Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Titanium tetrafluoride catalysis for the dehydrative conversion of diphenylmethanols to symmetric and unsymmetric ethers†
Aman G. Singh ,
Abdulkhaliq A. Alawaed and
P. Veeraraghavan Ramachandran
,
Abdulkhaliq A. Alawaed and
P. Veeraraghavan Ramachandran *
*
Department of Chemistry, Purdue University, West Lafayette, IN 47907, USA. E-mail: chandran@purdue.edu
First published on 2nd August 2024
Abstract
In contrast to the conversion of diphenylmethanol to the corresponding halides with an equivalent of titanium tetrachloride or -bromide, catalytic (50 mol%) titanium tetrafluoride converts benzhydrols in diethyl ether or dichloromethane to bis(benzhydryl) ethers within 0.5–1 h at room temperature. Cross ether formation with diphenylmethanols and primary aryl or aliphatic alcohols is achieved in the presence of 25 mol% TiF4 in refluxing toluene as solvent. A tentative mechanism involving a carbocation intermediate has been proposed.
Introduction
The formation of diphenylmethyl ethers (DPME) from alcohols and their trans-etherification have been subjects of investigation for several decades due to the interest in DPME protection of alcohols during multi-step organic syntheses.1 In addition, DPMEs are an integral part of several pharmacologically important molecules, such as the antihistamine diphenhydramine (benzhydryl dimethylaminoethyl ether) hydrochloride (Benadryl®),2 anti-cholinergic orphenadrine hydrochloride (Disipal®),3 anti-depressant tofenacin hydrochloride (Elamol®),3 etc. (Fig. 1). Several procedures for the direct self- and cross-etherification of benzyl alcohols, particularly diphenylmethanol have been reported in the literature.4,5 The preparation of DPMEs reported eight decades ago used tri-diphenylmethylphosphate as an alkylating agent, accelerated using trifluoracetic acid as a catalyst.6 Bis(diphenylmethyl) ether was also prepared using (diethylamino)sulfur trifluoride (DAST),7 zeolite,8 or p-toluenesulfonyl chloride (p-TsCl),9 etc. as catalysts. Cross ethers from DPM and alcohols can be prepared employing several catalysts, such as Fe(NO3)3,10 Fe(OTf)3,11 Cu(NO3)2,12 MoO2(acac)2,13 PdCl2,14–16 BF3–Et2O,17 Nafion-H,18 NaAuCl4,19 etc. Use of diphenyldiazomethane20 and trichloroacetimidate21 for the synthesis of DPMEs and trans-etherification of DPMEs with ytterbium triflate [Yb(OTF)3]22 and FeCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 have also been reported.
23 have also been reported.
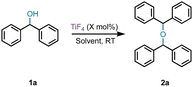
Our accidental discovery of the etherification of diphenylmethanols in the presence of titanium tetrafluoride (TiF4) originated from the reduction of benzophenone to diphenylmethanol (DPM, 1a) with borane-ammonia in the presence of titanium tetrachloride (TiCl4).24 We had observed that in addition to DPM, the corresponding diphenylmethyl chloride could also be prepared by altering the stoichiometry of TiCl4.25 This led to a titanium tetrachloride or -bromide-mediated conversion of benzhydrols to benzydryl halides (Scheme 1),25 which serve as precursors for several piperazine derivatives possessing biological properties.26 This dehydroxyhalogenation was extended to benzyl alcohol and other alcohols as well.25 We had postulated that the halogenation of DPM and alcohols proceeds via a carbocation intermediate and, indeed, recently reported on the use of benzyl alcohols as pre-electrophiles for Friedel–Crafts reactions in the presence of TiCl4.27 Based on a reported titanium tetrafluoride-mediated fluorination during Prins cyclization,28 we were interested in examining the potential for a dehydroxyfluorination of alcohols using TiF4. Unexpectedly, the reaction of DPM with a molar equiv. of TiF4 in diethyl ether (Et2O) at room temperature (RT) resulted in the formation of the corresponding bis(benzhydryl) ether (2a) in 91% yield within 30 minutes. Further examination of this reaction has led to an efficient dehydrative dimerization of substituted DPMs and cross-etherification with primary alcohols. An examination of the plausible mechanism of this reaction was also undertaken.
Results and discussion
The effect of stoichiometry, solvent, concentration, etc. on the TiF4-mediated room-temperature self-etherification was assessed first (Table 1). Optimization of the catalyst stoichiometry revealed that 50 mol% of TiF4 is sufficient to complete the dehydrative dimerization. The reaction was very facile at RT in Et2O, dichloromethane (DCM), and hexanes. A reaction in toluene at RT gave the bis(diphenylmethyl) ether 2a and the Friedel–Crafts product 5 in an 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 ratio (vide infra). The reaction in other solvents, such as tetrahydrofuran (THF), and nitromethane show product formation, but fail to undergo completion (TLC). Solvents such as dimethoxyethane (DME) and acetonitrile do not facilitate self-etherification, probably due to complexation with the catalyst.29 The solubility of the catalyst in the solvents was not favourable for a higher concentration reaction and optimal yields were achieved in 0.33 M Et2O, DCM, and n-hexane. The best yields were obtained when using DCM as solvent.
16 ratio (vide infra). The reaction in other solvents, such as tetrahydrofuran (THF), and nitromethane show product formation, but fail to undergo completion (TLC). Solvents such as dimethoxyethane (DME) and acetonitrile do not facilitate self-etherification, probably due to complexation with the catalyst.29 The solubility of the catalyst in the solvents was not favourable for a higher concentration reaction and optimal yields were achieved in 0.33 M Et2O, DCM, and n-hexane. The best yields were obtained when using DCM as solvent.
| Entry | TiF4, mol% | Solvent | Reaction time, h | bProduct 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a (yield%) 2a (yield%) |
|---|---|---|---|---|
| a All reactions were carried out at 1 mmol scale with 0.33 M solvent.b Isolated yields.c Friedel–Crafts reaction product. | ||||
| 1 | 10 | Et2O | 24 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 43 |
| 2 | 25 | Et2O | 24 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
| 3 | 50 | Et2O | 0.5 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (91) 100 (91) |
| 4 | 50 | Hexanes | 0.5 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (85) 100 (85) |
| 5 | 50 | DCM | 1 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (97) 100 (97) |
| 6 | 50 | Toluene | 2 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 84 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16c 16c |
| 7 | 50 | CH3CN | 0.5 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 8 | 50 | DME | 0.5 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 9 | 50 | THF | 1 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 10 | 50 | NO2Me | 1 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 78 |
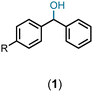
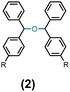
Having standardized the reaction, a series of diphenylmethanols, prepared via the sodium borohydride reduction of the corresponding benzophenones or Grignard reaction of the corresponding benzaldehydes bearing an electron-donating and -with-drawing substituent on the phenyl ring, were converted to the corresponding bis(benzydryl) ethers in Et2O or CH2Cl2. Thus, DPMs with a 4-bromo- (1b), 4-methoxy- (1c), 4-methyl- (1d), 4-nitro- (1e), and 4-fluoro-(1f) substituent on one of the phenyl rings were converted to the bis-ethers 2b–2f in 92–99% yields (Table 2). DPMs substituted with electron-withdrawing groups and halogens provided the corresponding bis-ethers in near quantitative yields. However, those with electron-donating groups provided slightly lower yields. Evidently, this may be attributed to the stability of the intermediate carbocations (vide infra).
Curious whether benzyl alcohol (3a) can be converted to dibenzyl ether in the presence of TiF4, a reaction was performed in Et2O at RT. Unlike the reaction of 3a with titanium tetrachloride and -bromide which led to the corresponding benzyl halides,25 the reaction with TiF4 did not yield any of the fluoride nor the corresponding dibenzyl ether products; the alcohol was recovered completely.
We sought to exploit this lack of reactivity of a primary alcohol to develop a direct cross-etherification/protection of alcohols by preparing the DPM ether via TiF4 catalysis. Unfortunately, a reaction of 1a and 3a in diethyl ether in the presence of 50 mol%, or even 100 mol% TiF4 resulted only in the formation of 2a and none of the cross ether (4aa). Fortuitously, when the above reaction was performed in the presence of 25 mol% TiF4 at higher temperature, in refluxing toluene, 4aa was isolated in 91% yield within 2 h. Notably, not even traces of 2a were observed during this reaction. To verify whether the formation of 4aa is proceeding via a trans-etherification of 2a,11 a solution of 2a and 3a in toluene was refluxed for 2 h, with and without TiF4. None of 4aa was formed in the latter reaction, but the former reaction revealed the formation of 4aa, albeit at a slow rate. The reversibility of the bis-ether formation step is discussed later (vide infra: mechanism). A similar reaction with methanol (3b) in refluxing toluene provided 96% of the cross ether (4ab) and none of the dimer 2a (Table 3).
| # | 1 | ROH (3) | Ether (4) | |||
|---|---|---|---|---|---|---|
| # | R | # | Structure | bYield% | ||
| a Reaction conditions: 1 mmol scale, reflux in 0.33 M toluene for 2 h with 25 mol% TiF4.b Isolated yields.c With 1.25 equiv. of 3.d NR = no reaction. | ||||||
| 1 | 1a | 3a | Bn | 4aa |  |
91 |
| 2 | 1a | 3b | Me | 4ab | 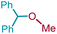 |
96c |
| 3 | 1b | 3b | Me | 4bb |  |
99c |
| 4 | 1a | 3c | Et | 4ac |  |
91c |
| 5 | 1a | 3d | n-Bu | 4ad |  |
96 |
| 6 | 1a | 3e | ClCH2CH2 | 4ae |  |
91 |
| 7 | 1a | 3f | p-ClBn | 4af |  |
98 |
| 8 | 1a | 3g | n-C6H11 | 4ag | NRd | |
| 9 | 1a | 3h | t-Bu | 4ah | NRd | |
| 10 | 1a | 3i | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2 CHCH2 |
4ai |  |
94c |
| 11 | 1b | 3i | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2 CHCH2 |
4bi |  |
97c |
Ethanol (3c), and n-butanol (3d) provided the corresponding ethers 4ac and 4ad, in 91% and 96% yields, respectively. Similarly, 4-bromo-substituted benzhydrol (1b) provided the corresponding methoxy ether (4bb) in 99% yield. 2-Chloroethanol (3e) and p-chlorobenzyl alcohol (3f) were also treated with 1a, which provided high yields of 91% and 98% respectively for the corresponding DPM ethers, 4ae and 4af, respectively. Chloroether 4ae is an intermediate for the preparation of Benadryl®.2 More hindered 2°- and 3°-alcohols, cyclohexanol (3g) and tert-butanol (3h), respectively failed to provide the desired etherification products 4ag and 4ah respectively in toluene as solvent, but 2a was formed. On the other hand, allyl alcohol (3i) when reacted with the DPMs 1a and 1b yielded 94% and 97% of ethers 4ai and 4bi, respectively.
Reaction mechanism
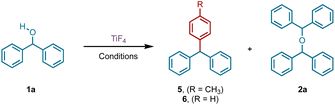
Having developed efficient protocols for the preparation of symmetrical and unsymmetrical ethers from DPMs, we turned our attention to rationalize the difference in behaviour of the tetrafluoro-reagent compared to the tetrachloro- and tetrabromotitanium derivatives. We had earlier established that the chlorination and bromination occurs via a carbocation,25 which was confirmed by carrying out a Friedel–Crafts reaction with pro-electrophiles, such as alcohols in the presence of the latter reagents.27 It is known that alcohols and amines form a complex with titanium tetrafluoride.29 Once this occurs, an SN1 pathway can be envisaged for the formation of the ether involving an intermediate carbocation (Scheme 2).The intermediacy of the carbocation can be presumed from the Friedel–Crafts alkylation product during the reaction of 1a in toluene as solvent at RT (Table 1, entry 6). Indeed, to demonstrate the presence of the carbocation unambiguously, a Friedel–Crafts reaction of DPM and an equivalent of TiF4 was conducted in refluxing benzene, anticipating the formation of triphenylmethane (5). The reaction proceeded to completion in 2 h and the 1H NMR of the product revealed the formation of 5 along with 2a in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. To facilitate the Friedel–Crafts alkylation, we carried out a similar reaction with DPM and 50 mol% TiF4 in refluxing toluene, which is a better substrate for Friedel–Crafts due to the increased electron density of the phenyl ring. Indeed, we isolated (p-tolylmethylene)dibenzene (5) exclusively in 96% yield, confirming the presence of a carbocation intermediate (Table 4). It is noteworthy that the triphenylmethane moiety forms the backbone for several dyes,30,31 and drugs possessing antiseptic,32 antihelmintic, and antimicrobial properties.33 They are also present in photodynamic therapy34 agents.
1 ratio. To facilitate the Friedel–Crafts alkylation, we carried out a similar reaction with DPM and 50 mol% TiF4 in refluxing toluene, which is a better substrate for Friedel–Crafts due to the increased electron density of the phenyl ring. Indeed, we isolated (p-tolylmethylene)dibenzene (5) exclusively in 96% yield, confirming the presence of a carbocation intermediate (Table 4). It is noteworthy that the triphenylmethane moiety forms the backbone for several dyes,30,31 and drugs possessing antiseptic,32 antihelmintic, and antimicrobial properties.33 They are also present in photodynamic therapy34 agents.
| Entry | Product | Reaction conditions | |||
|---|---|---|---|---|---|
| # | 5/6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a (yield %) 2a (yield %) |
Solvent | Temp. | TiF4 mol% | |
| a Reactions carried out at 1 mmol scale. | |||||
| 1 | 5 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 84 84 |
Toluene | RT | 50 |
| 2 | 5 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (96) 0 (96) |
Toluene | Reflux | 50 |
| 3 | 6 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
Benzene | Reflux | 100 |
Conclusion
In conclusion, we have developed a facile titanium tetrafluoride-catalysed dehydration protocol for the synthesis of symmetric and unsymmetric ethers from diphenylmethanol and related compounds by themselves at RT or with primary alcohols in refluxing toluene. This quick, room temperature synthesis of symmetrical ethers affords yields in the range of 92–99% and the cross-ethers in refluxing toluene in 91–99% yields. Mechanistic studies point to a carbocation pathway, which is confirmed by a TiF4-mediated Friedel–Crafts reaction. Although the process is efficient in preparing ethers, it fails when amines are used as the nucleophile, perhaps due to the complexation of TiF4 with amines. Continued studies on a potential dehydrative amination are underway.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
P. V. Ramachandran: funding acquisition, conceptualization, project administration, writing – review and editing; A. G. Singh: data curation, investigation, methodology, validation; A. A. Alawaed: data curation, investigation, methodology, validation.Conflicts of interest
There are no conflicts to declare.Notes and references
- M. T. Thornton and L. C. Henderson, Org. Prep. Proced. Int., 2013, 45, 395–420 CrossRef CAS.
- R. S. Vardanyan and V. J. Hruby, in Synthesis of Essential Drugs, ed. R. S. Vardanyan and V. J. Hruby, Elsevier, Amsterdam, 2006, pp. 219–235 Search PubMed.
- N. Capstick and H. Pudney, J. Int. Med. Res., 1976, 4, 435–440 CrossRef CAS PubMed.
- R. Paredes and R. L. Pérez, Tetrahedron Lett., 1998, 39, 2037–2038 CrossRef CAS.
- J. M. Altimari, J. P. Delaney, L. Servinis, J. S. Squire, M. T. Thornton, S. K. Khosa, B. M. Long, M. D. Johnstone, C. L. Fleming, F. M. Pfeffer, S. M. Hickey, M. P. Wride, T. D. Ashton, B. L. Fox, N. Byrne and L. C. Henderson, Tetrahedron Lett., 2012, 53, 2035–2039 CrossRef CAS.
- L. Lapatsanis, Tetrahedron Lett., 1978, 19, 3943–3944 CrossRef.
- A. L. Johnson, J. Org. Chem., 1982, 47, 5220–5222 CrossRef CAS.
- J. Cooke, E. J. Henderson and O. C. Lightbody, J. Chem. Educ., 2009, 86, 610 CrossRef CAS.
- G. Brahmachari and B. Banerjee, Org. Med. Chem. Lett., 2013, 3, 1 CrossRef PubMed.
- V. V. Namboodiri and R. S. Varma, Tetrahedron Lett., 2002, 43, 4593–4595 CrossRef CAS.
- P. K. Sahoo, S. S. Gawali and C. Gunanathan, ACS Omega, 2018, 3, 124–136 CrossRef CAS PubMed.
- M.-H. Bian, L.-Y. Lu, P. Zhang and Z.-X. Guo, Tetrahedron Lett., 2022, 99, 153838 CrossRef CAS.
- R. R. Singh, A. Whittington and R. S. Srivastava, J. Mol. Catal., 2020, 492, 110954 CrossRef CAS.
- Y. Bikard, J.-M. Weibel, C. Sirlin, L. Dupuis, J.-P. Loeffler and P. Pale, Tetrahedron Lett., 2007, 48, 8895–8899 CrossRef CAS.
- Y. Bikard, R. Mezaache, J.-M. Weibel, A. Benkouider, C. Sirlin and P. Pale, Tetrahedron, 2008, 64, 10224–10232 CrossRef CAS.
- M. Saudi and A. Van Aerschot, Molecules, 2013, 18, 8524–8534 CrossRef CAS PubMed.
- J. Li, X. Zhang, H. Shen, Q. Liu, J. Pan, W. Hu, Y. Xiong and C. Chen, Adv. Synth. Catal., 2015, 357, 3115–3120 CrossRef CAS.
- M. A. Stanescu and R. S. Varma, Tetrahedron Lett., 2002, 43, 7307–7309 CrossRef CAS.
- A. B. Cuenca, G. Mancha, G. Asensio and M. Medio-Simón, Chem.–Eur. J., 2008, 14, 1518–1523 CrossRef CAS PubMed.
- D. Best, S. F. Jenkinson, S. D. Rule, R. Higham, T. B. Mercer, R. J. Newell, A. C. Weymouth-Wilson, G. W. J. Fleet and S. Petursson, Tetrahedron Lett., 2008, 49, 2196–2199 CrossRef CAS.
- K. T. Howard, B. C. Duffy, M. R. Linaburg and J. D. Chisholm, Org. Biomol. Chem., 2016, 14, 1623–1628 RSC.
- G. V. M. Sharma, T. Rajendra Prasad and A. K. Mahalingam, Tetrahedron Lett., 2001, 42, 759–761 CrossRef CAS.
- V. H. Tran, M. T. La and H.-K. Kim, Org. Biomol. Chem., 2019, 17, 6221–6228 RSC.
- P. V. Ramachandran, A. A. Alawaed and H. J. Hamann, J. Org. Chem., 2022, 87, 13259–13269 CrossRef CAS PubMed.
- P. V. Ramachandran, A. A. Alawaed and H. J. Hamann, Org. Lett., 2023, 25, 4650–4655 CrossRef CAS PubMed.
- A. Venkat Narsaiah and P. Narsimha, Med. Chem. Res., 2012, 21, 538–541 CrossRef CAS.
- P. V. Ramachandran, R. Lin, A. A. Alawaed and H. J. Hamann, RSC Adv., 2024, 14, 15554–15559 RSC.
- S. Bondalapati, U. C. Reddy, D. S. Kundu, A. K. Saikia and J. Fluor, Chem, 2010, 131, 320–324 CAS.
- G. B. Nikiforov, H. W. Roesky and D. Koley, Coord. Chem. Rev., 2014, 258–259, 16–57 CrossRef CAS.
- M. S. Shchepinov and V. A. Korshun, Chem. Soc. Rev., 2003, 32, 170–180 RSC.
- X. Lu, Q. Che, X. Niu, Y. Zhang, Y. e. Chen, Q. Han, M. Li, S. Wang and J. Lan, Molecules, 2023, 28, 5401 CrossRef CAS PubMed.
- I. J. Kligler, J. Exp. Med., 1918, 27, 463–476 CrossRef CAS PubMed.
- K. T. Chen, C. S. Lu, T. H. Chang, Y. Y. Lai, T. H. Chang, C. W. Wu and C. C. Chen, J. Hazard. Mater., 2010, 174, 598–609 CrossRef CAS PubMed.
- K. Li, W. Lei, G. Jiang, Y. Hou, B. Zhang, Q. Zhou and X. Wang, Langmuir, 2014, 30, 14573–14580 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Optimization details, experimental procedures, product characterization, and 1H, 13C, and 19F NMR spectra of products. See DOI: https://doi.org/10.1039/d4ra04712e |
| This journal is © The Royal Society of Chemistry 2024 |