Open Access Article
Open Access ArticleElevating the upconversion performance of a multiple resonance thermally activated delayed fluorescence emitter via an embedded azepine approach†
Yi-Kuan
Chen‡
a,
Jian
Lei‡
 ab and
Tien-Lin
Wu
ab and
Tien-Lin
Wu
 *ac
*ac
aDepartment of Chemistry, National Tsing Hua University, No. 101, Sec. 2, Kuang-Fu Rd., Hsinchu 300044, Taiwan. E-mail: tlwu@mx.nthu.edu.tw
bInstitute of Atomic and Molecular Sciences, Academia Sinica, No. 1, Sec. 4, Roosevelt Rd., Taipei, 106319, Taiwan
cCollege of Semiconductor Research, No. 101, Sec. 2, Kuang-Fu Rd., Hsinchu 300044, Taiwan
First published on 24th May 2024
Abstract
Multiple resonance thermally activated delayed fluorescence (MR-TADF) emitters hold promise for efficient organic light-emitting diodes (OLEDs) and wide gamut displays. An azepine donor is introduced into the boron–nitrogen system for the first time. The highly twisted conformation of a seven-ring embedded new molecule, TAzBN, increases the intermolecular distances, suppressing self-aggregation emission quenching. Meanwhile, the azepine donor is crucial to achieve a narrow singlet-triplet gap (0.03 eV) as well as boost the reverse intersystem crossing (RISC) rate to 8.50 × 105 s−1. It is noteworthy that TAzBN demonstrates an impressive photoluminescence quantum yield of 94%. In addition, its nonsensitized OLED displayed a remarkable external quantum efficiency (EQEmax) with values peaking at 27.3%, and an EQE of 21.4% at 500 cd m−2. This finding shows that when TAzBN is used at a high concentration of 10 wt%, its device maintains efficiency even at higher brightness levels, highlighting TAzBN's resistance to aggregation quenching. Furthermore, TAzBN enantiomers showed circularly polarized photoluminescence characteristics with dissymmetry factors |gPL| of up to 1.07 × 10−3 in doped films. The curved heptagonal geometry opens an avenue to design the MR-TADF emitters with fast spin-flip and chiroptical properties.
Introduction
Thermally activated delayed fluorescence (TADF) technology has become a hot topic in the field of organic light-emitting diodes (OLEDs). Especially for the newly developed multiple resonance (MR) emitters, their features of exciton up-conversion and narrow emission can meet the requirements of high-performance and high-resolution displays.1–4 In 2016, Hatakeyama et al.5 presented the DABNA series, the first MR-TADF materials. The design strategy is to utilize the opposite resonance effects of boron and nitrogen atoms to separate the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) distributions. As a result, a rigid heteroatom polyaromatic framework simultaneously exhibits TADF properties and a narrow full width at half maximum (FWHM) of 30 nm. Recently, MR-TADF materials with five,6 six,7,8 or seven-membered9 rings with boron, nitrogen or oxygen atoms were developed and reported. Those state-of-the-art molecules contain high photoluminescence quantum yields (PLQYs) and a narrow emission bandwidth of around 20 nm.10–12 However, most MR-TADF materials display relatively wide singlet–triplet gaps (e.g., ΔEST ≥ 0.12 eV) due to the inherent short-range charge transfer (SRCT) of the excited state.13,14 Thus, reverse intersystem crossing (RISC) processes from triplet to singlet excited states in MR-TADF systems are slower than in conventional TADF emitters. And their rate constants are generally lower than 104 s−1, resulting in serious device efficiency roll-offs at high luminance.15–17 So far, the design strategy for preparing MR-TADF materials with a fast triplet exciton conversion is still an enormous challenge.Based on prior MR molecular designs, some successful strategies have emerged to enhance RISC rates (kRISC) and mitigate the efficiency roll-off in OLEDs. For example, extended MR frameworks18–23 can effectively lower the value of ΔEST. With incorporating heavy atoms,24–27 MR emitters enhance the strength of spin–orbit coupling (SOC), accelerating kRISC. In addition, the exciplex formation from the host and guest also boosts the TADF efficiency in MR molecules.28 These above methods could expedite the spin-flip process and further reduce the efficiency roll-off in their OLEDs. However, the planar structures of most MR emitters cause strong π–π stacking interactions, resulting in serious aggregation-caused quenching (ACQ) effects and spectral broadening in the solid state. The well-known strategy is to increase the steric hindrance (i.e. tert-butyl or carbazole groups) on the peripheral side of a molecule.22,29–32 The bulky substituents can suppress π–π stacking interactions between planar MR cores, inhibiting the ACQ quenching. However, there is no practical way to simultaneously resolve the two issues of low up-conversion rate and aggregation quenching. Herein, we proposed a molecular design strategy with three azepine groups (Scheme 1) that concurrently decreases the singlet–triplet gap (ΔEST), increases SOC33 and kRISC, and suppresses the ACQ effect. A newly designed compound, TAzBN, exhibited superior photophysical properties with a PLQY (94%) and narrowband emission (FWHM of 44 nm). TAzBN was determined to have a low singlet–triplet difference of 0.03 eV and a high RISC rate of 8.50 × 105 s−1, which revealed the efficient up-conversion pathway among reported MR-TADF emitters. Its optimized device with 10 wt% TAzBN showed the highest external quantum efficiency (EQEmax) of 27.3% and a mild efficiency roll-off of 22% at 500 nits. Moreover, TAzBN's enantiomers display circularly polarized luminescence (CPL) with PL dissymmetry factors (gPLs) of +0.79 × 10−3/−1.07 × 10−3 for PP-TAzBN and MM-TAzBN, respectively. This work provides a novel strategy using a twisted seven-ring to design a highly efficient and narrowband emitter, which has excellent potential in CPL applications.
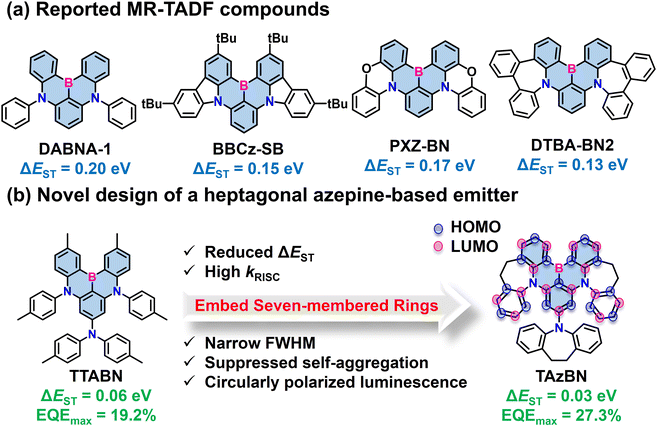 | ||
| Scheme 1 (a) Reported MR-TADF compounds. (b) Novel design of a heptagonal azepine-based emitter and its features. | ||
Results and discussion
At first, we selected a reported material with three di-p-tolyl amine groups, 2,12-dimethyl-N,N,5,9-tetra-p-tolyl-5,9-dihydro-5,9-diaza-13b-boranaphtho[3,2,1-de]anthracen-7-amine (TTABN), as a model for synthesis and comparison.34 Synthetic routes to the two compounds, TTABN and TAzBN, are provided in Fig. 1a, and both desired products were synthesized within two steps. In the first step, the synthesis of TAz is achieved in 83% yield via the Buchwald–Hartwig reaction. Then, the following direct electrophilic borylation using boron tribromide gave TAzBN in 23% yield at 195 °C. Because the TAz's crowded three azepine groups generate a highly congested structure, the reaction barrier of borylation might be much higher than TTA. Therefore, the borylation at 170 °C afforded TAzBN in an even lower yield of 11%. Characterization by 1H/13C NMR, elemental analysis, high-resolution mass spectroscopy, and single-crystal X-ray diffraction is provided in the ESI.† Furthermore, the thermal properties are determined via thermal gravimetric analysis (TGA) and differential scanning calorimetry (DSC) measurements, as shown in Fig. S1.† They both have excellent thermal stability, and the decomposition temperatures are higher than 380 °C, respectively. The glass transition temperatures are 228 °C and 252 °C respectively for TTABN and TAzBN, implying favorable solid-state behavior during device operation. Cyclic voltammetry (CV) measurement was then carried out to estimate their frontier orbitals, highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbitals (LUMO) levels. In Fig. S2,† HOMO and LUMO energy levels of TTABN and TAzBN are determined as −4.97/−2.26 and −5.10/−2.39 eV, respectively. As a result, they both revealed the regular electrochemical stability in the solution.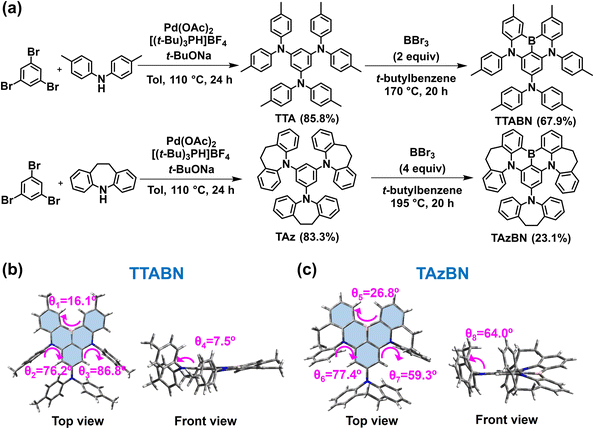 | ||
| Fig. 1 (a) Synthetic routes to TTABN and TAzBN. (b) The single crystal of TTABN. (c) The single crystal of TAzBN. | ||
Fig. 1b and c show the X-ray single-crystal structure and analysis of TTABN and TAzBN. TAzBN exhibited a relatively twisted DABNA core (marked in blue), compared to that of TTABN (Tables S1 and S2†). The torsion angles of the triangle DABNA core (blue region) were measured to be θ1 of 16.1° for TTABN and θ5 of 26.8° for TAzBN. Furthermore, we introduced the additional auxiliary azepine donor to induce steric hindrance with a relatively larger dihedral angle (θ8 = 64.0°) (Fig. S3 and S4†), which might suppress the intramolecular rotational vibrations. In contrast, the auxiliary di-p-tolyl amine of TTABN could freely rotate, leading to a more planar structure. In Fig. S5,† there is no intense intermolecular π–π stacking interaction between TAzBN's torsional MR cores. The results revealed that intermolecular exciton distances might be effectively increased by the introduction of azepine groups, which could suppress the ACQ effect in the solid state and reduce the efficiency roll-off of OLEDs.
To investigate the effect of the introduced azepine, we carried out the density functional theory (DFT), time-dependent DFT (TD-DFT), and Tamm–Dancoff approximation DFT (TDA-DFT) calculations using the B3LYP/6-31G(d) (Fig. S6, S7 and Table S3†).11,35 The calculated HOMO/LUMO energy levels of TTABN and TAzBN are −4.71/−1.25 eV and −4.82/−1.38 eV, respectively. As shown in Fig. S8,† the HOMO is mainly located on the nitrogen atoms, and the LUMO is located primarily on boron atoms. According to the charge distributions in TAzBN, there are only weak conjugations between azepine units and the DABNA core. Thus, the HOMO and LUMO levels of TAzBN are deeper than those of TTABN. The TAzBN's frontier molecular orbitals (FMOs) indicate SRCT characteristics and the effectiveness of the MR feature.36,37 The oscillator strengths (f) of the S1–S0 transition of TTABN and TAzBN are 0.2710 and 0.2395, respectively. In addition, the two emitters show small root-mean-square deviation (RMSD) values between the optimized S1 and S0 geometries in the solid phase (Fig. S9†). The low RMSD values and reorganization energies (λ) of TTABN and TAzBN are 0.03/0.19 and 0.04/0.29 Å eV−1 in the solid phase, respectively. As a result, the simulations suggest that the molecular relaxation of TAzBN is well suppressed, and it emits narrowband blue light.
The natural transition orbitals (NTOs) of the singlet (S1) and triplet excited states (T1/T2/T3) and their spin–orbit couplings (SOC) are performed as shown in Fig. 2. For the first singlet and triplet excited states, NTO distributions closely resemble each other, resulting in a low SOC value between the S1 and T1 excited states. The values of SOCs (S1T1) are 0.03 and 0.08 cm−1 for TTABN and TAzBN, respectively. In this case, these two states S1 and T1 have almost identical transition orbital distributions, which hinder the RISC path from T1 to S1. In contrast, the two higher triplet excited states (T2 and T3) of the TAzBN exhibit distinctly different NTO distribution patterns from S1. Interestingly, the “hole” and “electron” of TAzBN are not only distributed over the π-conjugated DABNA core but also localized on the azepine region, indicating the hybrid behavior of SRCT and long-range charge transfer (LRCT) nature.36,37 Thus, the values of SOC(S1T2) and SOC(S1T3) of TAzBN are 1.33 and 0.10 cm−1, which are higher than those of TTABN (0.14 and 0.01 cm−1). Specifically, the SOC(S1T2) values of the two compounds are higher than the SOC(S1T1) and SOC(S1T3) values, indicating a possible up-conversion from T2 to S1 state in Table S5†. A similar RISC pathway has been revealed among several MR-TADF materials,10,35,38 and it is expected that the higher triplet states could accelerate the overall RISC rate. Moreover, we summarize the calculated excited levels in Table S4†. The calculated ΔE(S1T2) values of TTABN and TAzBN are 0.09 and 0.02 eV in toluene, and 0.10 and 0.01 eV in the solid phase, respectively, implying the possible path of an efficient RISC process.
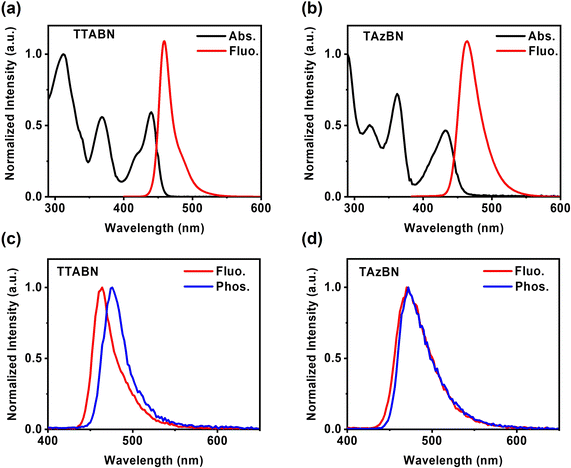
The ultraviolet-visible (UV-vis) absorption and photoluminescence (PL) spectra of TTABN and TAzBN were measured in toluene (1 × 10−5 M). As shown in Fig. 3a and b, strong absorption bands originating from HOMO–LUMO transitions were observed at 439 and 433 nm for TTABN and TAzBN, respectively. The intramolecular charge transfer (ICT) characteristics were confirmed by emission spectra in various solvents in Fig. S10.†TAzBN's red-shifted and broadened bands reveal a slightly stronger ICT effect than TTABN, possibly due to the auxiliary azepine. Both PL spectra of TTABN and TAzBN display narrow bandwidth emissions at 459 and 464 nm, respectively, which agrees with the TD-DFT calculations (Fig. S11†). Notably, the Stokes shifts of TTABN and TAzBN are 19 and 32 nm, and the full-width at half-maximum (FWHM) values of TTABN and TAzBN PL bands are 21 and 35 nm, respectively. In Fig. 3c and d, we further measured the fluorescence and phosphorescence spectra of 2 wt% emitters doped in a 9-(3-(9H-carbazol-9-yl)phenyl)-9H-carbazole-3-carbonitrile (mCPCN) host. The experimental ΔEST values of TTABN and TAzBN are determined as 60 and 32 meV, respectively. Especially for the low gap of TAzBN, it is the lowest value among reported MR-TADF molecules. The results are consistent with the trend of theoretical calculations and imply the participation of higher triplet states.3,38,39 The summary of photophysical data is listed in Table 1.
| Emitter | λ Abs,max [nm] | λ PL,max [nm] | FWHMb [nm] | HOMOc [eV] | LUMOd [eV] | T d/Tgd (°C) | S1/T1e [eV] | ΔESTe [meV] | τ p f [ns] | τ d [μs] |
|---|---|---|---|---|---|---|---|---|---|---|
a Absorption and photoluminescence spectra measured in toluene (1.0 × 10−5 M) at 300 K.
b FWHM of PL bands in toluene.
c The HOMO level was determined from the CV; LUMO = HOMO + Eg.
d
T
d as decomposition temperature (5% weight loss) and Tg as glass transition temperature.
e S1 and T1 were determined by analyzing the onset of the fluorescence band (at 300 K) and phosphorescence band (at 77 K) in thin films (2 wt% compound doped in mCPCN); ΔEST = S1 − T1.
f Fluorescence lifetimes of the prompt part (τp) and delayed part (τd) in doped mCPCN films (2 wt% doping ratio); The average lifetime was calculated according to the equation: 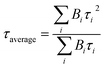 , where Bi is the pre-exponential for lifetime τi.40–42 , where Bi is the pre-exponential for lifetime τi.40–42
|
||||||||||
| TTABN | 312![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 440 |
459 | 21 | −4.97 | −2.26 | 381/228 | 2.80/2.74 | 60 | 8.3 | 65.5 |
| TAzBN | 290![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 432 432 |
464 | 35 | −5.10 | −2.39 | 400/252 | 2.79/2.76 | 32 | 9.2 | 15.8 |
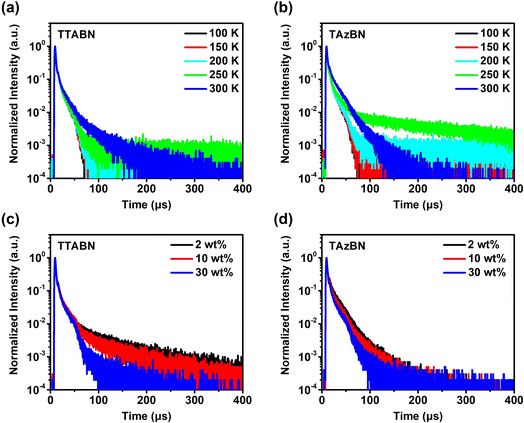
The transient PL measurements for the two materials in doped films were performed to investigate the features of TADF (Fig. 4, S12 and S13†). In Fig. 4a and b, the delayed fluorescence component increases along with the rising temperature, which strongly supports the TADF mechanism. The prompt and delayed lifetimes (τp and τd) were determined by fitting the decay curves of 300 K. The delayed fluorescence decay curves of TTABN and TAzBN-based films were analyzed and fitted. The methods and results of the fitting process are illustrated in Fig. S12 and S13.† Further details are provided in the ESI.† As shown in the results, the τp values of TTABN and TAzBN were 8.3 and 9.2 ns, indicating that the twisted structure of TAzBN still retains the LE feature of fast decay. Moreover, TAzBN has a much lower τd of 15.8 μs, compared to that of TTABN (65.5 μs). The trend of delayed components of the two emitters is highly correlated with their ΔEST values (Fig. S14†). Next, we investigated the concentration effect of the emitters in the solid phase, and vacuum-evaporated films with 2, 10, and 30 wt% doping concentrations were prepared for the transient PL and PLQY measurements. The decay curves of TAzBN at various concentrations exhibit relatively minor differences compared to the results of TTABN, as shown in Fig. 4c and d. This indicates a high consistency in the fluorescence behavior of TAzBN with increasing concentration. For the low-doped concentration films (2 wt% in mCPCN), TTABN and TAzBN showed high PLQYs (higher than 90%), compared to those of only 39% (TTABN) and 25% (TAzBN) in degassed toluene. Such high values in doped films indicate efficient energy transfer from the host and superior emission behaviors of the organoboron emitters. For 10 wt% doping films, the PLQY value of TAzBN significantly increased to 94%. However, that of TTABN dropped to 65%, revealing the serious emission quenching caused by aggregation. Moreover, the kRISC of TAzBN (8.50 × 105 s−1) is much higher than that of TTABN (4.79 × 104 s−1). Compared with planar TTABN, such an efficient up-conversion behavior of TAzBN could be attributed to the reduced ΔEST and strong spin–orbital coupling via the introduction of the twisted heptagonal azepine. Furthermore, the PLQY of the 30 wt% TAzBN doped film remained 90%, and its kRISC reached 4.83 × 105 s−1. Importantly, it is one of the highest kRISC values among blue MR-TADF emitters, even without heavy atoms or assisted sensitizers. The corresponding PL properties are summarized in Table S6†. Due to the high kRISC and slightly reduced PLQY under high doping concentrations, we believe that the extensively twisted molecular configurations and the fast spin-flip process of TAzBN play a role in inhibiting the ACQ effect and non-radiative transition. The advantages of TAzBN in singlet and triplet exciton conversion could benefit the luminous efficiency of OLEDs and alleviate the decay of efficiency at high brightness.
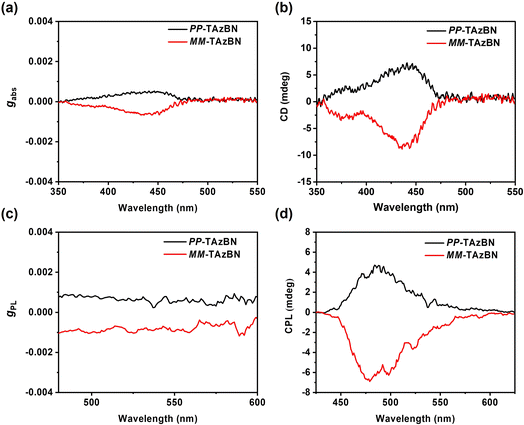
Furthermore, we notice the helical structure of TAzBN from its single crystal, which results in enantiomers. To investigate the chiroptical properties, we measured circular dichroism (CD) and circularly polarized photoluminescence (CPL) spectra for thin films (Fig. 5). As shown in Fig. 5b, mirror-image CD signals are observed for PP-TAzBN and MM-TAzBN with an obvious cotton effect in the n–π*/π–π* region from 350 to 450 nm. The CPL spectra of PP-TAzBN and MM-TAzBN enantiomers are symmetrical in their co-doped films (Fig. 5d), reflecting the chirality of the singlet excited state. Notably, the photoluminescence dissymmetry factors (gPL) of PP-TAZBN and MM-TAzBN were determined as +0.79 × 10−3 and −1.07 × 10−3. To discuss the relationship between the chiral structure and CPL properties of the emitter, relative calculations were performed to analyze the dissymmetry factor (Fig. S15, S16 and Table S7†). The PL dissymmetry factor is described as eqn (1):43
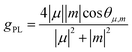 | (1) |
μ, m and θμ,m are the transition electric dipole moment, magnetic dipole moment, and their angle respectively. Obviously, |μ|, |m|, and cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θμ,m are the three key parameters for evaluating the gPL. The simulated gPL values of PP/MM-TAzBN are +2.26 × 10−3/−2.26 × 10−3 in the solid phase, respectively, which are consistent with the experimental results. Overall, the strategy of the embedded heptagonal ring achieves a novel MR-TADF emitter, TAzBN, with efficient TADF, reduced ACQ, and chiroptical features.
θμ,m are the three key parameters for evaluating the gPL. The simulated gPL values of PP/MM-TAzBN are +2.26 × 10−3/−2.26 × 10−3 in the solid phase, respectively, which are consistent with the experimental results. Overall, the strategy of the embedded heptagonal ring achieves a novel MR-TADF emitter, TAzBN, with efficient TADF, reduced ACQ, and chiroptical features.
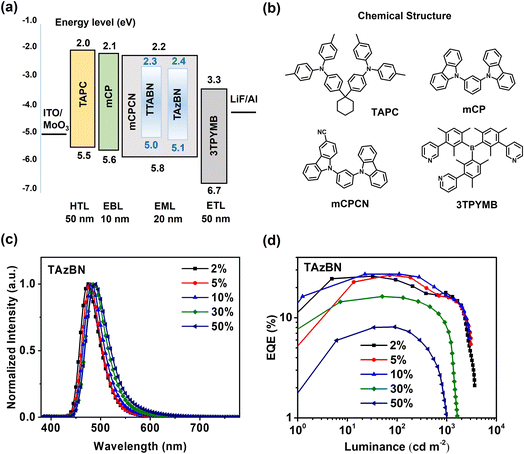
Next, we proceeded to create a series of OLEDs, utilizing the two compounds as emitters, with a device configuration of ITO (indium tin oxide)/MoO3 (1 nm)/TAPC (N,N-bis(p-tolyl)aniline) (50 nm)/mCP (1,3-di(9H-carbazol-9-yl)benzene) (10 nm)/mCPCN: 2–50 wt% emitters (20 nm)/tris-[3-(3-pyridyl)mesityl]borane (3TPYMB) (50 nm)/LiF (0.5 nm)/Al (100 nm) (see Fig. 6a). mCPCN was chosen as the host material due to its high T1 state (2.96 eV) and balanced bipolar carrier transport capability. Indium tin oxide (ITO) and Al are the anode and cathode, respectively. Di-[4-(N,N-ditolyl-amino)-phenyl]-cyclohexane (TAPC) and N,N-dicarbazolyl-3,5-benzene (mCP) are hole-transport layers (HTL). The mCPCN host with each dopant emitter is composed of the emitting layer (EML). 3TPYMB is the electron-transport layer (ETL). MoO3 and LiF are the hole-injection and electron-injection layers, respectively. The chemical structures of the utilized materials for OLED fabrication are depicted in Fig. 6b. The corresponding EL characteristics of OLED devices are summarized in Table S8† and Fig. S17.† In Fig. 6c and d, the TAzBN-based device (2 wt%) displayed the performance with an ELmax of 475 nm, EQEmax of 26.0%, and EQE500 of 17.2%. Compared with TAzBN-based devices, a TTABN-based device with the same doping ratio exhibited an ELmax of 464 nm and EQEmax of 19.2%. However, its EQE500 quickly dropped to 8.4% due to the relatively slow RISC process of TTABN. (Fig. S18†). Furthermore, nearly ideal Lambertian distributions were determined for both 2 wt%-based devices, as shown in Fig. S19.† The FWHM values of TTABN and TAzBN-based (2 wt%) devices are 31 and 45 nm, respectively. TAzBN exhibited a broader bandwidth than that of TTABN due to the twisted structure. Moving forward, we will emphasize the importance of mitigating this adverse effect, such as by introducing suitable functional groups,44 deuteration,45 hyper-fluorescence systems,37etc.
To study the behavior of emission quenching under electrical excitation, OLEDs with various doping ratios of EML were fabricated and measured, and the results are summarized in Table S8†. We observed that the major bands of EL spectra were slightly broadened in TAzBN-based devices. Notably, the device with a high doping (10 wt%) of TAzBN demonstrated EQEmax and EQE500 values of 27.3% and 21.4%, respectively. Even at 1000 cd m−2, the EQE remains at 16.6%, which is quite outstanding compared to most reported nonsensitized MR-TADF OLEDs, as summarized in Table S9†. TAzBN-based devices exhibited the resistance of aggregation-quenching and alleviated efficiency roll-off. In addition, the driving voltage decreased from 3.3 V to 2.6 V along with increasing concentrations. The fast RISC and the steric hindrance from the twisted azepine motif could reduce the triplet–triplet annihilation (TTA) or triplet-polaron annihilation (TPA). It is worth noting that the device with 30 wt% TAzBN still maintained 16.3% EQE. Such high concentration of emitting layer among reported MR-TADF OLEDs is rare, and the performance of OLEDs with a high doping of TAzBN is excellent among most MR-TADF OLEDs (Fig. S20†). The result implies the successful approach to improving SOC, accelerating RISC, and further reducing ACQ via the molecular design of introduced heptagonal geometry.
Conclusions
In summary, we have developed a molecular design to enhance kRISC values and suppress the ACQ effect in MR-TADF emitters. A newly developed emitter containing heptagonal rings, TAzBN, shows a narrow FWHM of 45 nm, a low ΔEST of 0.03 eV, a high PLQY of over 90%, and a high kRISC of 8.50 × 105 s−1 in doped films. Introducing the emitter as a dopant in OLEDs led to remarkable enhancements in EL performance, achieving high efficiency and reduced efficiency roll-off (EQEmax = 27.3%; EQE500 = 21.4%) at a high doping ratio of 10 wt%. Moreover, the azepine-induced helical structure can show distinct CPL signals with gPLs of +0.79 × 10−3 for PP-TAzBN and −1.07 × 10−3 for MM-TAzBN. The strategy of the twisted heptagonal geometry opens the avenue to solve the issues of the slow RISC process among recent MR-TADF studies. The results showcase the promising application of this strategy in constructing efficient and chiral MR-TADF emitters.Data availability
The theoretical calculation, experimental procedure, and device fabrication details have been provided within the manuscript and ESI.† The data that support the findings of this study are available from the corresponding authors upon reasonable request.Author contributions
Y.-K. Chen: synthesis, measurement of photophysical properties, and device fabrication; J. Lei: theoretical calculation and writing – original draft; T.-L. Wu: supervision, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Science and Technology Council of Taiwan (NSTC 110-2113-M-007-028-MY3). The authors acknowledge Prof. Liang-Yan Hsu for providing the supercomputing system at the Institute of Atomic and Molecular Sciences, Academia Sinica. We appreciate the support of chiroptical measurement by Prof. Yu-Chiang Chao at National Taiwan Normal University, Department of Physics. T.-L.W. acknowledges the support of the Yushan Young Scholar Program by the Ministry of Education, Taiwan.Notes and references
- A. Pershin, D. Hall, V. Lemaur, J.-C. Sancho-Garcia, L. Muccioli, E. Zysman-Colman, D. Beljonne and Y. Olivier, Nat. Commun., 2019, 10, 597 CrossRef CAS PubMed.
- Y. C. Cheng, X. C. Fan, F. Huang, X. Xiong, J. Yu, K. Wang, C. S. Lee and X. H. Zhang, Angew. Chem., Int. Ed., 2022, 134, e202212575 CrossRef.
- H. J. Cheon, S. J. Woo, S. H. Baek, J. H. Lee and Y. H. Kim, Adv. Mater., 2022, 34, 2207416 CrossRef CAS PubMed.
- M. Yang, I. S. Park and T. Yasuda, J. Am. Chem. Soc., 2020, 142, 19468–19472 CrossRef CAS PubMed.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Adv. Mater., 2016, 28, 2777–2781 CrossRef CAS PubMed.
- Y. Zhang, D. Zhang, J. Wei, Z. Liu, Y. Lu and L. Duan, Angew. Chem., Int. Ed., 2019, 58, 16912–16917 CrossRef CAS PubMed.
- G. Liu, H. Sasabe, K. Kumada, A. Matsunaga, H. Katagiri and J. Kido, J. Mater. Chem. C, 2021, 9, 8308–8313 RSC.
- X. Wu, J. W. Huang, B. K. Su, S. Wang, L. Yuan, W. Q. Zheng, H. Zhang, Y. X. Zheng, W. Zhu and P. T. Chou, Adv. Mater., 2022, 34, 2105080 CrossRef CAS PubMed.
- B. Lei, Z. Huang, S. Li, J. Liu, Z. Bin and J. You, Angew. Chem., Int. Ed., 2023, 135, e202218405 CrossRef.
- H. Tanaka, S. Oda, G. Ricci, H. Gotoh, K. Tabata, R. Kawasumi, D. Beljonne, Y. Olivier and T. Hatakeyama, Angew. Chem., Int. Ed., 2021, 60, 17910–17914 CrossRef CAS PubMed.
- K. R. Naveen, J. H. Oh, H. S. Lee and J. H. Kwon, Angew. Chem., Int. Ed., 2023, 62, e202306768 CrossRef CAS PubMed.
- X. Cai, J. Xue, C. Li, B. Liang, A. Ying, Y. Tan, S. Gong and Y. Wang, Angew. Chem., Int. Ed., 2022, 61, e202200337 CrossRef CAS PubMed.
- H. J. Kim and T. Yasuda, Adv. Opt. Mater., 2022, 10, 2201714 CrossRef CAS.
- Y. J. Yu, Z. Q. Feng, X. Y. Meng, L. Chen, F. M. Liu, S. Y. Yang, D. Y. Zhou, L. S. Liao and Z. Q. Jiang, Angew. Chem., Int. Ed., 2023, 135, e202310047 CrossRef.
- Y. Zhang, J. Wei, D. Zhang, C. Yin, G. Li, Z. Liu, X. Jia, J. Qiao and L. Duan, Angew. Chem., Int. Ed., 2022, 61, e202113206 CrossRef CAS PubMed.
- P. Jiang, J. Miao, X. Cao, H. Xia, K. Pan, T. Hua, X. Lv, Z. Huang, Y. Zou and C. Yang, Adv. Mater., 2022, 34, 2106954 CrossRef CAS PubMed.
- S. Madayanad Suresh, L. Zhang, D. Hall, C. Si, G. Ricci, T. Matulaitis, A. M. Slawin, S. Warriner, Y. Olivier and I. D. Samuel, Angew. Chem., Int. Ed., 2023, 135, e202215522 CrossRef.
- P. Jiang, L. Zhan, X. Cao, X. Lv, S. Gong, Z. Chen, C. Zhou, Z. Huang, F. Ni and Y. Zou, Adv. Opt. Mater., 2021, 9, 2100825 CrossRef CAS.
- F. Huang, X. C. Fan, Y. C. Cheng, H. Wu, X. Xiong, J. Yu, K. Wang and X. H. Zhang, Angew. Chem., Int. Ed., 2023, 135, e202306413 CrossRef.
- S. Oda, B. Kawakami, Y. Yamasaki, R. Matsumoto, M. Yoshioka, D. Fukushima, S. Nakatsuka and T. Hatakeyama, J. Am. Chem. Soc., 2021, 144, 106–112 CrossRef PubMed.
- H. Jiang, J. Jin and W. Y. Wong, Adv. Funct. Mater., 2023, 33, 2306880 CrossRef CAS.
- K. Zhang, X. Wang, Y. Chang, Y. Wu, S. Wang and L. Wang, Angew. Chem., Int. Ed., 2023, 135, e202313084 CrossRef.
- K. R. Naveen, H. Lee, R. Braveenth, K. J. Yang, S. J. Hwang and J. H. Kwon, Chem. Eng. J., 2022, 432, 134381 CrossRef.
- Y. Lee and J.-I. Hong, J. Mater. Chem. C, 2022, 10, 11855–11861 RSC.
- T. Hua, J. Miao, H. Xia, Z. Huang, X. Cao, N. Li and C. Yang, Adv. Funct. Mater., 2022, 32, 2201032 CrossRef CAS.
- X. Cao, K. Pan, J. Miao, X. Lv, Z. Huang, F. Ni, X. Yin, Y. Wei and C. Yang, J. Am. Chem. Soc., 2022, 144, 22976–22984 CrossRef CAS PubMed.
- M. Nagata, H. Min, E. Watanabe, H. Fukumoto, Y. Mizuhata, N. Tokitoh, T. Agou and T. Yasuda, Angew. Chem., Int. Ed., 2021, 133, 20442–20447 CrossRef.
- X. Wu, B.-K. Su, D.-G. Chen, D. Liu, C.-C. Wu, Z.-X. Huang, T.-C. Lin, C.-H. Wu, M. Zhu and E. Y. Li, Nat. Photonics, 2021, 15, 780–786 CrossRef CAS.
- K. R. Naveen, H. Lee, L. H. Seung, Y. H. Jung, C. K. Prabhu, S. Muruganantham and J. H. Kwon, Chem. Eng. J., 2023, 451, 138498 CrossRef CAS.
- X. Liang, Z. P. Yan, H. B. Han, Z. G. Wu, Y. X. Zheng, H. Meng, J. L. Zuo and W. Huang, Angew. Chem., Int. Ed., 2018, 130, 11486–11490 CrossRef.
- T.-L. Wu, J. Lei, C.-M. Hsieh, Y.-K. Chen, P.-Y. Huang, P.-T. Lai, T.-Y. Chou, W.-C. Lin, W. Chen and C.-H. Yu, Chem. Sci., 2022, 13, 12996–13005 RSC.
- H. Mubarok, A. Amin, T. Lee, J. Jung, J. H. Lee and M. H. Lee, Angew. Chem., Int. Ed., 2023, 135, e202306879 CrossRef.
- J. E. Adams, W. Mantulin and J. R. Huber, J. Am. Chem. Soc., 1973, 95, 5477–5481 CrossRef CAS.
- S. Oda, W. Kumano, T. Hama, R. Kawasumi, K. Yoshiura and T. Hatakeyama, Angew. Chem., Int. Ed., 2021, 133, 2918–2922 CrossRef.
- X. Lv, J. Miao, M. Liu, Q. Peng, C. Zhong, Y. Hu, X. Cao, H. Wu, Y. Yang and C. Zhou, Angew. Chem., Int. Ed., 2022, 134, e202201588 CrossRef.
- F. Huang, X. C. Fan, Y. C. Cheng, Y. Xie, S. Luo, T. Zhang, H. Wu, X. Xiong, J. Yu and D. D. Zhang, Adv. Opt. Mater., 2023, 11, 2202950 CrossRef CAS.
- G. Meng, H. Dai, Q. Wang, J. Zhou, T. Fan, X. Zeng, X. Wang, Y. Zhang, D. Yang and D. Ma, Nat. Commun., 2023, 14, 2394 CrossRef CAS PubMed.
- K. Stavrou, A. Danos, T. Hama, T. Hatakeyama and A. Monkman, ACS Appl. Mater. Interfaces, 2021, 13, 8643–8655 CrossRef CAS PubMed.
- K. Shizu and H. Kaji, Commun. Chem., 2022, 5, 53 CrossRef CAS PubMed.
- Q. Zhang, B. Li, S. Huang, H. Nomura, H. Tanaka and C. Adachi, Nat. Photonics, 2014, 8, 326–332 CrossRef CAS.
- Q. Zhang, H. Kuwabara, W. J. Potscavage Jr, S. Huang, Y. Hatae, T. Shibata and C. Adachi, J. Am. Chem. Soc., 2014, 136, 18070–18081 CrossRef CAS PubMed.
- T.-L. Wu, M.-J. Huang, C.-C. Lin, P.-Y. Huang, T.-Y. Chou, R.-W. Chen-Cheng, H.-W. Lin, R.-S. Liu and C.-H. Cheng, Nat. Photonics, 2018, 12, 235–240 CrossRef CAS.
- J. Lei, T. A. Lou, C. R. Chen, C. H. Chuang, H. Y. Liu, L. Y. Hsu, Y. C. Chao and T. L. Wu, Chem.–Asian J., 2024, 19, e202300940 CrossRef CAS PubMed.
- J. Liu, Y. Zhu, T. Tsuboi, C. Deng, W. Lou, D. Wang, T. Liu and Q. Zhang, Nat. Commun., 2022, 13, 4876 CrossRef CAS PubMed.
- T. Huang, Q. Wang, H. Zhang, Y. Zhang, G. Zhan, D. Zhang and L. Duan, Nat. Photonics, 2024, 18, 516–523 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2347003 and 2347004. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc02351j |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |