for the plane wave basis is 400 eV in all the calculations, and the energy and force convergence criterion are respectively set to 10−5 eV and 10−2 eV Å−1. We used VASPsol for the implicit solvation calculations.44 Water is considered as the continuum solvent throughout, with a bulk static dielectric constant εs of 80.45 In addition, canonical ab initio molecular dynamics simulations (AIMDs) with a Nosé thermostat and integrating time with the Verlet algorithm at a time step of 1 fs are employed to investigate the thermodynamic stability of the catalyst.46The isolated CO2 molecule is simulated in a large cubic cell of 15 Å in length. The adsorption energies are calculated according to the equation Eads = Eadsorbed-system − Emolecule − Ecatalyst, where Eadsorbed-system, Emolecule, and Ecatalyst are the total energies of the catalyst with CO2*, the isolates CO2 molecule and Cun@WTe2 (n = 3, 8, 13), respectively. The Bader charge, charge density difference, and DOS are calculated to clarify the interactions between the adsorbed molecule and the catalyst surface. To calculate the free energy profiles of the electrocatalytic CO2RR, the computational electrode model (CHE) is employed.47 The free energy change (ΔG) at each elementary step of the CO2RR process is calculated using the equation ΔG = ΔE + ΔZPE − TΔS, where ΔE is the total energy change calculated by DFT, ΔZPE is the zero-point energy correction through frequency analysis, and T and ΔS are the reaction temperature (T = 298.15 K) and the entropy value change, respectively. The limiting potential (UL) is defined as the maximum free energy change using the relation UL = −ΔGPDS/e, where ΔGPDS is the maximum free energy increase in a potential determining step (PDS). In addition, we utilized the post-processing functionalities provided by qvasp and VASPKIT to analyze the computational results.48
For isolated Cun clusters, our calculations show finite magnetic moments (1μB for Cu3, 0μB for Cu8, and 5μB for Cu13), in line with previous data.49,50 However, when supported on the T′-WTe2 substrate, the whole system becomes nonmagnetic. The charge transfer between the clusters and substrate fully quenches the magnetism.
3. Results and discussion
3.1. Structural models of Cun (n = 3, 8, 13) clusters, CO2 adsorption on Cun and CO2RR on Cun
The most stable structures of Cu3, Cu8, and Cu13 are an equilateral triangle geometry with C2v symmetry,34 a tetragonal crystal with D2d symmetry,36 and an icosahedron geometry with Ih symmetry (see Fig. 1a–c),35,51 respectively. The calculated Cu–Cu bond lengths (LCu–Cu) are 2.34 Å for Cu3, 2.30–2.50 Å for Cu8, and 2.53 Å for Cu13, which agree well with previously reported values.36,49,50,52We first examine CO2 adsorption on Cun (n = 3, 8, 13) clusters. The most stable adsorption configurations are depicted in Fig. 1d–f, revealing a bent CO2 geometry on all Cun clusters, which indicates effective activation of the CO2 molecule. For the CO2RR, CH4 is the sole product across all cluster sizes; however, the potential-determining step (PDS) varies with cluster size, and the overpotential increases as the cluster size grows (see Table 1). The calculated free energy profile and the corresponding reaction intermediates along the most favorable CO2RR pathway are provided in the ESI† (see Fig. S1 and S2). Additionally, we further investigate the HER on the Cun cluster (Fig. 1g–i). A comparison of UL between CO2RR and HER, presented in Table 1, shows that the HER suppresses the CO2RR on all pure Cun clusters (see Fig. S13, ESI†).
| Configurations | CO2RR | HER | ||
|---|---|---|---|---|
| PDS | U L (V) | Product | U L (V) | |
| Cu3 | *CO + e− + H+ → *CHO | 0.64 | CH4 | −0.51 |
| Cu8 | *OCHOH + e− + H+ → *CHO + H2O | 0.85 | CH4 | 0.73 |
| Cu13 | *CO + e− + H+ → *CHO | 0.89 | CH4 | −0.14 |
3.2. Geometric structures of T′-WTe2
To investigate whether substrate selection can enhance CO2RR performance, we build on our prior findings identifying T′-WTe2 as an effective substrate that could interact with clusters sufficiently.30,53 Consequently, we selected T′-WTe2 as the substrate for loading clusters to further evaluate its CO2RR properties.The monoclinic metal T′ phase of WTe2, a semimetal with no band gap (Fig. 2b), was synthesized decades ago (Fig. 2a),32 a characteristic corroborated by the calculated partial density of states (PDOS) in Fig. S6 (ESI†). T′-WTe2 features three atomic layers, with nonequivalent top (Te1 and Te2) and bottom (Te3 and Te4) Te layers. The Te1 atom sits higher than Te2 in the top layer, forming what we term as a ‘nonuniform surface’,33 with bond lengths of 2.72 Å (LTe2–W) and 2.83 Å (LTe1–W).
 | ||
| Fig. 2 (a) Schematic of the 3 × 2 supercell of T′-WTe2. W and Te atoms are denoted by grey and orange spheres, respectively. (b) Band structure for the T′-WTe2 monolayer. | ||
3.3. CO2 adsorption and electroreduction for Cun@T′-WTe2 (n = 3, 8, 13)
Firstly, we investigate the adsorption of Cun (n = 3, 8, 13) clusters on the T′-WTe2 substrate, with all optimized configurations presented in Fig. S3–S5 (ESI†). The most stable structures for Cu3@T′-WTe2 (Fig. S3b, ESI†), Cu8@T′-WTe2 (Fig. S4h, ESI†), and Cu13@T′-WTe2 (Fig. S5d, ESI†) are highlighted, in Fig. 3 for further studying the CO2RR. Bader charge analysis (Table S1, ESI†) reveals electron transfers of 0.28e, 0.32e and 0.18e from Cu3, Cu8, and Cu13 to T′-WTe2, respectively, indicating significant substrate–cluster interactions, further evidenced by orbital hybridization in the PDOS (Fig. S7, ESI†). As noted in Section 2, while isolated Cun clusters exhibit size-dependent magnetism, the interaction with the T′-WTe2 substrate leads to complete spin quenching. The AIMD simulation at 500 K over 5ps (Fig. S11, ESI†) shows no structural degradation, confirming the excellent thermal stability of the Cun@T′-WTe2 catalyst under ambient conditions.To assess the substrate's role in the CO2RR performance, we studied CO2 adsorption on the Cu3@T′-WTe2, Cu8@T′-WTe2, and Cu13@T′-WTe2 surfaces. Various adsorption configurations of CO2 on the catalyst surfaces are considered as shown in Fig. S8 (ESI†). Strong adsorption energy typically elongates the C–O bond length in the adsorbed CO2 molecule.17,54,55Fig. 3 presents the most favorable sites for CO2 adsorption, showing LC–O extended to 1.22 Å and 1.88 Å (Fig. 3a), 1.25 Å and 1.28 Å (Fig. 3d), and 1.26 Å (Fig. 3g) for the Cu3@T′-WTe2, Cu8@T′-WTe2 and Cu13@T′-WTe2 systems, respectively, suggesting enhanced activation of CO2. After CO2 adsorption, the linear O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O geometry bends into a V-shape, with the (∠OCO angles decreasing from 179.9° to 134.2° for Cu3@WTe2) (Table 2). Similarly, the same trend can be observed for Cu8@WTe2 and Cu13@WTe2, indicating effective CO2 activation by Cun@WTe2. The PDOS analysis shown in Fig. 3c, f, and i reveals orbital hybridization and charge transfer between Cu, C, and O atoms, underscoring the strong interaction between the CO2 molecules and the Cun@T′-WTe2 catalysts.11,56,57 The charge density difference (see Fig. 3) and the Bader charge analysis (Table 2) further confirm electron donation from Cun@WTe2 to the CO2 molecule (around 0.62e–0.79e). The broadly dispersed PDOS peaks for C and O relative to isolated CO2 molecules (Fig. S9, ESI†) and the hybridization with Cu atoms also support the strong interaction between CO2 and Cun.
O geometry bends into a V-shape, with the (∠OCO angles decreasing from 179.9° to 134.2° for Cu3@WTe2) (Table 2). Similarly, the same trend can be observed for Cu8@WTe2 and Cu13@WTe2, indicating effective CO2 activation by Cun@WTe2. The PDOS analysis shown in Fig. 3c, f, and i reveals orbital hybridization and charge transfer between Cu, C, and O atoms, underscoring the strong interaction between the CO2 molecules and the Cun@T′-WTe2 catalysts.11,56,57 The charge density difference (see Fig. 3) and the Bader charge analysis (Table 2) further confirm electron donation from Cun@WTe2 to the CO2 molecule (around 0.62e–0.79e). The broadly dispersed PDOS peaks for C and O relative to isolated CO2 molecules (Fig. S9, ESI†) and the hybridization with Cu atoms also support the strong interaction between CO2 and Cun.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O angles (∠OCO), the corresponding C
O angles (∠OCO), the corresponding C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths (LC
O bond lengths (LC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of Cun@WTe2 (n = 3, 8, 13), and the net charge transferred from adsorbents to CO2 (Δq is calculated based on the Bader charges)
O) of Cun@WTe2 (n = 3, 8, 13), and the net charge transferred from adsorbents to CO2 (Δq is calculated based on the Bader charges)
| Adsorption configurations | E ads-free (eV) | ∠OCO | L C–O (Å) | Δq (e) |
|---|---|---|---|---|
| CO2–Cu3@WTe2 | −0.10 | 134.2° | 1.22; 1.28 | 0.62 |
| CO2–Cu8@WTe2 | −0.33 | 130.6° | 1.25; 1.28 | 0.79 |
| CO2–Cu13@WTe2 | 0.02 | 132.3° | 1.26 | 0.74 |
3.4. CO2 reduction reaction pathways on the Cun@WTe2 (n = 3, 8, 13)
We next explore the CO2RR pathways on Cun@WTe2. Fig. 4 illustrates the calculated free energy profile for the most favorable CO2RR pathways on Cu3@WTe2, Cu8@WTe2, and Cu13@WTe2, with the corresponding optimal reaction configurations shown in Fig. S10 (ESI†). For CO2–Cu3@WTe2, the initial H+ and e− pair attacks the carbon atom of CO2, forming HCOO* with a free energy change of −0.27 eV. Subsequent hydrogenation yields HCOOH*, followed by an exothermic step forming CH2OOH* as shown in Fig. 4a, which is energetically more preferred than the formation of CHOHOH* or HCO* + H2O*. Further hydrogenation of CH2OOH* favors CH2OHOH* (ΔG < 0) over CH2O* + H2O*. Two subsequent additions of the H+ and e− pair will attack the O atom, releasing the H2O molecule and leaving *CH2OH, which undergoes successive hydrogenation steps to produce CH3OH molecules. Overall, the PDS is the formation of CH2OHOH* from CH2OOH* with a UL of −0.38 V.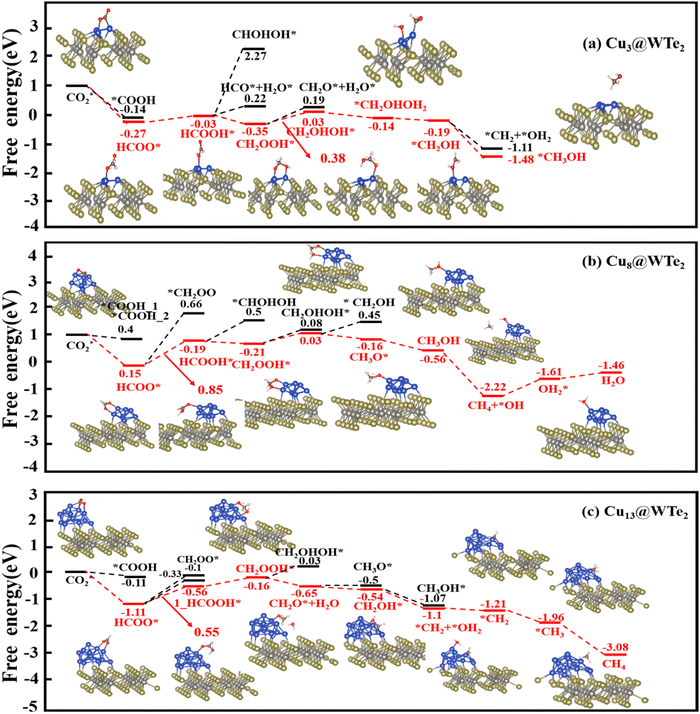 | ||
| Fig. 4 Free energy diagram for the electrochemical CO2RR on (a) Cu3@WTe2, (b) Cu8@WTe2, and (c) Cu13@WTe2. | ||
For the Cu8@WTe2 and Cu13@WTe2 surface, the initial step of the CO2RR is the formation of HCOO*, followed by hydrogenation of HCOO* to form HCOOH* (see Fig. 4b and c), which is more energetically favorable than that of CH2OO*. HCOOH* then converts to CH2OOH*. On Cu8@WTe2, CH2OOH* after hydrogenation will transform into CH2O* + *OH2, proceeding to CH3OH* and ultimately producing CH4. On Cu13@WTe2, CH2OOH will be further hydrogenated to CH2O* with H2O release, followed by hydrogenation to *CH4 with the final release of the CH4 molecule. The PDS for both the Cu8@WTe2 and Cu13@WTe2 systems is HCOO* → HCOOH*, with UL values of −0.85 V and −0.55 V, respectively. The free energy values (G(T)) for these pathways are detailed in Table S3 (ESI†).
The analysis reveals that Cu3@WTe2, Cu8@WTe2, and Cu13@WTe2 all exhibit robust CO2RR activity. On Cu3@WTe2, CO2RR proceeds via a 6e− charge transfer pathway, yielding CH3OH, whereas Cu8@WTe2 and Cu13@WTe2 favor an 8e− charge transfer reaction pathway, producing CH4 as the primary product. Comparison of the overpotentials with pure Cun clusters (Table S2, ESI†) shows that Cun on the T′-WTe2 substrate outperforms their standalone counterparts, with lower |UL| values indicating enhanced CO2RR efficiency. Specifically, |UL| for Cu3@WTe2 is 0.38 V (vs. 0.64 V for pure Cu3, a 0.26 V reduction), while |UL| for Cu13@WTe2 is 0.55 V (vs. 0.89 V for pure Cu13, a 0.34 V reduction). This suggests that the T′-WTe2 substrate significantly boosts the CO2RR performance, with the enhancement scaling with Cu cluster size.
3.5. Analysis of the hydrogen evolution reaction and solvent effects
To evaluate the competing HER,18 we analyzed the HER performance on Cun@WTe2 (n = 3, 8, 13), as detailed in Fig S12 and S14 (ESI†). In all the optimized configurations, H atoms preferentially bind to Cu clusters rather than the WTe2 surface. Catalytic selectivity was assessed using the difference in UL values, i.e., |UL(CO2)| − |UL(H2)|,37 where a positive value of |UL(CO2)| − |UL(H2)| indicates a poor CO2RR selectivity over the HER. For the Cu13@T′-WTe2, the |UL(CO2)| − |UL(H2)| is calculated to be −0.30 V (see Table 3), suggesting a better CO2RR selectivity over the HER. Given that HCOO* is a critical intermediate in all CO2RR pathways, we then considered the competition between H* and HCOO*.55,58–60 For the Cu13@WTe2, HCOO* formation (Fig. 4c) has a free energy change of −1.11 eV, more favorable than −0.85 eV for the HER process (see Table 3), indicating greater HCOO* stability over H*.| Configurations | CO2RR | HER | |||
|---|---|---|---|---|---|
| |UL| (V) | ΔGHCOO* (eV) | Product | |UL| (V) | ΔGH* (eV) | |
| Cu3@WTe2 | 0.38 | −0.27 | CH3OH | 0.10 | 0.10 |
| Cu8@WTe2 | 0.86 | −1.04 | CH4 | 0.05 | −0.05 |
| Cu13@WTe2 | 0.55 | −1.11 | CH4 | 0.85 | −0.85 |
To further gain an in-depth understanding of the catalyst effects, we compared the DOS curves of isolated and adsorbed HCOO* intermediates on the catalyst. As shown in Fig S15 (ESI†), the 2p orbitals of O and C atoms after adsorption shift toward lower energy region near the Fermi level, with larger shifts reflecting stronger intermediate–substrate interaction, and the lower adsorption energy.17,61,62 Cu13@WTe2 exhibits the lowest adsorption energy (−1.11 eV) compared to −0.27 eV for Cu3@WTe2. This suggests that the buckled T′-WTe2 surface enhances CO2RR activity in the supported Cun nanocluster, with larger cluster size strengthening substrate–Cu interactions and boosting electrocatalytic CO2 reduction.
Overall, Cu13@WTe2 emerges as the optimal CO2RR electrocatalyst among the studied configurations. We further examine its electrochemical CO2RR performance using free energy diagrams with an implicit solvation model (Fig. S16, ESI†). The PDS remains unchanged, with the UL value shifting by only 0.09 V upon incorporating the solvation effect. These findings suggest that the electrolyte environment exerts minimal influence on the CO2RR performance of the Cu13@WTe2 catalysts.
4. Conclusions
In conclusion, we theoretically investigate the CO2 electrocatalytic properties on pure Cu clusters and their performance when supported on a T′-WTe2 substrate. Our results reveal that the introduction of the WTe2 substrate significantly reduces the CO2RR overpotential across the system, with Cu13@WTe2 exhibiting particularly notable suppression of the competing hydrogen evolution reaction (HER). Our findings elucidate the size-dependent electrocatalytic behavior of the CO2RR and HER across different Cu clusters while highlighting the critical role of the substrate in modulating electrochemical performance. Collectively, this work offers valuable insights and a strategic framework for designing efficient CO2RR catalysts, paving the way for future experimental advancement in electrocatalytic systems.Author contributions
Qian Sun: data curation, writing – original draft. Huiru Yang: revise the manuscript. Chunmei Zhang: writing – original draft, writing – review & editing. Aijun Du: revise the manuscript and technical support. All authors contributed to the results interpretation and manuscript preparation.Data availability
The data presented in this work is available from the corresponding author upon reasonable request.Conflicts of interest
There is no conflict to declare.References
- Y. Shi, M. Chen and F. Wang, In Research on energy-saving of the pebble thermoregulation greenhouse, Global Conference on Civil, Structural and Environmental Engineering/3rd International Symp on Multi-field Coupling Theory of Rock and Soil Media and its Applications, China Three Gorges Univ, Yichang, PEOPLES R CHINA, 2012, Oct 20–21, China Three Gorges Univ, Yichang, PEOPLES R CHINA, 2012, pp. 2112–2115.
- Y. Shi and M. Chen, In Study on temperature distribution regularity of pebble thermoregulation greenhouse, Global Conference on Civil, Structural and Environmental Engineering/3rd International Symp on Multi-field Coupling Theory of Rock and Soil Media and its Applications, China Three Gorges Univ, Yichang, PEOPLES R CHINA, 2012 Oct 20–21, China Three Gorges Univ, Yichang, PEOPLES R CHINA, 2012, pp. 2116–2119.
- D. J. D. Pimlott, A. Jewlal, Y. Kim and C. P. Berlinguette, Oxygen-Resistant CO2 Reduction Enabled by Electrolysis of Liquid Feedstocks, J. Am. Chem. Soc., 2023, 145(48), 25933–25937 CrossRef CAS PubMed.
- Y. Zhang, In On Study of Teaching Reform of Organic Chemistry Course in Applied Chemical Industry Technology, 2017 3rd International Conference on Energy, Environment and Materials Science (EEMS), Northwestern Polytechnical University, Singapore, SINGAPORE, 2017 Jul 28–30, Northwestern Polytechnical University, Singapore, SINGAPORE, 2017.
- G. Liu, X. Mao, B. Yang, J. Shang and Z. Wu, Research progress on chemical looping reforming of macromolecular components of volatiles from biomass pyrolysis based on decoupling strategy, Fuel Process. Technol., 2022, 235, 107375 CrossRef CAS.
- S. Fu, I. Angelidaki and Y. Zhang, In situ Biogas Upgrading by CO2-to-CH4 Bioconversion, Trends Biotechnol., 2021, 39(4), 336–347 CrossRef CAS PubMed.
- M. Shen, L. Zhang and J. Shi, Defect Engineering of Photocatalysts towards Elevated CO2 Reduction Performance, ChemSusChem, 2021, 14(15), 3226 CrossRef CAS PubMed.
- X. Feng, Y. Pi, Y. Song, C. Brzezinski, Z. Xu, Z. Li and W. Lin, Metal-Organic Frameworks Significantly Enhance Photocatalytic Hydrogen Evolution and CO2 Reduction with Earth-Abundant Copper Photosensitizers, J. Am. Chem. Soc., 2020, 142(2), 690–695 CrossRef CAS PubMed.
- A. R. Woldu, Z. Huang, P. Zhao, L. Hu and D. Astruc, Electrochemical CO2 reduction (CO2RR) to multi-carbon products over copper-based catalysts, Coord. Chem. Rev., 2022, 454, 214340 CrossRef CAS.
- Z.-Y. Du, K. Wang, S.-B. Li, Y.-M. Xie, J.-H. Tian, Q.-N. Zheng, W. F. Ip, H. Zhang, J.-F. Li and Z.-Q. Tian, In Situ Raman Spectroscopic Studies of Electrochemical CO2 Reduction on Cu-Based Electrodes, J. Phys. Chem. C, 2024, 128(28), 11741–11755 CrossRef CAS.
- Z. Zhao, Z. Chen, X. Zhang and G. Lu, Generalized Surface Coordination Number as an Activity Descriptor for CO2 Reduction on Cu Surfaces, J. Phys. Chem. C, 2016, 120(49), 28125–28130 CrossRef CAS.
- T. Liu, G. Song, X. Liu, Z. Chen, Y. Shen, Q. Wang, Z. Peng and G. Wang, Insights into the mechanism in electrochemical CO2 reduction over single-atom copper alloy catalysts: A DFT study, Iscience, 2023, 26(10), 107953 CrossRef CAS PubMed.
- H. Dong, Y. Li and D.-E. Jiang, First-Principles Insight into Electrocatalytic Reduction of CO2 to CH4 on a Copper Nanoparticle, J. Phys. Chem. C, 2018, 122(21), 11392–11398 CrossRef CAS.
- M. Rozenberg, A. Loewenschuss and C. J. Nielsen, H-Bonding of Formic Acid with Its Decomposition Products: A Matrix Isolation and Computational Study of the HCOOH/CO and HCOOH/CO2 Complexes, J. Phys. Chem. A, 2015, 119(31), 8497–8502 CrossRef CAS PubMed.
- S. Xue, X. Liang, Q. Zhang, X. Ren, L. Gao, T. Ma, A. Liu and I. A. Pasti, Density Functional Theory Study of CuAg Bimetal Electrocatalyst for CO2RR to Produce CH3OH, Catalysts, 2024, 14(1), 7 CrossRef CAS.
- C. Christophe, Silica-supported PdGa Nanoparticles: Metal Synergy for Highly Active and Selective CO2-to-CH3OH Hydrogenation, JACS Au, 2022, 2(8), 1946–1947 CrossRef PubMed.
- P. Saha, S. Amanullah and A. Dey, Selectivity in Electrochemical CO2 Reduction, Acc. Chem. Res., 2022, 55(2), 134–144 CrossRef CAS PubMed.
- C. Kim, J. Kim, S. Joo, Y. Yang, J. Shin, M. Liu, J. Cho and G. Kim, Highly Efficient CO2 Utilization via Aqueous Zinc- or Aluminum- CO2 Systems for Hydrogen Gas Evolution and Electricity Production, Angew. Chem., Int. Ed., 2019, 58(28), 9506–9511 CrossRef CAS PubMed.
- J. Gao, H. Wang, K. Feng, C. Xiang, H. Wang, H. Qi, Y. Liu, H. Tian, J. Zhong and Z. Kang, Cu atomic clusters on N-doped porous carbon with tunable oxidation state for the highly-selective electroreduction of CO2, Mater. Adv., 2020, 1(7), 2286–2292 RSC.
- H. Xie, T. Wang, J. Liang, Q. Li and S. Sun, Cu-based nanocatalysts for electrochemical reduction of CO2, Nano Today, 2018, 21, 41–54 CrossRef CAS.
- Y. Xia, Q. Zhang, F. Guo, J. Wang, W. Li and J. Xu, Ag@Cu with Cu-CuO interface prepared by air cold-plasma promoting the electrocatalytic reduction of CO2 to low-carbon alcohols, Vacuum, 2022, 196, 110767 CrossRef CAS.
- S. Choe, J. Kim, S. Y. Kim and S. H. Ahn, Controlling the surface oxidation state of halogenated Cu-based catalyst for electrochemical reduction of carbon dioxide to ethylene, J. Alloys Compd., 2024, 1005 Search PubMed.
- X.-X. Li, L. Zhang, J. Liu, L. Yuan, T. Wang, J.-Y. Wang, L.-Z. Dong, K. Huang and Y.-Q. Lan, Design of Crystalline Reduction-Oxidation Cluster-Based Catalysts for Artificial Photosynthesis, JACS Au, 2021, 1(8), 1288–1295 CrossRef CAS PubMed.
- Y. Gao, M. Zhao, L. Jiang and Q. Yu, Electrochemical CO2 reduction of graphene single-atom/cluster catalysts, Mol. Catal., 2024, 562, 114225 CrossRef CAS.
- S.-Y. Wu, T.-C. Chuang and H.-T. Chen, Electrochemical CO2 reduction on Single-Atom aluminum catalysts supported on graphene and N-doped Graphene: Mechanistic insights and hydration effects, Appl. Surf. Sci., 2025, 681, 161523 CrossRef CAS.
- E. Plaza-Mayoral, V. Okatenko, K. N. Dalby, H. Falsig, I. Chorkendorff, P. Sebastian-Pascual and M. Escudero-Escribano, Composition effects of electrodeposited Cu-Ag nanostructured electrocatalysts for CO2 2 reduction, Iscience, 2024, 27(6), 109933 CrossRef CAS PubMed.
- X.-G. Zhang, S. Feng, C. Zhan, D.-Y. Wu, Y. Zhao and Z.-Q. Tian, Electroreduction Reaction Mechanism of Carbon Dioxide to C2 Products via Cu/Au Bimetallic Catalysis: A Theoretical Prediction, J. Phys. Chem. Lett., 2020, 11(16), 6593–6599 CrossRef CAS PubMed.
- D. Gao, S. Rao, Y. Li, N. Liu and D. Wang, Enhancement of CO adsorption energy on defective graphene-supported Cu13 cluster and prediction with an induction energy model, Appl. Surf. Sci., 2023, 615, 156368 CrossRef CAS.
- A. Y. Ermilov, A. V. Soloviev, Y. N. Morosov and T. I. Shabatina, Interaction of Copper Clusters with Dioxidine, Moscow Univ. Chem. Bull., 2024, 79(4), 233–238 CrossRef.
- H. Yang, W. Zou, C. Zhang and A. Du, Ab Initio Studies of Electrocatalytic CO2 Reduction for Small Cu Cluster Supported on Polar Substrates, ACS Appl. Mater. Interfaces, 2024, 16(26), 33688–33695 CrossRef CAS PubMed.
- C. Huang, A. Narayan, E. Zhang, Y. Liu, X. Yan, J. Wang, C. Zhang, W. Wang, T. Zhou, C. Yi, S. Liu, J. Ling, H. Zhang, R. Liu, R. Sankar, F. Chou, Y. Wang, Y. Shi, K. T. Law, S. Sanvito, P. Zhou, Z. Han and F. Xiu, Inducing Strong Superconductivity in WTe2 by a Proximity Effect, ACS Nano, 2018, 12(7), 7185–7196 CrossRef CAS PubMed.
- P. Zhang, P. Li, Q. Ma, M. Shen, Z. Tian and Y. Liu, Interfacial properties of In-plane monolayer 2H-MoTe2/1T? -WTe2 heterostructures, Appl. Surf. Sci., 2023, 623, 157022 CrossRef CAS.
- Y. Maximenko, Y. Chang, G. Chen, M. R. Hirsbrunner, W. Swiech, T. L. Hughes, L. K. Wagner and V. Madhavan, Nanoscale studies of electric field effects on monolayer 1T′-WTe2, Npj Quant. Mater., 2022, 7(1), 29 CrossRef CAS.
- V. E. Matulis, O. A. Ivashkevich and V. S. Gurin, DFT study of electronic structure and geometry of anionic copper clusters, J. Mol. Struct. Theochem., 2004, 681(1–3), 169–176 CrossRef CAS.
- Q. Zeng, X. Wang, M. L. Yang and H. B. Fu, Interplay between geometrical and electronic stability of neutral and anionic Cu13 clusters: a first-principles study, Eur. Phys. J., 2010, 58(1), 125–129 CAS.
- C. A. Barboza, A. Gambetta, R. Arratia-Perez, P. L. Rodriguez-Kessler, A. Munoz-Castro and D. MacLeod-Carey, Structural, electronic and magnetic properties of copper(I) cubic clusters, Polyhedron, 2021, 195, 114878 CrossRef CAS.
- G. Henkelman, B. P. Uberuaga and H. Jónsson, A climbing image nudged elastic band method for finding saddle points and minimum energy paths, J. Chem. Phys., 2000, 113(22), 9901–9904 CrossRef CAS.
- K. Furthmuller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54(16), 11169–11186 CrossRef PubMed.
- J. Hafner, Ab-initio simulations of materials using VASP:: Density-functional theory and beyond, J. Comput. Chem., 2008, 29(13), 2044–2078 CrossRef CAS PubMed.
- K. Burke, J. P. Perdew and M. Levy, Improving energies by using exact electron densities, Phys. Rev. A: At., Mol., Opt. Phys., 1996, 53(5), R2915–R2917 CrossRef CAS PubMed.
- X. Xu and W. A. Goddard, The extended Perdew-Burke-Ernzerhof functional with improved accuracy for thermodynamic and electronic properties of molecular systems, J. Chem. Phys., 2004, 121(9), 4068–4082 CrossRef CAS PubMed.
- P. E. Blöchl, Projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50(24), 17953–17979 CrossRef PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys., 2010, 132(15), 154104 CrossRef PubMed.
- H. S. Lee and M. E. Tuckerman, Ab initio molecular dynamics with discrete variable representation basis sets: Techniques and application to liquid water, J. Phys. Chem. A, 2006, 110(16), 5549–5560 CrossRef CAS PubMed.
- G. Fisicaro, L. Genovese, O. Andreussi, N. Marzari and S. Goedecker, A generalized Poisson and Poisson-Boltzmann solver for electrostatic environments, J. Chem. Phys., 2016, 144(1), 014103 CrossRef CAS PubMed.
- A. Lahanas and V. Tsaoussidis, Exploiting the efficiency and fairness potential of AIMD-based congestion avoidance and control, Comput. Networks, 2003, 43(2), 227–245 CrossRef.
- J. K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jónsson, Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode, J. Phys. Chem. B, 2004, 108(46), 17886–17892 CrossRef PubMed.
- V. Wang, N. Xu, J.-C. Liu, G. Tang and W.-T. Geng, VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code, Comput. Phys. Commun., 2021, 267, 108033 CrossRef CAS.
- G. Guzman-Ramirez, F. Aguilera-Granja and J. Robles, DFT study of the fragmentation channels and electronic properties of Cunν (ν = ±1,0,2; n = 3-13) clusters, Eur. Phys. J. D, 2010, 57(3), 335–342 CrossRef CAS.
- V. L. Mazalova, A. V. Soldatov, S. Adam, A. Yakovlev, T. Moeller and R. L. Johnston, Small Copper Clusters in Ar Shells: A Study of Local Structure, J. Phys. Chem. C, 2009, 113(21), 9086–9091 CrossRef CAS.
- V. E. Matulis, D. M. Palagin, A. S. Mazheika and O. A. Ivashkevich, DFT study of electronic structure and geometry of anionic copper clusters Cun− (n = 11, 12, 13), J. Mol. Struct. Theochem., 2008, 857(1–3), 66–71 CrossRef CAS.
- E. E. Karagiannis, C. E. Kefalidis, I. Petrakopoulou and C. A. Tsipis, Density Functional Study of Structural, Electronic, and Optical Properties of Small Bimetallic Ruthenium-Copper Clusters, J. Comput. Chem., 2011, 32(7), 1241–1261 CrossRef CAS PubMed.
- H. Yang, W. Zou, K. Ostrikov, C. Zhang and A. Du, Tuning electrocatalytic nitrogen reduction on supported nickel cluster via substrate phase engineering, Appl. Surf. Sci., 2023, 640, 158277 CrossRef CAS.
- Y. Gao, X. Tu, X. Liu, Y. Zhang, M. Huang, J. Zhu and H. Jiang, Advances in DFT study of electronic structure and geometry of anionic copper clusters CO2 Electroreduction over Hollow Fiber Gas Diffusion Electrodes, ChemCatChem, 2024, 16, 17 Search PubMed.
- M. Hu, L. Li, J. Li, K. Zahra and Z. Zhang, Two-dimensional Cu-based materials for electrocatalytic carbon dioxide reduction, Iscience, 2024, 27(3), 109313 CrossRef CAS PubMed.
- W. Choi, Y. Chae, E. Liu, D. Kim, W. S. Drisdell, H.-S. Oh, J. H. Koh, D. K. Lee, U. Lee and D. H. Won, Exploring the influence of cell configurations on Cu catalyst reconstruction during CO2 electroreduction, Nat. Commun., 2024, 15(1), 8345 CrossRef PubMed.
- W. Nie, G. P. Heim, N. B. Watkins, T. Agapie and J. C. Peters, Organic Additive-derived Films on Cu Electrodes Promote Electrochemical CO2 Reduction to C2+ Products Under Strongly Acidic Conditions, Angew. Chem., Int. Ed., 2023, 62(12), 202216102 Search PubMed.
- C. Christophe, Silica-supported PdGa Nanoparticles: Metal Synergy for Highly Active and Selective CO2-to-CH3OH Hydrogenation, JACS Au, 2021, 2(8), 1946–1947 Search PubMed.
- Q. Xu, J. Jiang, X. Sheng, Q. Jing, X. Wang, L. Duan and H. Guo, Understanding the synergistic effect of piezoelectric polarization and the extra electrons contributed by oxygen vacancies on an efficient piezo-photocatalysis CO2 reduction, Inorg. Chem. Front., 2023, 10(10), 2939–2950 RSC.
- R. Cai, M. Sun, J. Ren, M. Ju, X. Long, B. Huang and S. Yang, Unexpected high selectivity for acetate formation from CO2 reduction with copper based 2D hybrid catalysts at ultralow potentials, Chem. Sci., 2021, 12(46), 15382–15388 RSC.
- O. F. Lopes and H. Varela, Effect of Annealing Treatment on Electrocatalytic Properties of Copper Electrodes toward Enhanced CO2 Reduction, ChemistrySelect, 2018, 3(31), 9046–9055 CrossRef CAS.
- Q. Chang, J. H. Lee, Y. Liu, Z. Xie, S. Hwang, N. S. Marinkovic, A.-H. A. Park, S. Kattel and J. G. Chen, Electrochemical CO2 Reduction Reaction over Cu Nanoparticles with Tunable Activity and Selectivity Mediated by Functional Groups in Polymeric Binder, Jacs Au, 2022, 2(1), 214–222 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cp01541c |
| This journal is © the Owner Societies 2025 |


