Open Access Article
Open Access ArticleUnraveling the atomic structure and dissociation of interfacial water on anatase TiO2 (101) under ambient conditions with solid-state NMR spectroscopy†
Longxiao
Yang‡
a,
Min
Huang‡
 b,
Ningdong
Feng
*a,
Meng
Wang
b,
Ningdong
Feng
*a,
Meng
Wang
 c,
Jun
Xu
c,
Jun
Xu
 a,
Ying
Jiang
a,
Ying
Jiang
 d,
Ding
Ma
d,
Ding
Ma
 *c and
Feng
Deng
*c and
Feng
Deng
 *a
*a
aState Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, National Center for Magnetic Resonance in Wuhan, Wuhan Institute of Physics and Mathematics, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences, University of Chinese Academy of Sciences, Wuhan 430071, Beijing 100049, P. R. China. E-mail: ningdong.feng@wipm.ac.cn; dengf@wipm.ac.cn
bSchool of Physics, Hubei University, Wuhan 430062, P. R. China
cBeijing National Laboratory for Molecular Sciences, New Cornerstone Science Laboratory, College of Chemistry and Molecular Engineering, Peking University, Beijing, China. E-mail: dma@pku.edu.cn
dInternational Center for Quantum Materials, School of Physics, Peking University, Beijing, P. R. China
First published on 26th June 2024
Abstract
Anatase TiO2 is a widely used component in photo- and electro-catalysts for water splitting, and the (101) facet of anatase TiO2 is the most commonly exposed surface. A detailed understanding of the behavior of H2O on this surface could provide fundamental insights into the catalytic mechanism. This, however, is challenging due to the complexity of the interfacial environments, the high mobility of interfacial H2O, and the interference from outer-layer H2O. Herein, we investigate the H2O/TiO2 interface using advanced solid-state NMR techniques. The atomic-level structures of surface O sites, OH groups, and adsorbed H2O have been revealed and the detailed interactions among them are identified on the (101) facet of anatase TiO2. By following the quantitative evolution of surface O and OH sites along with H2O loading, it is found that more than 40% of the adsorbed water spontaneously dissociated under ambient conditions on the TiO2 surface at a loading of 0.3 mmol H2O/g, due to the delicate interplay between water–surface and water–water interactions. Our study highlights the importance of understanding the atomic-level structures of H2O on the surface of TiO2 in catalytic reactions. Such knowledge can promote the design of more efficient catalytic systems for renewable energy production involving activation of water molecules.
Introduction
The behavior of water on the surface of metal oxide or metal is a subject of immense importance in a variety of fields such as heterogeneous catalysis, energy science, and materials science.1–8 This is because water plays a critical role in various chemical processes that occur on the surface of these materials.9–19 A detailed understanding of the structure and behavior of interfacial H2O on these surfaces can help researchers to develop more efficient and effective catalytic systems and materials for energy storage and conversion.One material that has been extensively studied in this regard is titanium dioxide (TiO2). TiO2 is widely used in various fields, including photocatalysis, solar cells, and sensors.1–3,20–22 The surface of TiO2 is highly hydrophilic, and therefore water molecules readily adsorb onto the surface. However, details of the interaction of water with the surface, the reactivity of the TiO2 surface, the rupture of the water O–H bond and consequently the formation of the water–oxide interface structure are essential for the understanding of the interfacial water behavior on the TiO2 surface.
A number of studies have been conducted to investigate the behavior of water on the TiO2 surface. By using scanning tunneling microscopy (STM), it was reported that water molecules form a highly ordered structure on the TiO2 surface, and depending on the conditions, one/two/three-dimensional water structures (monomer, dimer, trimer, tetramer, chains, and networks) could be formed on the surface.23–27 In other reports, it was suggested that a water molecule could react with the oxygen vacancy/defect or rupture over the low coordination surface Ti sites of TiO2, and forms two hydroxyls.11,28–31 Very recently, by an elegant environmental TEM method, Wang et al. reported that the four-coordinated Ti on a (1 × 4) reconstructed TiO2 (001) surface is highly active for water activation.32 However, water adsorption and dissociation on non-defect TiO2 has been disputed for decades.33–36 For the interfacial H2O on the (101) facet of anatase TiO2, a number of theoretical calculations37–40 and some experimental studies, including temperature-programmed desorption (TPD)41 and STM,23 have concluded an intact molecular adsorption of H2O on the TiO2 surface. In contrast, a partial dissociation of H2O has been inferred through the observation of hydroxyls formed on the TiO2 surface by using spectroscopic techniques, such as X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), sum frequency generation (SFG) and so on.42–49 However, it is challenging to distinguish the OH groups formed by H2O dissociation on the non-defect TiO2 surface from either the OH groups generated by H2O reaction with defect sites or the original OH groups present on TiO2. Therefore, while progress has been made, the atomic-level structures of interfacial H2O and especially its detailed interaction with the TiO2 surface are still to be resolved. Significantly, several challenges need to be addressed50 to get the atomic-level structures of the interplay, including how to reveal the interaction of surface oxygen and titanium atoms with interfacial H2O, how the water splitting and hydroxyl formation occur, and the quantitative evolution of these surface oxygen species at the water/TiO2 interface during the hydration process. All these problems are difficult to solve under working reaction conditions due to the interference from outer-layer H2O, the high mobility of interfacial H2O, and the complexity of interfacial environments.15,51,52
17O NMR has long been a powerful and attractive approach for characterizing the atomic-level structure of various oxygen-containing materials due to the wide 17O chemical shift range.53–63 For nano-oxides, the relatively low gyromagnetic ratio, low 17O abundance (0.037%), quadrupolar nature, and especially low proportion of surface oxygen atoms lead to a great challenge to investigate the detailed structure of surface oxygen atoms. The sensitivity and resolution were still limited despite 17O isotopic enrichment. Recently, Peng and Grey et al. selectively enriched surface oxygen atoms on metal oxides (including CeO2, TiO2, and ZnO) with H217O, and distinguished surface O/OH sites from bulk oxygen by using one-dimensional (1D) 17O MAS NMR spectra combined with DFT calculations.64–66 We identified surface OH groups and (sub-)surface O sites on γ-Al2O3 by using the 2D proton-detected 1H–17O heteronuclear correlation technique to improve the sensitivity and resolution of 17O NMR spectra,67 which would make it possible to study interactions between interfacial H2O and oxide surfaces. To date, the detailed structures of the surface O/OH sites and their interactions with interfacial H2O are still ambiguous, let alone quantitative evolution of the structure of these surface O/OH sites in the presence of interfacial H2O, which should be the key to understand the H2O–oxide interaction.
In this study, we choose anatase TiO2 nanoparticles as the oxide model owing to their wide practical application in photo- and photoelectro-catalysis, on which the most frequently exposed surface is the (101) facet with the lowest energy, and investigated the ability of anatase (101) facet-dominated TiO2 nanoparticles to adsorb and activate water on their surface. By using the 2D 17O MQMAS and 1H{17O} J-HMQC NMR methods and other techniques, we examined the atomic-level structures of interfacial water and surface O/OH sites of TiO2 at different water loading levels, as well as the through-bond interactions between them. Our findings show that due to the delicate interplay between water–surface and water–water interactions, the O–H bond of the adsorbed water is broken through the joint effort of coordination-unsaturated Ti5C and the adjacent surface O2C sites, resulting in a terminal OH group (Ti5C–OH) and a proton accommodated on the surface O2C site to form a bridging hydroxyl (O2CH). By following the quantitative evolution of surface O and OH sites along with H2O loading, it is demonstrated that at a loading of 0.3 mmol H2O g−1, over 40% of the adsorbed water was dissociated spontaneously on the TiO2 surface. The understanding over the structure and behavior of interfacial H2O is helpful for developing more efficient and effective catalytic systems for energy storage and conversion.
Results and discussion
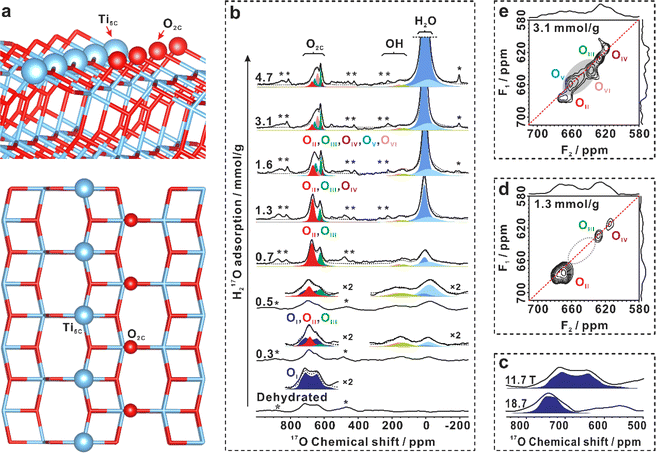
Interaction of surface oxygen sites with H2O on the (101) facet of TiO2
Anatase TiO2 (Fig. S1–S4 in ESI†) with predominantly (101) facets (95% percent) has been synthesized. 68,69 No oxygen vacancies were detected at liquid nitrogen temperature by the ESR spectra (Fig. S5†) before and after dehydration of the TiO2 sample at 160 °C. The morphology of the (101) facet of anatase is well-documented.23 It features a saw-tooth-like shape, and its top layer is composed of two coordination-unsaturated species in close proximity: the five-coordinated Ti (Ti5C) and two-coordinated surface O (O2C) atoms, as illustrated in Fig. 1a.To investigate the surface sites on the (101) facet of TiO2 that interact with H2O, one-dimensional (1D) and two-dimensional (2D) 17O MAS NMR experiments were performed on 17O-enriched TiO2 (with the surface layer of TiO2 enriched with 17O, see methods for details) with different H217O loadings. As shown in Fig. 1b, the resonances at 600–800 ppm in the 17O MAS NMR spectrum of bare TiO2 were observed, the chemical shift of which is well above the three-coordinated oxygen in the bulk (around 400–600 ppm, Fig. S6†).65 The signal shows a broad second-order quadrupolar interaction lineshape (11.7 T), with its line width sharply decreasing with the increase of magnetic field (18.7 T, Fig. 1c). We assign the resonance to a surface O2C site without interaction with H2O on bare TiO2, marked as OI. Upon loading 0.03 mmol H217O over 100 mg TiO2 (0.3 mmol g−1), the shape of the signal at 600–800 ppm changes significantly, with a tip appearing at 700 ppm (Fig. 1b). At the same time, two new resonances centered at 0 and 150 ppm emerge. To gain insight into the structure of the surface oxygen sites interacting with H2O, we used the 2D 17O 3Q MAS NMR technique to remove the quadrupolar broadening and enhance the spectral resolution. For TiO2 loaded with 0.3 mmol g−1 H217O, clearly, the 2D 3Q MAS spectrum resolved the overlapped resonances in the 17O MAS NMR spectrum (Fig. S7†), revealing two new two-coordinated O2C sites (OII and OIII). However, the large quadrupolar interaction of the OI site made it hardly observable in the 2D 3Q MAS spectrum. When the H217O loading increased to 1.3 mmol g−1 and then to 3.1 mmol g−1, up to five signals were identified, representing five types of O2C sites (OII, OIII, OIV, OV, and OVI) (Fig. 1d and e). The appearance of new O2C sites at the expense of OI sites demonstrates that a fraction of two-coordinated surface oxygen sites can interact with water molecules, leading to changes in their coordination environments.
The NMR parameters of these O2C sites (OI–OVI) obtained from 1D and 2D 17O 3Q MAS NMR spectra (Table S1†) were used to deconvolute the 1D 17O MAS NMR signals acquired at 11.7 T and 18.7 T (Fig. 1b and S8†). The different oxygen species (OII–OVI) obtained upon water loading indicate different interplays between the O2c sites and water. Notably, the 17O MAS NMR spectrum of bare TiO2 does not exhibit any signals of adsorbed H2O or surface OH groups (usually at −200 to 200 ppm) (Fig. 1b). At a water loading of 0.3 mmol g−1, besides the change in O2C sites, the adsorbed H2O at −50–10 ppm and two new overlapped species of surface hydroxyls at around 150 ppm emerge (Fig. 1b), which can be well resolved by the following 2D 1H{17O} J-HMQC NMR experiments. The appearance of hydroxyls suggests that water splitting occurs, which is also confirmed by 2H MAS NMR (see the following). These results demonstrate that: (1) water can interact with the surface O2C site, resulting in a change in its chemical environment, although the type and strength of the interaction cannot be determined at the present time; and (2), 17O MAS NMR experiments confirm the formation of hydroxyls arising from the dissociation of water on the TiO2 surface.
Detailed interaction of surface oxygen/hydroxyl sites with interfacial H2O
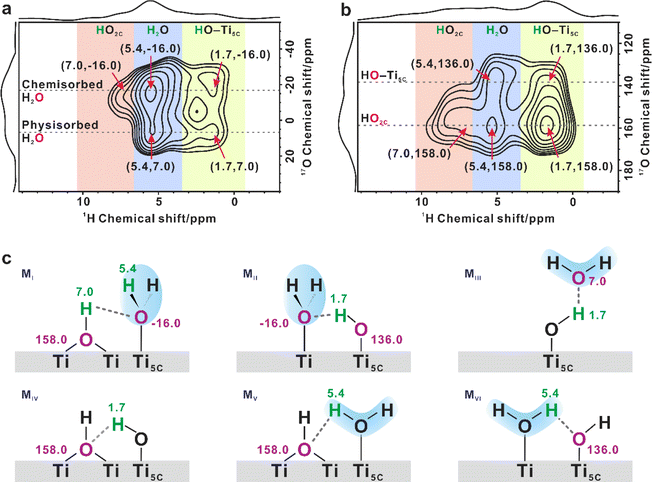
To better understand the structure of the interfacial scenario during water adsorption, a series of 2D 1H{17O} J-HMQC NMR experiments were conducted. These experiments allowed for the identification of 1H–17O correlation/connectivity through chemical bonds and strong hydrogen bonds. There are three types of 1H NMR signals at 1.7, 5.4, and 7.0 ppm, which can be assigned to the proton of terminal hydroxyl, adsorbed H2O, and bridging hydroxyl, respectively.13,14 At H217O loadings of 0.5, 1.3 and 3.1 mmol g−1, the result showed that O2C sites were strongly correlated with adsorbed H2O (5.4 ppm, Fig. S9†), indicating that the surface coordination-unsaturated O2C sites were responsible for the interaction with water. Interestingly, no correlation was observed between the O2C sites and terminal/bridging hydroxyls, indicating that the hydrogen in surface hydroxyls is not connected to the adjacent O2C site via hydrogen-bonds.The spatial relationship between the adsorbed water and the two surface hydroxyls was also investigated. Fig. 2a and b display the 1H–17O correlations between adsorbed H2O and surface OH groups on TiO2 with a 0.5 mmol g−1 H217O loading in different 17O chemical shift ranges. As shown in Fig. 2a, two cross peaks were visible at (5.4, −16) ppm and (5.4, 7.0) ppm in the correlation experiment, indicating the presence of two types of adsorbed H2O on the TiO2 surface. These were ascribed to chemisorbed and physisorbed H2O, respectively, which was validated by variable-temperature 2H static NMR experiments (Fig. S10†). In the 1D 17O MAS NMR spectra (Fig. 1b), when the H2O loading increases from 0.3 to 4.7 mmol g−1, the 17O signal of adsorbed H2O gradually narrows and shifts from −16.0 to 7.0 ppm, suggesting that the H2O molecule is preferentially adsorbed on the unsaturated Ti5C site to form chemisorbed H2O (Ti5C–OH2, 17O signal at −16 ppm), while excess H2O molecules adsorb on the outer layer of chemisorbed H2O and hydroxyls through hydrogen bonds to form physisorbed H2O (17O signal at 7.0 ppm).
Interestingly, the 1H signal (7.0 ppm) of bridging hydroxyl (HO2C) only correlates with the 17O signal of chemisorbed H2O (−16.0 ppm; with the most possible structure illustrated in Fig. 2c MI), while the 1H signal (1.7 ppm) of the terminal hydroxyl (Ti5C–OH) correlates with the 17O signals of both chemisorbed (Fig. 2c MII) and physisorbed H2O (7.0 ppm, Fig. 2c MIII). At the same time, as shown in Fig. 2b, there are two types of oxygen of hydroxyls present at 136 and 158 ppm, respectively. The 1H (7.0 ppm) signal of HO2C is only correlated with the 17O signal at 158 ppm. As such, we assigned the cross peak at (7.0, 158) ppm to the 1H–17O correlation from HO2C, and ascribed the cross peak at (1.7, 136) ppm to the Ti5C–OH correlation. The cross peak at (1.7, 158) ppm represents the correlation between them (Fig. 2c MIV). It is worth noting that the 1H signal of adsorbed H2O (5.4 ppm) correlates with the 17O signals of both bridging OH (158 ppm, Fig. 2c MV) and terminal OH groups (136 ppm, Fig. 2c MVI), leading to two cross peaks at (5.4, 158) and (5.4, 136) ppm. As the H2O loading increases to 1.3 mmol g−1, the cross peaks become more prominent, but the interplay remains the same (Fig. S11†).
These results indicate that water splitting is easy to happen under ambient conditions over the practical TiO2 sample under conditions close to the working catalytic conditions, and more importantly, the interplay mode between the physisorbed or chemisorbed H2O and the different types of hydroxyls is very complicated (Fig. 2c MI to MVI), but it could be well resolved by NMR methods. However, questions remain to answer are, how the O–H bond of water ruptured over the TiO2 surface and whether the vacancy gets involved.
Quantitative evolution of surface oxygen/hydroxyl sites at the water/TiO2 interface during the hydration process
To answer the questions, quantitative information about different sites and species during water adsorption was estimated from 1D 17O MAS NMR spectra (Fig. 3a). As shown in Fig. 3a, the only surface O2C site present on the bare TiO2 sample is OI. When the H2O loading is 0.3–0.5 mmol g−1, two new O2C sites (OII, OIII) appear at the expense of the OI site. When the H2O loading grows up to 0.7 mmol g−1, the OII and OIII sites reach maximum, while the OI site disappears completely. As shown in Fig. 1b, the chemisorbed H2O (Ti5C–OH2) is predominant in the H2O loading range of 0–0.7 mmol g−1. Therefore, the OII and OIII structures should originate from the OI site interacting with chemisorbed H2O. With further increasing the H2O loading to 1.3 and then to 1.6 mmol g−1, the adsorbed H2O exists mainly in the form of physisorbed H2O (those without direct bonding with Ti sites), and meanwhile new O2C sites (OIV, OV, OVI) appear (Fig. 3a). In addition, the amount of OII and OIII sites remains almost unchanged, implying that the physisorbed H2O molecules are mainly adsorbed on the outer-layer of chemisorbed H2O and OH groups rather than the surface O2C site. When the H2O loading is increased to 3.1 mmol g−1, the OVI site increases remarkably at the expense of OII, OIII, and OV sites, indicating the further evolution of surface O2C sites interacting with the additional physisorbed H2O. The relative content of the OI–OVI sites eventually remains unchanged even when the H2O loading is further increased to 4.7 mmol g−1. With the above information, we constructed six O2C sites with possible H2O adsorption patterns (Fig. 3b), which are derived from a series of calculated structures with different H2O adsorption configurations on (2 × 2) surface slabs (Fig. S12–S24†). Obviously, the calculated 17O NMR chemical shifts of these O2C sites just correspond to experimental observation (Fig. S25†).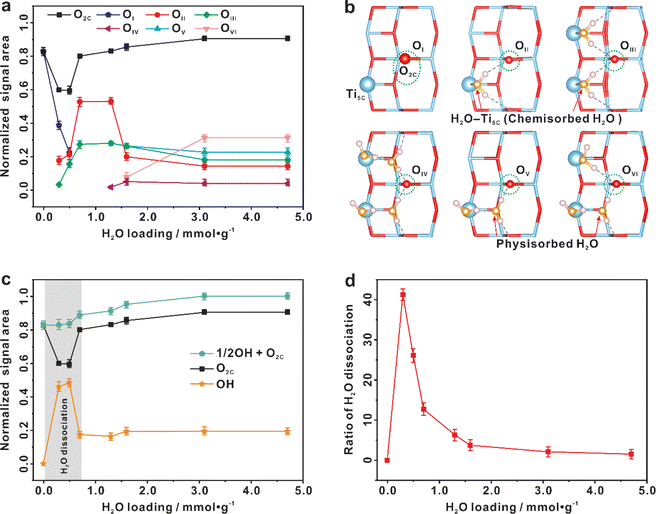 | ||
| Fig. 3 Spontaneous dissociation of interfacial H2O on the (101) facet of TiO2 (100 mg) at room temperature. (a) Quantitative evolution of various surface two-coordinated oxygen sites (O2C, including OI–OVI) with the increase of H2O loading. (b) The possible configurations of six O2C sites (OI, OII, OIII, OIV, OV, and OVI) on the (101) facet of TiO2 optimized from theoretically calculations. (c) Quantitative evolution of surface O2C sites and hydroxyls with the increase of H2O loading. (d) The proportion of H2O dissociation with the increase of H2O loading. Quantification of the O2C/OH sites is conducted by fitting the main peaks and their spinning sidebands in the 1D 17O MAS NMR spectra (Fig. 1b). Error bars in a, c, and d represent s.d. for each data point (three independent experiments), and points are the average values. | ||
The sum of O2c (O2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OI + OII + OIII + OIV + OV + OVI) sites shows quantitative information about the surface O2C sites (Fig. 3a and c). It is relatively constant except at the water loading of 0.3–0.5 mmol g−1, where the total content of surface O2C sites is decreased by ca. 20% (see the grey region highlighted in Fig. 3c). Very interestingly, this is just the time for the appearance of OH groups whose content is increased by ca. 40% (Fig. 3c). This, together with the above results (Fig. 2), shows that the chemisorbed H2O molecule on the Ti5C site (H2O–Ti5C) is apt to dissociate into a terminal OH group (Ti5C–OH) and a proton (H+), and the latter protonates an adjacent surface O2C site to form a bridging OH group (HO2C), which “consumes” an adjacent O2C site (see below Fig. 4). The proportion of water dissociation relative to total water loaded was also calculated, as depicted in Fig. 3d. Intriguingly, the highest proportion of H2O dissociation, at 41.2%, was found at a water loading of 0.3 mmol g−1. With increasing the H2O loading from 0.5 to 4.7 mmol g−1, the content of surface O2C sites gradually recovered and that of the hydroxyls decreased (Fig. 3c), The results point to the reaction of HO2C groups with nearby Ti5C–OH groups, leading to the regeneration of H2O–Ti5C and the recovery of surface O2C sites. The schematic diagram of H2O dissociation and regeneration on the TiO2 surface is illustrated in Fig. 4.
OI + OII + OIII + OIV + OV + OVI) sites shows quantitative information about the surface O2C sites (Fig. 3a and c). It is relatively constant except at the water loading of 0.3–0.5 mmol g−1, where the total content of surface O2C sites is decreased by ca. 20% (see the grey region highlighted in Fig. 3c). Very interestingly, this is just the time for the appearance of OH groups whose content is increased by ca. 40% (Fig. 3c). This, together with the above results (Fig. 2), shows that the chemisorbed H2O molecule on the Ti5C site (H2O–Ti5C) is apt to dissociate into a terminal OH group (Ti5C–OH) and a proton (H+), and the latter protonates an adjacent surface O2C site to form a bridging OH group (HO2C), which “consumes” an adjacent O2C site (see below Fig. 4). The proportion of water dissociation relative to total water loaded was also calculated, as depicted in Fig. 3d. Intriguingly, the highest proportion of H2O dissociation, at 41.2%, was found at a water loading of 0.3 mmol g−1. With increasing the H2O loading from 0.5 to 4.7 mmol g−1, the content of surface O2C sites gradually recovered and that of the hydroxyls decreased (Fig. 3c), The results point to the reaction of HO2C groups with nearby Ti5C–OH groups, leading to the regeneration of H2O–Ti5C and the recovery of surface O2C sites. The schematic diagram of H2O dissociation and regeneration on the TiO2 surface is illustrated in Fig. 4.
The dissociation process of interfacial H2O could be also confirmed by 1D 2H MAS NMR experiments on the TiO2 samples (Fig. 5 and S26†). The dehydrated 2H-enriched TiO2 was prepared by exchanging TiO2 with 4.7 mmol g−1 of 2H2O at room temperature for 2 h, and then the 2H-enriched sample was dehydrated at 160 °C and then loaded with different amounts (0.3–4.7 mmol g−1) of 2H2O at room temperature. Since the proton of the bridging OH group is readily exchanged with 2H of D2O, only the 2H signal of the deuterated bridging OH group (DO2C) is observable at 7.4 ppm in the 1D spectrum of dehydrated TiO2 (Fig. 5a). When 0.3 mmol g−1 D2O is adsorbed on the deuterated TiO2, in addition to the increase of the deuterated bridging OH group, a new signal at 1.6 ppm due to the deuterated terminal OH group (Ti5C-OD) appears, which originates from the dissociation of D2O (Fig. 5a). The broad signal at 4.4 ppm corresponds to adsorbed D2O. With the increase of D2O loading, there is a similar evolution trend for the DO2C and Ti5C-OD groups, especially their signal increment is roughly equal (Fig. 5b), consistent with the theoretical content ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of the two types of deuterated hydroxyls originating from the D2O dissociation. As shown in Fig. 5b, the content of DO2C and Ti5C-OD groups on the TiO2 surface reaches maximum at a 0.5 mmol g−1 H2O loading, in line with the result of 1D 17O MAS NMR analysis (Fig. 3c).
1) of the two types of deuterated hydroxyls originating from the D2O dissociation. As shown in Fig. 5b, the content of DO2C and Ti5C-OD groups on the TiO2 surface reaches maximum at a 0.5 mmol g−1 H2O loading, in line with the result of 1D 17O MAS NMR analysis (Fig. 3c).
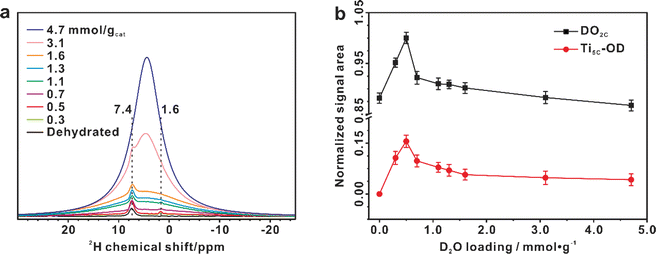 | ||
| Fig. 5 Dissociation of interfacial H2O validated by 1D 2H MAS NMR spectra. (a) 2H MAS NMR spectra of dehydrated 2H-enriched TiO2 samples with 2H2O loading from 0 to 4.7 mmol g−1. (b) Quantitative evolution of the deuterated bridging OH (DO2C, at 7.4 ppm) and terminal OH (Ti5C-OD, at 1.6 ppm) groups with the increase of H2O loading, derived from the simulation of the 1D 2H MAS NMR spectra (Fig. S26†). | ||
Theoretical insight into the adsorption and dissociation of interfacial H2O
Based on our NMR results, a series of structures with different H2O adsorption configurations on (2 × 2) surface slabs are optimized (Fig. S12–S24†) by density functional theory (DFT) calculations. From the adsorption energy points of view, it can be found that when the same amount of H2O is adsorbed onto the TiO2 (101) (2 × 2) surface slabs, the more the chemisorbed H2O (Ti5C–OH2), the greater the adsorption energy of H2O will be released (Table S2†), indicating that H2O is preferentially chemisorbed on the Ti5C site, forming Ti5C–OH2, and then excess H2O is physisorbed on the Ti5C–OH2 site through the hydrogen bond, consistent with our NMR experimental results. Thus, with the increase of H2O loading, the constructed 1H2O/1Ti5C, 2H2O/2Ti5C, 3H2O/3Ti5C, 4H2O/4Ti5C, 5H2O/4Ti5C, 6H2O/4Ti5C, and 8H2O/4Ti5C configurations are energy optimal and appear gradually on the TiO2 (101) surface with the surface O2C sites consisting of the OI–OVI sites (Fig. 6a).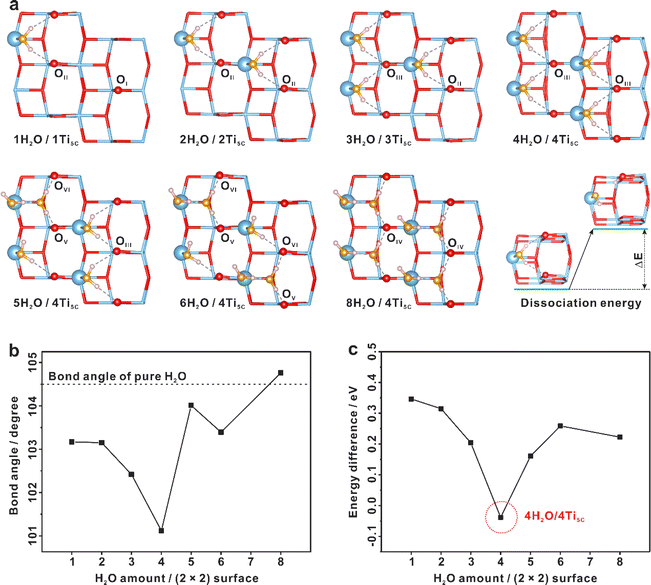 | ||
| Fig. 6 Dissociation of interfacial H2O on the TiO2 surface revealed by DFT calculations. (a) Calculated structures of the TiO2 (101) (2 × 2) surface slabs with different H2O adsorption configurations. Titanium and oxygen atoms of TiO2 are plotted in blue (Ti) and red (O), oxygen atoms originating from the adsorbed H2O are plotted in yellow (H2O), and hydrogen atoms are plotted in the pink (H). (b) The average bond angle of interfacial H2O on the (2 × 2) surface slabs with different H2O adsorption configurations, calculated from Table S3.† (c) Dissociation energy of interfacial H2O in the optimized structures of different H2O adsorption configurations on (2 × 2) TiO2 (101) surface slabs. | ||
Our DFT calculations revealed that water dissociation is a coverage-dependent process. Increasing the H2O loading on the Ti5C site of (2 × 2) surface slabs from 1H2O/1Ti5C to 4H2O/4Ti5C, the H–O–H bond angle of chemisorbed H2O is gradually twisted (Fig. 6b), leading to a decline of the dissociation energy of chemisorbed H2O (Fig. 6c). At the optimal water coverage of 4H2O per TiO2 (101) (2 × 2) surface slab (4H2O/4Ti5C), water splitting is enhanced, with the dissociation energy dropping to as low as −0.03 eV. This suggests that H2O dissociation is both exothermic and spontaneous, aligning with our experimental findings. Obviously, the more chemisorbed H2O on the localized (101) facet of TiO2, the more favorable to the dissociation of H2O to form OH. With the further increase of the H2O loading on the Ti5C site of (2 × 2) surface slabs (5H2O/4Ti5C, 6H2O/4Ti5C, and 8H2O/4Ti5C), the presence of physisorbed H2O causes a recovery in the average bond angle of H2O (Fig. 6b) and an increase in dissociation energy (Fig. 6c), implying that physisorbed H2O impedes the dissociation of chemisorbed H2O.
To further address the importance of the interplay between OH and H2O, we also computed the optimized configurations of the H2O dissociation state on the (101) facet of TiO2 at 40% coverage by using a large (5 × 5 × 1) supercell slab with 300 atoms (100 Ti atoms and 200 O atoms), as presented in Fig. 7 and S27.† It's evident that the OH groups originating from water dissociation have strong interactions with the neighboring chemisorbed H2O in the MI–MVI conformations, corroborating with our 2D 1H{17O} J-HMQC NMR findings (Fig. 2). This robust interaction stabilizes H2O dissociation, serving as the driving force for the event. Therefore, at water loadings ranging from 0.3 to 0.5 mmol g−1 (approximately 28–46% coverage), massive H2O dissociation takes place. At elevated water loadings, physisorbed H2O molecules appear, potentially disrupting the interaction between bridging OH groups (Ti–HO2C–Ti) and chemisorbed H2O. This promotes the reaction of bridging OH groups with nearby terminal OH groups, leading to the reformation of molecular H2O (Fig. S28†). All these findings underscore the crucial role of the interplay between OH–H2O and H2O–H2O interactions in determining the behavior of interfacial H2O on the TiO2 surface.
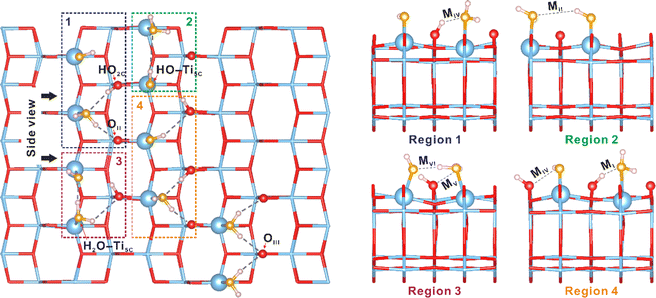 | ||
| Fig. 7 The role of the OH–H2O interactions in the dissociation of interfacial H2O revealed by DFT calculations. The interaction modes of the hydroxyls with chemisorbed H2O on the (101) facet of TiO2 at a 40% coverage of water by using a large (5 × 5 × 1) supercell slab with 300 atoms (100 Ti atoms and 200 O atoms), which highlights the optimized atomic structures (MI–MVI) of hydroxyls and adsorbed H2O molecules on the surface of TiO2 shown in Fig. 2c. Regions 1–4 show side views of those structures (MI–MVI) in the 1–4 regions. Titanium and oxygen atoms of TiO2 are plotted in blue (Ti) and red (O), oxygen atoms originating from the adsorbed H2O are plotted in yellow (H2O), and hydrogen atoms are plotted in pink (H). | ||
Conclusions
In summary, the atomic-level structures and quantitative evolution of surface O/OH sites along with H2O loading are identified on the TiO2 (101) surface by solid-state NMR spectroscopy coupled with theoretical calculations. Two types of H2O (chemisorbed H2O, i.e. Ti5C–OH2 and physisorbed H2O) are present on the H2O/TiO2 interface, and detailed interactions between the interfacial H2O and surface O2C/OH sites are ascertained. We need to mention that the results of the quantitative 17O NMR and 2H NMR tests have confirmed that, under ambient conditions, a maximum of 41.2% of the total adsorbed H2O on TiO2 spontaneously dissociates at a water loading of 0.3 mmol g−1. This means that approximately 0.124 mmol g−1 of water dissociates over TiO2 under these conditions. Our NMR findings and DFT calculations, together with ESR results, confirm that the responsible factor for the observed water rupture at room temperature over the TiO2 (101) surface is not defects such as oxygen vacancies and impurities, but the joint effect of the coordination-unsaturated Ti5C site and adjacent O2C site, as well as the delicate interplay between water–surface and water–water interactions.Data availability
The data that support the findings of this study are available within the article and the ESI.†Author contributions
F. D., N. F., and D. M. conceived the project. N. F. designed the studies. L. Y. synthesized the TiO2 photocatalysts. N. F., L. Y., J. X., M. W. performed NMR experiments. M. W., Y. J., D. M., L. Y., N. F. and F. D. analyzed all the experimental data. M. H. performed theoretical calculations. N. F., D. M. and F. D. wrote the manuscript. All authors interpreted the data and contributed to the preparation of the manuscript.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 22372177, 22127801, 22225205, and 22320102002), Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0540000), Natural Science Foundation of Hubei Province (2021CFA021), Hubei International Scientific and Technological Cooperation program (2022EHB021), and International Science & Technology Cooperation Base for Sustainable Catalysis and Magnetic Resonance (SH2303). This work has been supported by the New Cornerstone Science Foundation. D. M. acknowledges support from the Tencent Foundation through the XPLORER PRIZE.Notes and references
- A. Fujishima and K. Honda, Electrochemical Photolysis of Water at a Semiconductor Electrode, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- S. U. M. Khan, M. Al-Shahry and W. B. Ingler, Efficient Photochemical Water Splitting by a Chemically Modified n-TiO2, Science, 2002, 297, 2243–2245 CrossRef CAS PubMed.
- A. Kudo and Y. Miseki, Heterogeneous photocatalyst materials for water splitting, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- Z. Zou, J. Ye, K. Sayama and H. Arakawa, Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst, Nature, 2001, 414, 625–627 CrossRef CAS PubMed.
- R. D. Cortright, R. R. Davda and J. A. Dumesic, Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water, Nature, 2002, 418, 964–967 CrossRef CAS PubMed.
- Q. Fu, H. Saltsburg and M. Flytzani-Stephanopoulos, Active Nonmetallic Au and Pt Species on Ceria-Based Water-Gas Shift Catalysts, Science, 2003, 301, 935–938 CrossRef CAS PubMed.
- T. Takata, J. Jiang, Y. Sakata, M. Nakabayashi, N. Shibata, V. Nandal, K. Seki, T. Hisatomi and K. Domen, Photocatalytic water splitting with a quantum efficiency of almost unity, Nature, 2020, 581, 411–414 CrossRef CAS PubMed.
- Z. Liu, E. Huang, I. Orozco, W. Liao, R. M. Palomino, N. Rui, T. Duchon, S. Nemšák, D. C. Grinter, M. Mahapatra, P. Liu, J. A. Rodriguez and S. D. Senanayake, Water-promoted interfacial pathways in methane oxidation to methanol on a CeO2-Cu2O catalyst, Science, 2020, 368, 513–517 CrossRef CAS PubMed.
- J. Saavedra, H. A. Doan, C. J. Pursell, L. C. Grabow and B. D. Chandler, The critical role of water at the gold-titania interface in catalytic CO oxidation, Science, 2014, 345, 1599–1602 CrossRef CAS PubMed.
- L. R. Merte, G. Peng, R. Bechstein, F. Rieboldt, C. A. Farberow, L. C. Grabow, W. Kudernatsch, S. Wendt, E. Lægsgaard, M. Mavrikakis and F. Besenbacher, Water-Mediated Proton Hopping on an Iron Oxide Surface, Science, 2012, 336, 889–893 CrossRef CAS PubMed.
- H. Hussain, G. Tocci, T. Woolcot, X. Torrelles, C. L. Pang, D. S. Humphrey, C. M. Yim, D. C. Grinter, G. Cabailh, O. Bikondoa, R. Lindsay, J. Zegenhagen, A. Michaelides and G. Thornton, Structure of a model TiO2 photocatalytic interface, Nat. Mater., 2017, 16, 461–466 CrossRef CAS PubMed.
- O. Björneholm, M. H. Hansen, A. Hodgson, L.-M. Liu, D. T. Limmer, A. Michaelides, P. Pedevilla, J. Rossmeisl, H. Shen, G. Tocci, E. Tyrode, M.-M. Walz, J. Werner and H. Bluhm, Water at Interfaces, Chem. Rev., 2016, 116, 7698–7726 CrossRef PubMed.
- F. Liu, N. Feng, Q. Wang, J. Xu, G. Qi, C. Wang and F. Deng, Transfer Channel of Photoinduced Holes on a TiO2 Surface As Revealed by Solid-State Nuclear Magnetic Resonance and Electron Spin Resonance Spectroscopy, J. Am. Chem. Soc., 2017, 139, 10020–10028 CrossRef CAS PubMed.
- L. Yang, N. Feng, Q. Wang, Y. Chu, J. Xu and F. Deng, Surface Water Loading on Titanium Dioxide Modulates Photocatalytic Water Splitting, Cell Rep. Phys. Sci., 2020, 1, 100013 CrossRef.
- Y.-H. Wang, S. Zheng, W.-M. Yang, R.-Y. Zhou, Q.-F. He, P. Radjenovic, J.-C. Dong, S. Li, J. Zheng, Z.-L. Yang, G. Attard, F. Pan, Z.-Q. Tian and J.-F. Li, In situ Raman spectroscopy reveals the structure and dissociation of interfacial water, Nature, 2021, 600, 81–85 CrossRef CAS PubMed.
- S. Nihonyanagi, S. Yamaguchi and T. Tahara, Ultrafast Dynamics at Water Interfaces Studied by Vibrational Sum Frequency Generation Spectroscopy, Chem. Rev., 2017, 117, 10665–10693 CrossRef CAS PubMed.
- I. V. Stiopkin, C. Weeraman, P. A. Pieniazek, F. Y. Shalhout, J. L. Skinner and A. V. Benderskii, Hydrogen bonding at the water surface revealed by isotopic dilution spectroscopy, Nature, 2011, 474, 192–195 CrossRef CAS PubMed.
- Y. Wang and C. Wöll, IR spectroscopic investigations of chemical and photochemical reactions on metal oxides: bridging the materials gap, Chem. Soc. Rev., 2017, 46, 1875–1932 RSC.
- J. G. Davis, K. P. Gierszal, P. Wang and D. Ben-Amotz, Water structural transformation at molecular hydrophobic interfaces, Nature, 2012, 491, 582–585 CrossRef CAS PubMed.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Solar Water Splitting Cells, Chem. Rev., 2010, 110, 6446–6473 CrossRef CAS PubMed.
- M. Grätzel, Photoelectrochemical cells, Nature, 2001, 414, 338–344 CrossRef PubMed.
- A. L. Linsebigler, G. Lu and J. T. Yates Jr, Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- Y. He, A. Tilocca, O. Dulub, A. Selloni and U. Diebold, Local ordering and electronic signatures of submonolayer water on anatase TiO2 (101), Nat. Mater., 2009, 8, 585–589 CrossRef CAS PubMed.
- J. Matthiesen, J. O. Hansen, S. Wendt, E. Lira, R. Schaub, E. Laegsgaard, F. Besenbacher and B. Hammer, Formation and Diffusion of Water Dimers on Rutile TiO2 (110), Phys. Rev. Lett., 2009, 102, 226101 CrossRef CAS PubMed.
- J. Carrasco, A. Michaelides, M. Forster, S. Haq, R. Raval and A. Hodgson, A one-dimensional ice structure built from pentagons, Nat. Mater., 2009, 8, 427–431 CrossRef CAS PubMed.
- K. Onda, B. Li, J. Zhao, K. D. Jordan, J. Yang and H. Petek, Wet Electrons at the H2O/TiO2 (110) Surface, Science, 2005, 308, 1154–1158 CrossRef CAS PubMed.
- R. Mu, Z.-j. Zhao, Z. Dohnálek and J. Gong, Structural motifs of water on metal oxide surfaces, Chem. Soc. Rev., 2017, 46, 1785–1806 RSC.
- Y. Du, N. A. Deskins, Z. Zhang, Z. Dohnálek, M. Dupuis and I. Lyubinetsky, Two Pathways for Water Interaction with Oxygen Adatoms on TiO2 (110), Phys. Rev. Lett., 2009, 102, 096102 CrossRef CAS PubMed.
- H. H. Kristoffersen, J. Ø. Hansen, U. Martinez, Y. Y. Wei, J. Matthiesen, R. Streber, R. Bechstein, E. Lægsgaard, F. Besenbacher, B. Hammer and S. Wendt, Role of Steps in the Dissociative Adsorption of Water on Rutile TiO2 (110), Phys. Rev. Lett., 2013, 110, 146101 CrossRef CAS PubMed.
- C. Kamal, N. Stenberg, L. E. Walle, D. Ragazzon, A. Borg, P. Uvdal, N. V. Skorodumova, M. Odelius and A. Sandell, Core-Level Binding Energy Reveals Hydrogen Bonding Configurations of Water Adsorbed on TiO2 (110) Surface, Phys. Rev. Lett., 2021, 126, 016102 CrossRef CAS PubMed.
- S. Selcuk and A. Selloni, Facet-dependent trapping and dynamics of excess electrons at anatase TiO2 surfaces and aqueous interfaces, Nat. Mater., 2016, 15, 1107–1112 CrossRef CAS PubMed.
- W. Yuan, B. Zhu, X.-Y. Li, T. W. Hansen, Y. Ou, K. Fang, H. Yang, Z. Zhang, J. B. Wagner, Y. Gao and Y. Wang, Visualizing H2O molecules reacting at TiO2 active sites with transmission electron microscopy, Science, 2020, 367, 428–430 CrossRef CAS PubMed.
- M. A. Henderson, The interaction of water with solid surfaces: fundamental aspects revisited, Surf. Sci. Rep., 2002, 46, 1–308 CrossRef CAS.
- U. Diebold, The surface science of titanium dioxide, Surf. Sci. Rep., 2003, 48, 53–229 CrossRef CAS.
- C. L. Pang, R. Lindsay and G. Thornton, Structure of Clean and Adsorbate-Covered Single-Crystal Rutile TiO2 Surfaces, Chem. Rev., 2013, 113, 3887–3948 CrossRef CAS PubMed.
- Z. Dohnálek, I. Lyubinetsky and R. Rousseau, Thermally-driven processes on rutile TiO2 (110)-(1 × 1): a direct view at the atomic scale, Prog. Surf. Sci., 2010, 85, 161–205 CrossRef.
- A. Vittadini, A. Selloni, F. P. Rotzinger and M. Grätzel, Structure and Energetics of Water Adsorbed at TiO2 Anatase (101) and (001) Surfaces, Phys. Rev. Lett., 1998, 81, 2954–2957 CrossRef CAS.
- A. Tilocca and A. Selloni, Vertical and Lateral Order in Adsorbed Water Layers on Anatase TiO2 (101), Langmuir, 2004, 20, 8379–8384 CrossRef CAS PubMed.
- M. Sumita, C. Hu and Y. Tateyama, Interface Water on TiO2 Anatase (101) and (001) Surfaces: First-Principles Study with TiO2 Slabs Dipped in Bulk Water, J. Phys. Chem. C, 2010, 114, 18529–18537 CrossRef CAS.
- D. Selli, G. Fazio, G. Seifert and C. Di Valentin, Water Multilayers on TiO2 (101) Anatase Surface: Assessment of a DFTB-Based Method, J. Chem. Theory Comput., 2017, 13, 3862–3873 CrossRef CAS PubMed.
- G. S. Herman, Z. Dohnálek, N. Ruzycki and U. Diebold, Experimental Investigation of the Interaction of Water and Methanol with Anatase-TiO2 (101), J. Phys. Chem. B, 2003, 107, 2788–2795 CrossRef CAS.
- L. E. Walle, A. Borg, E. M. J. Johansson, S. Plogmaker, H. Rensmo, P. Uvdal and A. Sandell, Mixed Dissociative and Molecular Water Adsorption on Anatase TiO2 (101), J. Phys. Chem. C, 2011, 115, 9545–9550 CrossRef CAS.
- C. E. Patrick and F. Giustino, Structure of a Water Monolayer on the Anatase TiO2 (101) Surface, Phys. Rev. A, 2014, 2, 014001 CAS.
- M. J. Jackman, A. G. Thomas and C. Muryn, Photoelectron Spectroscopy Study of Stoichiometric and Reduced Anatase TiO2 (101) Surfaces: The Effect of Subsurface Defects on Water Adsorption at Near-Ambient Pressures, J. Phys. Chem. C, 2015, 119, 13682–13690 CrossRef CAS.
- C. Dette, M. A. Pérez-Osorio, S. Mangel, F. Giustino, S. J. Jung and K. Kern, Single-Molecule Vibrational Spectroscopy of H2O on Anatase TiO2 (101), J. Phys. Chem. C, 2017, 121, 1182–1187 CrossRef CAS.
- I. M. Nadeem, J. P. W. Treacy, S. Selcuk, X. Torrelles, H. Hussain, A. Wilson, D. C. Grinter, G. Cabailh, O. Bikondoa, C. Nicklin, A. Selloni, J. Zegenhagen, R. Lindsay and G. Thornton, Water Dissociates at the Aqueous Interface with Reduced Anatase TiO2 (101), J. Phys. Chem. Lett., 2018, 9, 3131–3136 CrossRef CAS PubMed.
- C. Dette, M. A. Pérez-Osorio, S. Mangel, F. Giustino, S. J. Jung and K. Kern, Atomic Structure of Water Monolayer on Anatase TiO2 (101) Surface, J. Phys. Chem. C, 2018, 122, 11954–11960 CrossRef CAS.
- M. F. Calegari Andrade, H.-Y. Ko, L. Zhang, R. Cara and A. Selloni, Free energy of proton transfer at the water–TiO2 interface from ab initio deep potential molecular dynamics, Chem. Sci., 2020, 11, 2335–2341 RSC.
- F. Fasulo, G. M. Piccini, A. B. Muñoz-García, M. Pavone and M. Parrinello, Dynamics of Water Dissociative Adsorption on TiO2 Anatase (101) at Monolayer Coverage and Below, J. Phys. Chem. C, 2022, 126, 15752–15758 CrossRef CAS.
- U. Diebold, Perspective: A controversial benchmark system for water-oxide interfaces: H2O/TiO2 (110), J. Chem. Phys., 2017, 147, 040901 CrossRef PubMed.
- Q. Guo, Z. Ma, C. Zhou, Z. Ren and X. Yang, Single Molecule Photocatalysis on TiO2 Surfaces, Chem. Rev., 2019, 119, 11020–11041 CrossRef CAS PubMed.
- J. Chen, M. A. Hope, Z. Lin, M. Wang, T. Liu, D. M. Halat, Y. Wen, T. Chen, X. Ke, P. C. M. M. Magusin, W. Ding, X. Xia, X.-P. Wu, X.-Q. Gong, C. P. Grey and L. Peng, Interactions of Oxide Surfaces with Water Revealed with Solid-State NMR Spectroscopy, J. Am. Chem. Soc., 2020, 142, 11173–11182 CrossRef CAS PubMed.
- S. Schramm and E. Oldfield, High-resolution oxygen-17 NMR of solids, J. Am. Chem. Soc., 1984, 106, 2502–2506 CrossRef CAS.
- S. Yang, K. D. Park and E. Oldfield, Oxygen-17 labeling of oxides and zeolites, J. Am. Chem. Soc., 1989, 111, 7278–7279 CrossRef CAS.
- T. J. Bastow and S. N. Stuart, 17O NMR in simple oxides, Chem. Phys., 1990, 143, 459–467 CrossRef CAS.
- A. V. Chadwick, I. J. F. Poplett, D. T. S. Maitland and M. E. Smith, Oxygen speciation in nanophase MgO from solid-state 17O NMR, Chem. Mater., 1998, 10, 864–870 CrossRef CAS.
- N. Kim and C. P. Grey, Probing Oxygen Motion in Disordered Anionic Conductors with 17O and 51V MAS NMR Spectroscopy, Science, 2002, 297, 1317–1320 CrossRef CAS PubMed.
- S. E. Ashbrook and M. E. Smith, Solid state 17O NMR—an introduction to the background principles and applications to inorganic materials, Chem. Soc. Rev., 2006, 35, 718–735 RSC.
- N. Merle, J. Trebosc, A. Baudouin, I. D. Rosal, L. Maron, K. Szeto, M. Genelot, A. Mortreux, M. Taoufik, L. Delevoye and R. M. Gauvin, 17O NMR gives unprecedented insights into the structure of supported catalysts and their interaction with the silica carrier, J. Am. Chem. Soc., 2012, 134, 9263–9275 CrossRef CAS PubMed.
- G. P. M. Bignami, D. M. Dawson, V. R. Seymour, P. S. Wheatley, R. E. Morris and S. E. Ashbrook, Synthesis, isotopic enrichment, and solid-state NMR characterization of zeolites derived from the assembly, disassembly, organization, reassembly process, J. Am. Chem. Soc., 2017, 139, 5140–5148 CrossRef CAS PubMed.
- E. N. Bassey, P. J. Reeves, I. D. Seymour and C. P. Grey, 17O NMR Spectroscopy in Lithium-Ion Battery Cathode Materials: Challenges and Interpretation, J. Am. Chem. Soc., 2022, 144, 18714–18729 CrossRef CAS PubMed.
- F. Tielens, C. Gervais, G. Deroy, M. Jaber, L. Stievano, C. C. Diogo and J.-F. Lambert, Characterization of Phosphate Species on Hydrated Anatase TiO2 Surfaces, Langmuir, 2016, 32, 997–1008 CrossRef CAS PubMed.
- J. Chen, X.-P. Wu, M. A. Hope, Z. Lin, L. Zhu, Y. Wen, Y. Zhang, T. Qin, J. Wang, T. Liu, X. Xia, D. Wu, X.-Q. Gong, W. Tang, W. Ding, X. Liu, L. Chen, C. P. Grey and L. Peng, Surface differences of oxide nanocrystals determined by geometry and exogenously coordinated water molecules, Chem. Sci., 2022, 13, 11083 RSC.
- M. Wang, X.-P. Wu, S. Zheng, L. Zhao, L. Li, L. Shen, Y. Gao, N. Xue, X. Guo, W. Huang, Z. Gan, F. Blanc, Z. Yu, X. Ke, W. Ding, X.-Q. Gong, C. P. Grey and L. Peng, Identification of Different Oxygen Species in Oxide Nanostructures with 17O Solid-State NMR Spectroscopy, Sci. Adv., 2015, 1, e1400133 CrossRef PubMed.
- Y. Li, X.-P. Wu, N. Jiang, M. Lin, L. Shen, H. Sun, Y. Wang, M. Wang, X. Ke, Z. Yu, F. Gao, L. Dong, X. Guo, W. Hou, W. Ding, X.-Q. Gong, C. P. Grey and L. Peng, Distinguishing Faceted Oxide Nanocrystals with 17O Solid-State NMR Spectroscopy, Nat. Commun., 2017, 8, 581 CrossRef PubMed.
- B. Song, Y. Li, X.-P. Wu, F. Wang, M. Lin, Y. Sun, A.-P. Jia, X. Ning, L. Jin, X. Ke, Z. Yu, G. Yang, W. Hou, W. Ding, X.-Q. Gong and L. Peng, Unveiling the Surface Structure of ZnO Nanorods and H2 Activation Mechanisms with 17O NMR Spectroscopy, J. Am. Chem. Soc., 2022, 144, 23340–23351 CrossRef CAS PubMed.
- Q. Wang, W. Li, I. Hung, F. Mentink-Vigier, X. Wang, G. Qi, X. Wang, Z. Gan, J. Xu and F. Deng, Mapping the oxygen structure of γ-Al2O3 by high-field solid-state NMR spectroscopy, Nat. Commun., 2020, 11, 3620 CrossRef CAS PubMed.
- L. Liu, X. Gu, Z. Ji, W. Zou, C. Tang, F. Gao and L. Dong, Anion-Assisted Synthesis of TiO2 Nanocrystals with Tunable Crystal Forms and Crystal Facets and Their Photocatalytic Redox Activities in Organic Reactions, J. Phys. Chem. C, 2013, 117, 18578–18587 CrossRef CAS.
- A. Jia, Y. Zhang, T. Song, Z. Zhang, C. Tang, Y. Hu, W. Zheng, M. Luo, J. Lu and W. Huang, Crystal-plane effects of anatase TiO2 on the selective hydrogenation of crotonaldehyde over Ir/TiO2 catalysts, J. Catal., 2021, 395, 10–22 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc02768j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |