Open Access Article
Open Access ArticleDirect ladderization of cyclooctatetraene-containing, processable conjugated ladder polymers from annulated bis-zirconacyclopentadienes†
August J.
Rothenberger
 a,
Harrison M.
Bergman
a,
Harrison M.
Bergman
 a,
He
Li
a,
He
Li
 bc,
Miao
Qi
bc,
Miao
Qi
 b,
Yunfei
Wang
b,
Yunfei
Wang
 b,
Yi
Liu
b,
Yi
Liu
 *bc and
T. Don
Tilley
*bc and
T. Don
Tilley
 *ad
*ad
aDepartment of Chemistry, University of California, Berkeley, California 94720, USA. E-mail: tdtilley@berkeley.edu
bMolecular Foundry, Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA. E-mail: yliu@lbl.gov
cMaterials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA
dChemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA
First published on 12th November 2024
Abstract
Conjugated ladder polymers (CLPs) are difficult yet captivating synthetic targets due to their fully unsaturated fused backbones. Inherent challenges associated with their synthesis often lead to low yields, structural defects, and insoluble products. Here a new method to form CLPs is demonstrated, utilizing a high-yielding dimerization of annulated zirconacyclopentadienes to form cyclooctatetraene (COT) monomer units. The resulting COT-containing polymers form rapidly in a single ladderization step from the bis-zirconacyclopentadiene precursors and display Mn up to 29.7 kg mol−1. The polymers represent rare examples of CLPs with negatively curved rings, resulting in the observation of unusual properties. The rigid tub-shaped COT units embedded in the backbone imbue the polymers with microporosity, exhibiting BET surface areas up to 555 m2 g−1. Additionally, the remarkable solubility of these CLPs in organic solvents enables the fabrication of thin films showcasing high dielectric performance with a discharged energy density as high as 6.54 J cm−3 at 650 MV m−1.
Introduction
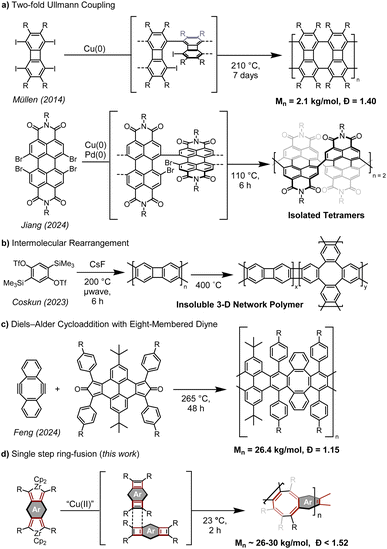
Ladder polymers are an intriguing class of materials where the polymer chain consists of multiple backbones connected at regular intervals like the rungs of a ladder.1,2 These polymers are generally rigid and can display disordered packing resulting in intrinsic microporosity3,4 that allows applications as membranes with high thermal stability5 and remarkable gas selectivity.6 A subset class of ladder polymer, conjugated ladder polymers (CLPs, also known as fully unsaturated ladder polymers) have a fully sp2-hybridized backbone and are of interest due to the electronic properties that arise from their partially delocalized electrons.7–11 However, CLPs are difficult to synthesize, particularly in high yields and with large molecular weights, as a result of the inherent difficulty of forming many fused rings. Additionally, many CLPs are sparingly soluble, further hindering their synthesis, characterization, and processing.The use of negatively-curved rings to form contorted and flexible nanographene materials has been the topic of many recent studies,12–17 but their use in CLPs is less explored.18 To date, there are still no methods of generating negatively curved rings in high enough yield for the synthesis of high molecular weight CLPs. Müllen and co-workers first incorporated eight-membered rings into graphene nanoribbons of Mn = 2.1 kg mol−1via Ullmann coupling (Fig. 1a),19 although these rings are planar and did not display the characteristic “tub-shape” of cyclooctatetraene (COT), possessing greater similarity to a biphenylene sheet.20 A similar perylene diimide (PDI) nanoribbon was synthesized by Zhong on an Au(111) surface.21 Recently, Jiang reported the use of Ullmann coupling to generate chiral PDI dimers and tetramers containing negatively curved eight-membered rings with high dissymmetry.22 This iterative approach to the synthesis of oligomers highlights the inherent challenges of generating linear COT-containing ladder polymers. In 2023, Coskun synthesized a three-dimensional polymer network containing COT and biphenylene moieties via benzyne dimerization (Fig. 1b).23 This initially generates a mostly linear biphenylene polymer, with appreciable COT crosslinks only being introduced during a separate thermal treatment at 400 °C to induce an intermolecular rearrangement of the polymer backbone. The resulting polymer is insoluble, limiting characterization of molecular weight or the efficiency of the COT generation step. The materials displayed high surface areas and gas absorption selectivity, showing promise for membrane applications of COT-containing materials. During the preparation of this manuscript, Feng and co-workers reported the synthesis of a ladder polymer by cycloaddition of a bis-cyclopentadienone with a cycloocta-1,5-diyne at 265 °C (Scheme 1c).24 The success of this approach is predicated on the use of established Diels–Alder chemistry,25,26 which requires a pre-made COT building block to install negative curvature. Additionally, the high Mn ∼26.4 kg mol−1 and low dispersity of 1.15 are achieved after separation of fractions by recycling size exclusion chromatography. In each of the former cases, molecular weight and defect density are non-ideal due to COT formation occurring via two distinct reactions, either via successive Ullmann couplings or an intermolecular rearrangement. The development of approaches that utilize a single, concerted step to generate COT promises to circumvent these issues.
 | ||
| Scheme 1 (a) Synthesis of COT 2 from zirconacycle 1. (b) COT-containing polymers Poly-3 and Poly-4 from bis-zirconacycle precursors 3 and 4. | ||
Such a reaction to generate a COT ring was previously described by this research group, whereby phenanthrene-annulated zirconacyclopentadiene (zirconacycle) 1 is converted to COT 2via a cyclobutadiene intermediate at room temperature (Scheme 1a).27 It is hypothesized that this occurs by dimerization of two transient phenanthrocyclobutadienes through a [4 + 2] cycloaddition and spontaneous rearrangement, affording a COT ring rapidly at room temperature. In this report we improve the efficiency of the reaction to enable its use for polymerization to obtain two COT-containing CLPs with different aromatic backbones, each displaying high molecular weight (Mn > 26 kg mol−1) and low dispersity (Đ ≤ 1.52) (Scheme 1b). Both resulting polymers are highly soluble in common organic solvents, enabling the fabrication of thin films as high-performance dielectrics.
Results and discussion
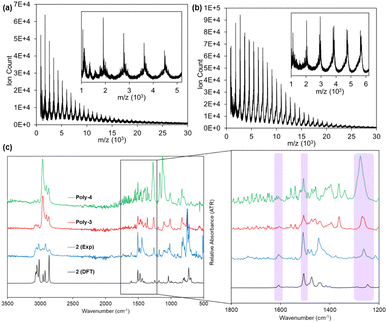
Cyclooctatetraene polymers Poly-3 and Poly-4 were synthesized in a single step by reaction of bis-zirconacycle precursors 3 and 4 with CuCl2 and 1,4-diazobicyclo[2.2.2]octane (DABCO) (Scheme 1b). The polymers differ in the aromatic group annulated to the COT units, either disubstituted[c,j]dibenz[a,h]anthracene or disubstituted[e,l]2,7-di(trifluoromethyl)pyrene. The ligand DABCO was chosen after screening a variety of Lewis bases for the dimerization of 1 and optimization of the yield of 2 (Table S1†). The polymerization reaction is scalable and efficient, giving the polymers in 84% and 81% yields on a 200 mg scale, respectively. To probe the polymerization process, aliquots from the reaction of Poly-3 were quenched with methanol at regular intervals and analyzed by size exclusion chromatography (SEC). After the polymerization begins, Poly-3 rapidly increases in molecular weight, ultimately forming chains with maximum Mn = 29.7 kg mol−1 and Đ = 1.52 (Fig. 2a and b). The polymerization that yields Poly-4 operates similarly, with a maximum Mn = 26.5 kg mol−1 with Đ = 1.22 within 2 h (Fig. S9 and S10†).The polymers were also characterized by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry, which gave molecular weights consistent with those determined by SEC analysis. Poly-3 displays a prominent repeat unit of 854 m/z corresponding to the mass of the monomer unit visible to about Xn = 26 (Fig. 3a). Similarly, the MALDI-TOF pattern for Poly-4 possesses a 914 m/z repeat unit visible until Xn = 28 (Fig. 3b). Infrared spectroscopy was utilized to confirm the presence of cyclooctatetraene moieties in the backbone of the polymers. The spectra of 2, Poly-3, and Poly-4 were compared to a simulated IR spectrum of 2 calculated at the B3LYP/6-31g level of theory. Representative COT vibrations were found at 1606, 1504, and 1241 cm−1 (Fig. 3c) across all spectra, demonstrating shared COT functionality between 2, Poly-3, and Poly-4.
The polymers display broad resonances in NMR spectra, due to the unsymmetrical substitution for the COT repeat unit. This is reflected in the 1H NMR spectrum of monomeric 2, which has 24 unique aryl proton resonance peaks.28 Introduction of repeat units leads to further complexity; thus, the ratio of aryl to alkyl resonances is more informative. For both polymers, the resonance ratio nearly matches the predicted values of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.38 for Poly-3 and 1
1.38 for Poly-3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.80 for Poly-4 (Fig. S1 and S3†). The 13C NMR spectra are similarly broad for both polymers, with peaks in the aryl region between 155 to 125 ppm and resonances for alkyl groups between 35 and 30 ppm. Very few other peaks are visible in the 1H spectrum of Poly-3 at 3.43 and 3.28 ppm, indicating a highly selective polymerization with minimal defects. Similarly, in the 1H spectrum of Poly-4, small defect peaks are present at 2.98 and 2.13 ppm. These likely correspond to annulated cyclobutene resonances, a plausible source of quenched chain ends.
1.80 for Poly-4 (Fig. S1 and S3†). The 13C NMR spectra are similarly broad for both polymers, with peaks in the aryl region between 155 to 125 ppm and resonances for alkyl groups between 35 and 30 ppm. Very few other peaks are visible in the 1H spectrum of Poly-3 at 3.43 and 3.28 ppm, indicating a highly selective polymerization with minimal defects. Similarly, in the 1H spectrum of Poly-4, small defect peaks are present at 2.98 and 2.13 ppm. These likely correspond to annulated cyclobutene resonances, a plausible source of quenched chain ends.
UV-vis spectroscopy was utilized to determine the absorption onset for samples of Poly-3 with increasing molecular weight (Fig. 2c). Normalized at the maximum absorbance peak for the sample of monomer 3 quenched before exposure to CuCl2/DABCO (labeled as 0 min) the samples show a red-shifted absorption onset as Mn increases up to about 14 kg mol−1 at 15 min, indicating that the effective conjugation length along the polymer backbone occurs between 11–16 repeat units. Similarly, a red shift is observed in the absorption onset for Poly-4 samples (Fig. S13†), corresponding to a maximum between 12–18 repeat units. Samples of COT 2, Poly-3, and Poly-4 are weakly fluorescent, displaying emission peaks at 455, 474, and 489 nm, respectively (Fig. S12†).
The incorporation of negatively curved COT rings embedded in a rigid ladder backbone makes Poly-3 and Poly-4 promising candidates as polymers with high intrinsic porosity.29 The polymers were subjected to nitrogen adsorption measurements at 77 K and showed substantial uptake at low relative pressure (P/P0). The calculated apparent Brunauer–Emmett–Teller surface areas (SABET)30 of 421 m2 g−1 and 555 m2 g−1 for Poly-3 and Poly-4 indicate that the polymers possess significant microporosity (Fig. S20†). Evaluation of pore sizes in the two polymers with non-local density functional theory (NLDFT) using a carbon-slit model revealed a microporous region with pores between 10.9–13.5 Å as well as some mesoporosity extending to pore diameters of 120–220 Å (Fig. S21 and S22†). Microporosity likely results from the negative curvature of the rigid COT monomer units forcing inefficient packing of polymer chains while the meso- to macroporosity may result from void space between polymer particles. The polymers have surface area metrics similar to other COT-containing networks,23 but are noteworthy due to their ideal solution processibility amenable to castings of thin films.
The rigid structures of Poly-3 and Poly-4 imbues them with high thermal stability, such that they display relatively high decomposition temperatures around 350 and 325 °C, respectively, as measured by thermal gravimetric analysis (Fig. S14 and S15†). Neither possesses observable glass transition temperatures within the thermally accessible range (Fig. S16 and S17†). Additionally, neither polymer displays reversible redox events as determined by cyclic voltammetry (Fig. S19†). This is consistent with the behavior of monomer 2, which shows four irreversible reductions between −2.2 and −3.5 V vs. Fc/Fc+ (Fig. S18†). The inability to access an aromatic COT dianion showcases the polymers' resistance to planarization and deformation of the polymeric structure.
The solution processability, thermal stability, and wide window of redox stability prompted us to consider these polymers as thin film-based dielectric materials for energy storage applications,31–33 for which ladder polymers have been identified as promising candidates.34,35 Indium tin oxide (ITO) coated glass substrates served as the conducting substrate (see ESI for casting procedure†). The films were analyzed by grazing-incidence wide-angle X-ray scattering (GI-WAXS) to reveal an amorphous distribution of polymer chains (Fig. S23†).
At 103 Hz, films of Poly-3 exhibit a dielectric constant of 3.68 and a loss tangent of 0.0546 while films of Poly-4 displayed a dielectric constant of 3.56 with a loss tangent of 0.0302 (Fig. 4a and b). This reduction is attributed to the presence of –CF3 substituents which leads to a decreased overall polarizability. This phenomenon has been observed in various CF3-containing dielectric polymers,36 such as fluorinated polyimides.37–39 Few traditional conjugated ladder polymers have been subjected to thin-film dielectric studies, but a planar poly(benzimidazobenzophenanthroline) CLP has a dielectric constant of 8.3.40 Due to the rigid microporous nature of Poly-3 and Poly-4, the lowered dielectric constants would be expected due to the decreased packing density of polymer chains.
 | ||
| Fig. 4 (a) Frequency-dependent dielectric constant; (b) frequency-dependent dielectric loss tangent; (c) discharged energy density; (d) energy efficiency of COT polymers. | ||
Poly-4 achieved substantial discharged energy density, up to 6.54 J cm−3 at 650 MV m−1 (Fig. 4c). In contrast, Poly-3 possessed a lower electrical-voltage endurance which limited the film device's capability to withstand higher electric fields, resulting in a maximum discharged energy density of 4.47 J cm−3 at an electric field of 550 MV m−1. Further details concerning the dielectric breakdown strength of the films are provided in the ESI.† Under the same electric field, Poly-3 exhibited an energy efficiency that is inferior to that of Poly-4 (Fig. 4d), which is likely attributable to the higher polarizability and larger dielectric loss with to the absence of –CF3 groups. With the increase in electric field, the lower energy efficiency of Poly-3 further decreased. In contrast, Poly-4 consistently exceeds 85% energy efficiency even at high electric fields of 600–650 MV m−1.
Conclusions
The remarkably efficient dimerization of annulated zirconacycles to generate COT units represents a new pathway to access conjugated ladder polymers, which can exhibit high Mn of nearly 30 kg mol−1. The incorporated COT units in the polymer backbone result in an unusual contorted, rigid ladder structure fully composed of unsaturated carbon atoms. The polymers possess an uncommon combination of properties due to their structure, showcasing intrinsic microporosity, remarkable solubility, and processibility into thin dielectric films which may prove useful for a range of potential applications from gas separation to capacitance.Data availability
The data supporting this article is included as part of the ESI.†Author contributions
A. J. R., H. M. B., and T. D. T. conceived of the idea. H. M. B. developed the methodology and A. J. R. used it for materials synthesis. A. J. R. acquired the data for materials synthesis and characterization. H. L. cast thin films and acquired dielectric characterization data. M. Q. acquired porosimetry data. Y. W. acquired X-ray scattering data. A. J. R., H. M. B., H. L., M. Q., Y. W., Y. L., and T. D. T. wrote and edited the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the National Science Foundation under Grant No. CHE-2103696. Work performed at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the U. S. Department of Energy under Contract No. DE-AC02-05CH11231. The LBNL Catalysis Laboratory, led by Dr Cooper Citek, provided photophysical instrumentation. The computational work was performed at the UC Berkeley Molecular Graphics and Computation Facility (MGCF), which is supported by the National Institute of Health (Grant No. NIH S10OD023532). The authors thank Dr Hasan Celik, Dr Raynald Giovine, and Pines Magnetic Resonance Center's Core NMR Facility (PMRC Core) for spectroscopic assistance. The instruments used in this work were supported by the PMRC Core. H. L. and Y. L. were supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Materials Sciences and Engineering Division, under Contract no. DE-AC02-05CH11231 within the Inorganic/Organic Nanocomposites Program (KC3104). The authors would also like to acknowledge Nathalie Co and Alex Wheeler for thoughtful discussions during the assembly of this manuscript.References
- Y. C. Teo, H. W. H. Lai and Y. Xia, Saynthesis of Ladder Polymers: Developments, Challenges, and Opportunities, Chem.–Eur. J., 2017, 23, 14101–14112 CrossRef CAS PubMed.
- Y. Xia, in Ladder Polymers, John Wiley & Sons, Ltd, 2023, pp. 1–12 Search PubMed.
- A. M. Robinson and Y. Xia, Regioisomeric Spirobifluorene CANAL Ladder Polymers and Their Gas Separation Performance, ACS Macro Lett., 2024, 13, 118–123 CrossRef CAS PubMed.
- M. Carta, in Ladder Polymers, John Wiley & Sons, Ltd, 2023, pp. 179–218 Search PubMed.
- P. M. Budd, E. S. Elabas, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib, C. E. Tattershall and D. Wang, Solution-Processed, Organophilic Membrane Derived from a Polymer of Intrinsic Microporosity, Adv. Mater., 2004, 16, 456–459 CrossRef CAS.
- H. W. H. Lai, F. M. Benedetti, J. M. Ahn, A. M. Robinson, Y. Wang, I. Pinnau, Z. P. Smith and Y. Xia, Hydrocarbon ladder polymers with ultrahigh permselectivity for membrane gas separations, Science, 2022, 375, 1390–1392 CrossRef CAS PubMed.
- J. Lee, Recent Progress in Synthesis of Conjugated Ladder Polymers, Asian J. Org. Chem., 2023, 12, e202300104 CrossRef CAS.
- J. Lee, A. J. Kalin, T. Yuan, M. Al-Hashimi and L. Fang, Fully conjugated ladder polymers, Chem. Sci., 2017, 8, 2503–2521 RSC.
- J. S.-J. Yang and L. Fang, Conjugated ladder polymers: advances from syntheses to applications, Chem, 2024, 10, 1668–1724 CAS.
- T. Ikai, S. Miyoshi, K. Oki, R. Saha, Y. Hijikata and E. Yashima, Defect-Free Synthesis of a Fully π-Conjugated Helical Ladder Polymer and Resolution into a Pair of Enantiomeric Helical Ladders, Angew. Chem., Int. Ed., 2023, 62, e202301962 CrossRef CAS PubMed.
- S. M. West, D. K. Tran, W. Kaminsky and S. A. Jenekhe, Conjugated Ladder Poly(thienobenzothiazine): Synthesis, Electronic Structure, Optical Properties, and Electrical Conductivity of a Narrow Bandgap p-Type Semiconducting Polymer, Macromolecules, 2024, 57, 8176–8186 CAS.
- S. H. Pun and Q. Miao, Toward Negatively Curved Carbons, Acc. Chem. Res., 2018, 51, 1630–1642 CrossRef CAS PubMed.
- G. G. Miera, S. Matsubara, H. Kono, K. Murakami and K. Itami, Synthesis of octagon-containing molecular nanocarbons, Chem. Sci., 2022, 13, 1848–1868 RSC.
- Z. Zhou and M. A. Petrukhina, Planar, curved and twisted molecular nanographenes: reduction-induced alkali metal coordination, Coord. Chem. Rev., 2023, 486, 215144 CrossRef CAS.
- Y. Zhang, D. Yang, S. H. Pun, H. Chen and Q. Miao, Merging a Negatively Curved Nanographene and a Carbon Nanoring, Precis. Chem., 2023, 1, 107–111 CrossRef CAS.
- S. M. Elbert, O. T. A. Paine, T. Kirschbaum, M. P. Schuldt, L. Weber, F. Rominger and M. Mastalerz, A Negatively Curved Nanographene with Four Embedded Heptagons, J. Am. Chem. Soc., 2024, 146, 27324–27334 CrossRef CAS PubMed.
- B. Borrisov, G. M. Beneventi, Y. Fu, Z. Qiu, H. Komber, Q. Deng, P. M. Greißel, A. Cadranel, D. M. Guldi, J. Ma and X. Feng, Deep-Saddle-Shaped Nanographene Induced by Four Heptagons: Efficient Synthesis and Properties, J. Am. Chem. Soc., 2024, 146, 27335–27344 CrossRef CAS PubMed.
- N. M.-W. Wu, M. C. Warndorf, A. Alexander-Katz and T. M. Swager, Oxepine-Based π-Conjugated Ladder/Step-Ladder Polymers with Excited -State Aromaticity, Macromolecules, 2024, 57, 991–1000 CrossRef CAS.
- F. Schlütter, T. Nishiuchi, V. Enkelmann and K. Müllen, Octafunctionalized Biphenylenes: Molecular Precursors for Isomeric Graphene Nanostructures, Angew. Chem., Int. Ed., 2014, 53, 1538–1542 CrossRef PubMed.
- Q. Fan, L. Yan, M. W. Tripp, O. Krejčí, S. Dimosthenous, S. R. Kachel, M. Chen, A. S. Foster, U. Koert, P. Liljeroth and J. M. Gottfried, Biphenylene network: A nonbenzenoid carbon allotrope, Science, 2021, 372, 852–856 CrossRef CAS PubMed.
- M. Liu, M. Liu, L. She, Z. Zha, J. Pan, S. Li, T. Li, Y. He, Z. Cai, J. Wang, Y. Zheng, X. Qiu and D. Zhong, Graphene-like nanoribbons periodically embedded with four- and eight-membered rings, Nat. Commun., 2017, 8, 14924 CrossRef CAS PubMed.
- Y. Liu, Z. Li, M.-W. Wang, J. Chan, G. Liu, Z. Wang and W. Jiang, Highly Luminescent Chiral Double π-Helical Nanoribbons, J. Am. Chem. Soc., 2024, 146, 5295–5304 CrossRef CAS PubMed.
- T. Ashirov, P. W. Fritz, Y. Lauber, C. E. Avalos and A. Coskun, Fully Conjugated Benzyne-Derived Three-Dimensional Porous Organic Polymers, Chem.–Eur. J., 2023, 29, e202301053 CrossRef CAS PubMed.
- X. Feng, S. Obermann, X. Zhou, L. A. Guerrero-León, G. Serra, S. Böckmann, Y. Fu, E. Dmitrieva, J.-J. Zhang, F. Liu, A. A. Popov, A. Lucotti, M. R. Hansen, M. Tommasini, Y. Li, P. W. M. Blom and J. Ma, Wavy Graphene Nanoribbons Containing Periodic Eight-Membered Rings for Light-Emitting Electrochemical Cells, Angew. Chem., Int. Ed., 2024, e202415670 Search PubMed.
- S. Ito, M. Wehmeier, J. D. Brand, C. Kübel, R. Epsch, J. P. Rabe and K. Müllen, Synthesis and Self-Assembly of Functionalized Hexa-peri-hexabenzocoronenes, Chem.–Eur. J., 2000, 6, 4327–4342 CrossRef CAS.
- Y. Zhang, Q. Tang, Z. Li and K. Zhang, Self-Accelerating Diels–Alder Reaction for Preparing Polymers of Intrinsic Microporosity, Angew. Chem., Int. Ed., 2023, 62, e202302527 CrossRef CAS PubMed.
- H. M. Bergman, D. D. Beattie, R. C. Handford, E. Rossomme, B. A. Suslick, M. Head-Gordon, T. R. Cundari, Y. Liu and T. D. Tilley, Copper(III) Metallacyclopentadienes via Zirconocene Transfer and Reductive Elimination to an Isolable Phenanthrocyclobutadiene, J. Am. Chem. Soc., 2022, 144, 9853–9858 CrossRef CAS PubMed.
- H. M. Bergman, D. D. Beattie, G. R. Kiel, R. C. Handford, Y. Liu and T. D. Tilley, A sequential cyclization/π-extension strategy for modular construction of nanographenes enabled by stannole cycloadditions, Chem. Sci., 2022, 13, 5568–5573 RSC.
- P. M. Budd, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib and C. E. Tattershall, Polymers of intrinsic microporosity (PIMs): robust, solution-processable, organic nanoporous materials, Chem. Commun., 2004, 230–231 RSC.
- K. E. Hart, L. J. Abbott and C. M. Colina, Analysis of force fields and BET theory for polymers of intrinsic microporosity, Mol. Simul., 2013, 39, 397–404 CrossRef CAS.
- M. Yang, M. Guo, E. Xu, W. Ren, D. Wang, S. Li, S. Zhang, C.-W. Nan and Y. Shen, Polymer nanocomposite dielectrics for capacitive energy storage, Nat. Nanotechnol., 2024, 1–16 Search PubMed.
- F. Le Goupil, V. Salvado, V. Rothan, T. Vidil, G. Fleury, H. Cramail and E. Grau, Bio-Based Poly(hydroxy urethane)s for Efficient Organic High-Power Energy Storage, J. Am. Chem. Soc., 2023, 145, 4583–4588 CrossRef CAS PubMed.
- C. L. Anderson, H. Li, C. G. Jones, S. J. Teat, N. S. Settineri, E. A. Dailing, J. Liang, H. Mao, C. Yang, L. M. Klivansky, X. Li, J. A. Reimer, H. M. Nelson and Y. Liu, Solution-processable and functionalizable ultra-high molecular weight polymers via topochemical synthesis, Nat. Commun., 2021, 12, 6818 CrossRef CAS PubMed.
- J. Chen, Y. Zhou, X. Huang, C. Yu, D. Han, A. Wang, Y. Zhu, K. Shi, Q. Kang, P. Li, P. Jiang, X. Qian, H. Bao, S. Li, G. Wu, X. Zhu and Q. Wang, Ladderphane copolymers for high-temperature capacitive energy storage, Nature, 2023, 615, 62–66 CrossRef CAS PubMed.
- A. M. M. Hasan, S. Bose, R. Roy, J. D. Marquez, C. Sharma, J. C. Nino, K. O. Kirlikovali, O. K. Farha and A. M. Evans, Electroactive Ionic Polymer of Intrinsic Microporosity for High-Performance Capacitive Energy Storage, Adv. Mater., 2024, 36, 2405924 CrossRef CAS PubMed.
- G. Maier, Low dielectric constant polymers for microelectronics, Prog. Polym. Sci., 2001, 26, 3–65 CrossRef CAS.
- M.-D. Damaceanu and M. Bruma, Local and segmental motion in highly transparent and low-k poly(ether-imide) films, J. Polym. Res., 2014, 22, 639 CrossRef.
- X. Wu, J. Cai and Y. Cheng, Synthesis and characterization of high fluorine-containing polyimides with low-dielectric constant, J. Appl. Polym. Sci., 2022, 139, 51972 CrossRef CAS.
- W.-L. Qu and T.-M. Ko, Studies of dielectric characteristics and surface energies of spin-coated polyimide films, J. Appl. Polym. Sci., 2001, 82, 1642–1652 CrossRef CAS.
- S. Kraner, C. Koerner, K. Leo, E. Bittrich, K.-J. Eichhorn, Y. Karpov, A. Kiriy, M. Stamm, K. Hinrichs and M. Al-Hussein, Dielectric function of a poly(benzimidazobenzophenanthroline) ladder polymer, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 195202 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc06902a |
| This journal is © The Royal Society of Chemistry 2024 |