Open Access Article
Open Access ArticleManganese tetraphenylporphyrin and carbon nanocoil interface-based electrochemical sensing of tyrosine†
Syeda Aqsa Batool Bukharia,
Abeera Aziza,
Habib Nasir*a,
Sharif Ullaha,
Tehmina Akhtara,
Sadia Iramab,
Effat Sitaraac,
Shehla Mushtaqad and
Syed Abdul Moize
aDepartment of Chemistry, School of Natural Sciences, National University of Sciences and Technology, H-12, Islamabad, 44000, Pakistan. E-mail: habibnasir@sns.nust.edu.pk
bDepartment of Chemistry, Rawalpindi Women University, Rawalpindi, Pakistan
cDepartment of Chemistry, Karakoram International University, Gilgit, Pakistan
dDepartment of Chemistry, University of Management and Technology, Sialkot, Pakistan
eDepartment of Electrical Engineering, Umm Al-Qura University, Saudi Arabia
First published on 1st August 2024
Abstract
Tyrosine is one of the essential metabolites present in the human body for nutritional maintenance and normal physiological functioning. Its concentration in the body is crucial in predicting various hereditary, emotional, and physiological disorders. Therefore, quantitative monitoring of tyrosine in clinical samples is indispensable. We state the use of carbon nanocoils/manganese tetraphenylporphyrin convened glassy carbon electrode (CNC/MnTPP/GC) for the streamlined electrochemical sensing of tyrosine. Cutting-edge analytical techniques were employed to perform a comprehensive physicochemical analysis of the synthesized materials. To investigate the electrochemical properties, various techniques such as cyclic voltammetry (CV), differential pulse voltammetry (DPV), electrochemical impedance spectroscopy, and chronocoulometry were employed. CNC/MnTPP/GC displayed an optimal response at pH 5 and exhibited remarkable linearity within the concentration range of 0.05 to 100 μM for tyrosine. Using DPV, it demonstrated a low limit of detection (21 nM ± 1.17) and a sensitivity of 0.12 μA μM−1 cm−2. CNC/MnTPP/GC displayed excellent performance in terms of repeatability, reproducibility, and stability for up to 30 days, making it suitable for real-time analysis, particularly in the analysis of tyrosine in blood serum. Notably, CNC/MnTPP/GC showcased a superior detection limit compared to previously reported methods.
1. Introduction
Tyrosine plays a crucial role as a fundamental component in human biochemistry.1 Along with its role in the production of proteins and melanin and the regulation of hormonal balance, tyrosine has a significant role in producing neurotransmitters in the body. Its concentration in the body assists in predicting various hereditary, emotional and physiological ailments such as hereditary tyrosinemia type I,2 lung disorder3 and cardiovascular diseases.4 Hence, it is crucial to monitor the tyrosine concentration in the blood samples.Several approaches are being utilized to estimate tyrosine5 including spectrophotometric analysis,6,7 chemiluminescence,8 Raman spectroscopy,9 GC-MS,10,11 HPLC-UV,12 ion-exchange chromatography13 and fluorescence spectroscopy.14 Although these methods provide an accurate and precise determination of tyrosine, they require sophisticated laboratory setups along with the tedious process to prepare the samples for analysis. This is the reason why electrochemical analysis has been selected to detect tyrosine more conveniently and does not require time-consuming preparation of samples similarly it is less expensive, has simple instrumentation, has good resolution, and proved to be effective in real-time analysis of the biomolecules.15 A wide variety of combinations of nanomaterials are available to serve as electrode material to detect tyrosine and transduce signals efficiently in the form of readable output and these materials encompass non-/noble metal nanoparticles,16 metallo-organic receptors17 to carbon nanostructures18 etc. Herein, we present the first-ever use of carbon nanocoils and MnTPP nanocomposite for the efficient detection of tyrosine.
Carbon nanocoils (CNC) are coiled structures that exist in a one-dimensional form, with diameters ranging from 10 to 100 nm and lengths varying from 10 to 100 mm. These CNCs have garnered significant interest in the realm of electrochemical analysis for applications such as sensors, supercapacitors, and fuel cells, primarily due to their distinctive chemical, mechanical, and optical properties.19,20 The expansive specific surface area of carbon nanocoils enables the binding of receptor molecules through non-covalent interactions.
Metallo-porphyrins are planar macro heterocyclic molecules and are considered as bio-inspired organometallic compounds due to their rich electro-catalytic properties for redox reactions of several classes of compounds like phenols etc.21,22 Synthetic metalloporphyrins with different transition metals imitate the working mechanism of enzymes and provide good opportunities to be explored in the electrochemical sensing of organic and inorganic compounds.23,24 Manganese tetraphenylporphyrin (MnTPP) and its derivatives exhibit excellent catalytic properties and have been used in electro-catalysis applications like water splitting and sensing of various organic compounds. The poor conductivity of tetraphenyl porphyrins limits their application to be used directly in electrochemical sensing devices, therefore, their composites with conducting substances like carbon nanomaterials are being used.
In the present research study, the nanocomposite of carbon nanocoils with manganese tetraphenylporphyrin was prepared and fully characterized. The charge transfer process at the surface of the as prepared electrode, CNC/MnTPP/GC, was studied using electrochemical impedance spectroscopy and chronocoulometry. Cyclic voltammetry and differential pulse voltammetry were employed to conduct tyrosine sensing studies under pH 5 conditions. The schematic representation is shown in Fig. 1.
 | ||
| Fig. 1 Graphical representation of CNC/MnTPP/GC modified sensor for the electrochemical detection of tyrosine. | ||
2. Materials and methodology
2.1 Materials
Tyrosine, dopamine, uric acid, ascorbic acid, pyrrole (96%), potassium ferricyanide (99%), and manganese acetate (98%) were obtained from Sigma-Aldrich while disodium hydrogen phosphate (Na2HPO4), sodium dihydrogen phosphate (NaH2PO4), methanol (99%), ethanol (99.8%), chloroform (99%), propanoic acid (≥99.5%) and benzaldehyde were procured from Merck. Deionized water (DI) was used to prepare aqueous solutions. The electrode polishing kit consisting of 0.05 μm remained very useful for the polishing of GC.2.2 Instrumentation
The attenuated total reflectance Fourier transform infrared spectrometer (Bruker, Alpha Platinum, Germany) was utilized for FTIR analysis, covering the range of 550–4000 cm−1. The solid sample was analyzed using ATR-FTIR at room temperature. Raman analysis was conducted using the i-Raman high resolution TE Cooled Fiber Optic Raman System (Model: 1064 nm fiber optics, USA) with a wavelength of 532 nm, spanning the range of 150 to 4000 cm−1. For Raman analysis powdered sample was used. UV-visible spectroscopic analysis of the materials was carried out using a PerkinElmer spectrometer (Model: Lambda-365) within the 250 to 700 nm region at room temperature and the solutions were prepared in DMF. To examine the morphology of the nanocomposites, a scanning electron microscope (SEM) (Jeol, JSM-6490A, Japan) was utilized, and the elemental composition of the materials was determined using energy-dispersive X-ray spectroscopy (EDS). SEM was conducted in at 20 kV and the powdered samples were coated with palladium. For the electrochemical experiments, including cyclic voltammetry (CV), differential pulse voltammetry (DPV), chronocoulometry, and electrochemical impedance spectroscopy (EIS), a Gamry 3000/3000AE workstation was employed. Throughout the electrochemistry experiments, a three-electrode system was utilized, consisting of a silver/silver chloride reference electrode filled with 0.3 M KCl, a platinum wire as the counter electrode, and a glassy carbon electrode as the working electrode.2.3 Methodology
3. Results and discussion
3.1 Physicochemical characterization of the materials
Fig. 2 displays the Fourier transform infrared spectra of the materials prepared. The FTIR analysis of TPP reveals the existence of N–H stretching and bending vibration bands at 3314 cm−1 and 967 cm−1, correspondingly.26 The successful metalation of TPP was confirmed by observing the absence of N–H stretching and bending vibration bands at 3314 cm−1 and 967 cm−1, respectively, as well as the presence of the Mn–N band at 1008 cm−1 in the FTIR spectrum of MnTPP.27 CNC/MnTPP showed corresponding peaks for both CNC and MnTPP at 1540 cm−1 for aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, at 1410 cm−1 for C
C, at 1410 cm−1 for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, at 1331 cm−1 for C–N, and at 1001 cm−1 for Mn–N stretching vibrations but due to the formation of pi–pi interaction between carbon nanocoils and MnTPP the bands are shifted and somehow masked.
N, at 1331 cm−1 for C–N, and at 1001 cm−1 for Mn–N stretching vibrations but due to the formation of pi–pi interaction between carbon nanocoils and MnTPP the bands are shifted and somehow masked.
Raman analysis was performed for CNC, MnTPP, and CNC/MnTPP as shown in Fig. 3. It is well known that Raman of carbon nanostructured materials exhibit the characteristic D and G bands present at around 1300–1400 cm−1 and 1500–1600 cm−1, respectively.28,29 Weak band at 998 cm−1 is also present in the spectrum of MnTPP for the Cph–H stretching that is shifted in the spectrum of CNC/MnTPP. Raman spectrum of CNC shows the presence of the intense bands i.e. D band at 1338 cm−1 and G band at 1572 cm−1 representing somehow crystalline and amorphous structure of nanocoils with sp3–sp2 mixed character.30 The shifting of bands in CNC/MnTPP as compared to the pristine MnTPP and CNC is an indication that the nanocomposite is formed due to the pi–pi conjugation between CNC and the MnTPP.
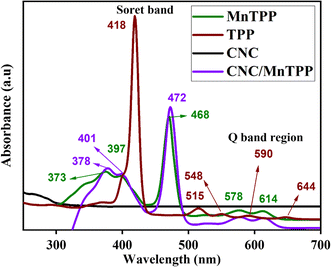
Ultraviolet-visible spectroscopy is helpful in the identification of chemically colored compounds. Tetraphenylporphyrin shows characteristic absorbance bands in the visible region with an intense Soret band present in the range of 300–500 nm and small Q-bands spanning 500 nm to 700 nm. Herein, the UV/vis. analysis of the samples was performed in DMF solvent. In Fig. 4 tetraphenylporphyrin shows a sharp Soret band at 418 nm arising from π–π* transitions involved in the molecule. The four Q-bands with weak absorption arise due to n–π* transitions and appear at 515, 548, 590, and 644 nm. After the complexation of TPP with manganese ion the absorption bands of MnTPP show a large bathochromic shift of the Soret band, as well as the number of Q-bands, decreased. MnTPP exhibits a Soret band located at 468 nm, accompanied by two additional bands at 373 nm and 397 nm.31 Moreover, two Q-bands can be seen at 578 nm and 614 nm. The diminution in the Q-bands for MnTPP depicts the formation of a more symmetrical molecule. The presence of two extra bands at 373 nm and 397 nm is an indication that the Mn ion has been incorporated into the TPP macrocycle.31 The nanocomposite, CNC/MnTPP, displays a bathochromic shift in both the Soret and Q-bands, attributed to the pi–pi interaction between the porphyrin ring and the carbon nanocoils.
Scanning electron microscopic (SEM) analysis of CNC/MnTPP has been shown in Fig. S2(a).† The coiled morphological structures show the presence of carbon nanocoils in the nanocomposite. The presence of Mn (weight% = 1.37) in the energy dispersive spectroscopy could be the indication that CNC/MnTPP nanocomposite is formed (Fig. S2(b)†).
3.2 Electrochemical characterization of modified electrodes
GC, MnTPP/GC, CNC/GC, and CNC/MnTPP/GC fabricated electrodes were subjected to analyze their electrochemical interfacial properties using an internal probe potassium ferricyanide and their electrocatalytic performance towards tyrosine in aqueous buffer (pH 5). | ||
| Fig. 5 Nyquist plot of GC, MnTPP/GC, CNC/GC, and CNC/MnTPP/GC in 5 mM potassium ferricyanide and 0.1 M KCl. | ||
Nyquist diagram consists of three main regions i.e. low frequency region, mid frequency region, and high frequency region. The high frequency region is associated with the presence diffusion-limited process (Warburg diffusion) and the low frequency region talks about the solution resistance (Ru) whereas the mid frequency region tells a picture about the resistance faced by the charge from the electrolyte to the electrode surface and known as charge transfer resistance (Rct). It indirectly provides information about the conductivities of the electrode. Rct values were predicted by the fitting of the EIS data with the Randles circuit. Rct for GC, MnTPP/GC, CNC/GC, and CNC/MnTPP/GC are 5.9 kΩ, 12.3 kΩ, 2.8 kΩ, and 901.9 Ω, respectively; indicating the conductive nature of CNC and the catalytic performance of MnTPP in the nanocomposite CNC/MnTPP and showing least charge transfer resistance when compared to other prepared electrodes.
The electrochemical surface area of the electrodes was obtained from cyclic voltammetric analysis of GC, CNC/GC, MnTPP/GC, and CNC/MnTPP/GC in 5 mM potassium ferricyanide solution in 0.1 M KCl, as shown in Fig. S3.† Applying the Randles–Sevcik equation, we sorted the electrochemical surface areas as; 0.007 cm2, 0.019 cm2, 0.025 cm2 and 0.076 cm2 for GC, MnTPP/GC, CNC/GC, and CNC/MnTPP/GC, respectively.
Chronocoulometric analysis (CC) also provides us with information on the more conductive nature of the CNC/MnTPP/GC electrode. CC was performed in the same conditions of potassium ferricyanide and potassium chloride electrolyte as EIS. CC curves demonstrate the change in the charge as a function of time for GC, MnTPP/GC, CNC/GC, and CNC/MnTPP/GC modified electrodes, as shown in Fig. 6. The lowest charge accumulation is observed for bare GC electrode (0.809 μC) and MnTPP (2.06 μC). When the GC is decorated with CNC and CNC/MnTPP, the amount of charge is increased from 9.80 μC to 21.91 μC as more active sites are available provided by both CNC and the nanocomposite. The amplified charge signal resulting from enhanced adsorption and accumulation of electroactive ferricyanide redox species on the electrode surface can be attributed to two factors: the high specific surface area provided by CNC and the increased electro catalytic ability of MnTPP.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tyrosine was probed in the phosphate buffer solution having the concentration of 0.1 M with pH 5 by voltammetric procedure (Fig. 7). No prominent oxidation peak was observed at both bare GC and MnTPP/GC in the presence of tyrosine due to the absence of any conducting support for MnTPP. The same experiment was also obtained at CNC/GC and CNC/MnTPP/GC in tyrosine and the oxidation process occurred. As there is no counter reduction peak in voltammograms so the electrochemical process of tyrosine is regarded as irreversible. At a potential of 0.79 V, tyrosine underwent oxidation, resulting in a peak intensity of 10.48 μA for CNC/MnTPP/GC. CNC/MnTPP/GC exhibited a higher current density compared to CNC, MnTPP/GC, and GC. This increase in current density was attributed to the combined effects of electron shuttling and the catalytic properties of carbon nanocoils and manganese centered tetraphenylporphyrin, which acted synergistically. Similarly, a significant enhancement in the peak current of tyrosine is observed in CNC/MnTPP/GC and represents the excellent electrocatalytic nature of the fabricated electrode towards tyrosine. Therefore, all the remaining electrochemical studies were performed using CNC/MnTPP/GC sensor.
tyrosine was probed in the phosphate buffer solution having the concentration of 0.1 M with pH 5 by voltammetric procedure (Fig. 7). No prominent oxidation peak was observed at both bare GC and MnTPP/GC in the presence of tyrosine due to the absence of any conducting support for MnTPP. The same experiment was also obtained at CNC/GC and CNC/MnTPP/GC in tyrosine and the oxidation process occurred. As there is no counter reduction peak in voltammograms so the electrochemical process of tyrosine is regarded as irreversible. At a potential of 0.79 V, tyrosine underwent oxidation, resulting in a peak intensity of 10.48 μA for CNC/MnTPP/GC. CNC/MnTPP/GC exhibited a higher current density compared to CNC, MnTPP/GC, and GC. This increase in current density was attributed to the combined effects of electron shuttling and the catalytic properties of carbon nanocoils and manganese centered tetraphenylporphyrin, which acted synergistically. Similarly, a significant enhancement in the peak current of tyrosine is observed in CNC/MnTPP/GC and represents the excellent electrocatalytic nature of the fabricated electrode towards tyrosine. Therefore, all the remaining electrochemical studies were performed using CNC/MnTPP/GC sensor.
 | ||
| Fig. 7 Cyclic voltammograms of GC, MnTPP/GC, CNC/GC, and CNC/MnTPP/GC in 100 μM tyrosine in 0.1 M PBS (conditions; scan rate = 0.05 V s−1; pH 5). | ||
The sensitivity of CNC/MnTPP/GC sensors for detecting tyrosine can be influenced by the pH value, making it an important parameter in electrochemical detection. To examine this effect, cyclic voltammetry (CV) was conducted using a sodium hydrogen phosphate/sodium dihydrogen phosphate (PBS) buffer with pH values ranging from 3 to 8, shown in Fig. 8(a). The oxidation peak current of tyrosine exhibited an initial increase with rising pH, reaching its maximum at pH 5, followed by a decrease as the pH increased further. These findings indicate that the electrochemical behavior of tyrosine is pH-dependent, with the reaction rate at the electrode being influenced by the concentration of hydrogen ions. Additionally, the reduction peak potential shifted towards more negative values with increasing pH as shown in Fig. 8(b), suggesting the involvement of H+ in the electrochemical reaction of tyrosine. This observation further confirms the reaction mechanism described in Scheme S1.† Consequently, a pH of 5 was chosen for subsequent experiments to investigate tyrosine detection.
Further, the kinetics of the electrode (CNC/MnTPP/GC) was analyzed by studying the effect of scan rate on the oxidation of tyrosine using CV. Increase in peak current is observed as the sweep rate increased from 0.01 V s−1 to 0.10 V s−1, as illustrated in Fig. 9(a). The results of scan rate were assessed by making two linear relationships i.e. square root of scan rate (ν1/2) vs. peak current (Ipa) and logarithmic relationship between scan rate (ν) vs. peak current (Ip). The value of regression coefficient (R2) obtained as a result of a plot of the square root of scan rate (ν1/2) vs. peak current (Ipa) was 0.98 (Fig. 9(b)), illustrating the charge transfer occurring at the surface of the electrode is due to the diffusion of tyrosine from the bulk to the surface of the electrode where the charge is transferred, and the analyte molecule is oxidized. The slope obtained from linear fitting of the log of scan rate vs. the log of the peak current Fig. 9(c) also provides useful information about the charge transfer process on the surface of the fabricated electrode. If slope is equal to 0.5 the electron transfer is occurring via diffusion process and if the slope is equal to 1 the charge transfer is due to the adsorbed material on the electrode thus showing an adsorption controlled mechanism. If this slope is between 0.5 and 1, then the process is both a mixed diffusion–adsorption controlled. From our experiments we can see the value of the slope is equal to 0.55, hence confirming the tyrosine detection at the electrode interface is diffusion controlled.
Similarly, it can be seen that as the scan rate increases, the peak potential also slightly shifts providing an indication of an irreversible electrocatalytic oxidation of tyrosine using CNC/MnTPP/GC electrode. Fig. 9(d) depicts the relationship between the (Epa) and the log of scan rate (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν) in the presence of tyrosine was developed. The regression equation is obtained as Epa (V) = 0.06 log
ν) in the presence of tyrosine was developed. The regression equation is obtained as Epa (V) = 0.06 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν + 0.90 (R2 = 0.99) in the range from 0.01 to 0.1 V s−1. The electrooxidation of tyrosine is an irreversible phenomenon so the Laviron equation is used to determine the relationship between Epa, and log
ν + 0.90 (R2 = 0.99) in the range from 0.01 to 0.1 V s−1. The electrooxidation of tyrosine is an irreversible phenomenon so the Laviron equation is used to determine the relationship between Epa, and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v as shown in following equation:
v as shown in following equation:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν vs. Ep which is equal to 1.002. The transfer coefficient (α) was calculated using the equation i.e. α = 47.7/Ep − Ep1/2 and obtained as 0.56. Further, the value calculated for n is 1.78 from nα which indicates that the tyrosine oxidation at the electrode surface is a two-electron irreversible process. The detail of the calculation is given in ESI above Scheme S1.†
ν vs. Ep which is equal to 1.002. The transfer coefficient (α) was calculated using the equation i.e. α = 47.7/Ep − Ep1/2 and obtained as 0.56. Further, the value calculated for n is 1.78 from nα which indicates that the tyrosine oxidation at the electrode surface is a two-electron irreversible process. The detail of the calculation is given in ESI above Scheme S1.†
Owing to the good electrochemical characteristics of the CNC/MnTPP/GC, its electrochemical behavior towards different concentrations of tyrosine was observed using both CV and differential pulse voltammetry (DPV). To account for CV curves for CNC/MnTPP/GC electrode in Fig. 10, the peak current is enhanced as the concentration of tyrosine is increased from 0.05 μM to 100 μM.
Further, the DPV was conducted to notice the trend in CNC/MnTPP/GC sensor characteristics towards varied tyrosine concentrations i.e. 0.05–100 μM, as illustrated in Fig. 11(a) and (b). Again, to conclude for sensitivity measurement and the limit of detection, a linear trend was established between peak current and concentration of tyrosine. The sensitivity of the sensor was 0.12 μA μM−1 cm−2 and the limit of detection was 21 nM (±1.17). To generate a comparison between calibration plots taken at pH 5 and normal physiological pH 7.4 DPV was also performed for different concentrations of tyrosine at pH 7.4 (Fig. 11(c) and (d)). The linear concentration range studied was the same as for pH 5 but in pH 7.4 the anodic peaks started to appear from 0.1 μM concentration. LoD obtained was 45 nM (±1.58) with sensitivity as 0.43 μA μM−1 cm−2.
Three different CNC/MnTPP/GC electrodes were prepared under identical conditions of ink deposition, drying temperature etc. The relative standard deviation incurred from the bar graphs in Fig. 13 is 2.16%, indicating excellent reproducibility of the prepared electrodes.
 | ||
| Fig. 14 (a) Repeatability studies and (b) stability experiment data of CNC/MnTPP/GC sensor in 100 μM tyrosine solution (pH 5). | ||
We also reconnoitered the stability of the CNC/MnTPP/GC sensor. On the very first day, CV was performed in 100 μM tyrosine solution (pH 5). The tested modified electrode was stored for 10 days in the buffer solution in the refrigerator at 4 °C, tested, and again stored for further 10 more days, and so on till 30 days. The loss in current intensity was only 6%, indicating that CNC/MnTPP/GC possesses terrific stability (Fig. 14(b)).
| Serum sample | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Sample 1 | 40 μM | 38.5 μM | 96% | 2.33 (n = 3) |
| Sample 2 | 50 μM | 47.13 μM | 94% | 4.53 (n = 3) |
| Sample 3 | 60 μM | 62.03 μM | 103% | 2.84 (n = 3) |
4. Conclusions
A nanocomposite, CNC/MnTPP, was synthesized by incorporating manganese tetraphenylporphyrin onto the surface of carbon nanocoils. This nanocomposite was then drop-coated onto a GC electrode to enable the electrochemical detection of tyrosine. The electrochemical results demonstrated that CNC/MnTPP/GC exhibited excellent electrocatalytic activity for tyrosine. The combination of highly conductive CNC and MnTPP, which possesses exceptional electron transfer abilities, led to a notable synergistic catalytic effect in the electrocatalytic reaction of tyrosine. As a result, this novel composite holds great potential for applications in the electrochemical detection of tyrosine in biological fluids. CNC/MnTPP/GC exhibited significant linear response in a concentration range of 0.05 to 100 μM with a low limit of detection (21 nM ± 1.17) and sensitivity of 0.12 μA μM−1 cm−2.Author contributions
SABB: conceptualization, experimentation, methodology, investigation, writing – original draft and data curation; AZ: writing – original draft; HN: conceptualization, resources, supervision, validation, writing – review & editing, project administration and funding acquisition; SU: methodology and formal analysis; TA: formal analysis; SI: characterizations; ES: visualization and formal analysis; SM: formal analysis and experimentation; SAM: review and formal analysis.Conflicts of interest
We declare no financial conflict of interests.Acknowledgements
This research was supported by Pakistan Science Foundation under Project Number: PSF-NSFC/Eng-C-NUST (03). Authors are highly obliged to National University of Sciences and Technology, Islamabad, Pakistan for provision of lab facilities in order to accomplish our research endeavors. The authors would like to extend their gratitude to Prof. Lujun Pan from the Department of Physics at Dalian University of Technology in China for his kindness in providing carbon nanocoils for this research. We are also thankful to Pakistan Science Foundation Islamabad, Pakistan for the availability of funds for our research.References
- K. Moulaee and G. Neri, Biosensors, 2021, 11, 502 CrossRef CAS PubMed.
- Y. Yang, Y. Song, X. Bo, J. Min, O. S. Pak, L. Zhu, M. Wang, J. Tu, A. Kogan and H. Zhang, Nat. Biotechnol., 2020, 38, 217–224 CrossRef CAS PubMed.
- J. T. Hartmann, M. Haap, H.-G. Kopp and H.-P. Lipp, Curr. Drug Metab., 2009, 10, 470–481 CrossRef CAS PubMed.
- A. B. Dollins, L. P. Krock, W. F. Storm, R. J. Wurtman and H. R. Lieberman, Physiol. Behav., 1995, 57, 223–230 CrossRef CAS PubMed.
- H.-Y. Zou, X.-Y. Lu, F.-Y. Kong, Z.-X. Wang, H.-Y. Li, H.-L. Fang and W. Wang, RSC Adv., 2020, 10, 28026–28031 RSC.
- B. E. A. Basheir and A. A. Elbashir, Chem. Express, 2015, 8, 95–101 Search PubMed.
- Y. Nozaki, Arch. Biochem. Biophys., 1990, 277, 324–333 CrossRef CAS PubMed.
- M. S. Alonso, L. L. Zamora and J. M. Calatayud, Talanta, 2003, 60, 369–376 CrossRef PubMed.
- Y. Lu, D. Lu, R. You, J. Liu, L. Huang, J. Su and S. Feng, Nanomaterials, 2018, 8, 400 CrossRef PubMed.
- A. L. Shroads, G. N. Henderson, J. Cheung, M. O. James and P. W. Stacpoole, J. Chromatogr. B, 2004, 808, 153–161 CrossRef CAS PubMed.
- C. Deng, Y. Deng, B. Wang and X. Yang, J. Chromatogr. B, 2002, 780, 407–413 CrossRef CAS PubMed.
- X.-m. Mo, Y. Li, A.-g. Tang and Y.-p. Ren, Clin. Biochem., 2013, 46, 1074–1078 CrossRef CAS PubMed.
- P. Allard, L. D. Cowell, T. H. Zytkovicz, M. S. Korson and M. G. Ampola, Clin. Biochem., 2004, 37, 857–862 CrossRef CAS PubMed.
- H. Yoshida, H. Nohta, Y. Harada, M. Yoshitake, K. Todoroki, K. Yamagata and M. Yamaguchi, J. Chromatogr. B, 2005, 821, 88–93 CrossRef CAS PubMed.
- J. Zhang, J. Feng, Y. Tian, Y. Wu, X. Liu and Q. He, Microchem. J., 2021, 171, 106867 CrossRef CAS.
- Z. Wei, Y. Yang, X. Xiao, W. Zhang and J. Wang, Sens. Actuators, B, 2018, 255, 895–906 CrossRef CAS.
- Q. Ma, S. Ai, H. Yin, Q. Chen and T. Tang, Electrochicia Acta, 2010, 55, 6687–6694 CrossRef CAS.
- J. Wei, J. Qiu, L. Li, L. Ren, X. Zhang, J. Chaudhuri and S. Wang, Nanotechnology, 2012, 23, 335707 CrossRef PubMed.
- A. S. Khan, L. Pan, A. Farid, M. Javid, H. Huang and Y. Zhao, Nanoscale, 2021, 13, 11943–11952 RSC.
- S. Xu, Z. Fan, S. Yang, Y. Zhao and L. Pan, Chem. Eng. J., 2021, 404, 126064 CrossRef CAS.
- R. Peng, A. Offenhäusser, Y. Ermolenko and Y. Mourzina, Sens. Actuators, B, 2020, 321, 128437 CrossRef CAS.
- S. A. B. Bukhari, H. Nasir, L. Pan, M. Tasawar, M. Sohail, M. Shahbaz, F. Gul and E. Sitara, Sci. Rep., 2021, 11, 1–13 CrossRef PubMed.
- X. Guo, B. Guo, C. Li and Y. L. Wang, J. Electroanal. Chem., 2016, 783, 8–14 CrossRef CAS.
- Y. Yang, R. Sun, M. Li, B. Geng, J. Deng and M. Tang, Int. J. Electrochem. Sci., 2016, 11, 7370–7379 CrossRef CAS.
- S. Sakthinathan, H. F. Lee, S.-M. Chen and P. Tamizhdurai, J. Colloid Interface Sci., 2016, 468, 120–127 CrossRef CAS PubMed.
- Z. Qian-Kun and X.-X. Gao, Electrochim. Acta, 1995, 40, 959–964 CrossRef.
- K. Ezzayani, A. B. Khelifa, E. Saint-Aman, F. Loiseau and H. Nasri, J. Mol. Struct., 2017, 1137, 412–418 CrossRef CAS.
- E.-X. Ding, J. Wang, H.-Z. Geng, W.-Y. Wang, Y. Wang, Z.-C. Zhang, Z.-J. Luo, H.-J. Yang, C.-X. Zou and J. Kang, Sci. Rep., 2015, 5, 1–9 Search PubMed.
- C. Deng, P. Wang, C. Li, X. Wang and L. Pan, Diamond Relat. Mater., 2019, 97, 107426 CrossRef CAS.
- R. Cui, L. Pan, D. Li, H. Ma and W. Peng, Carbon, 2014, 76, 455–458 CrossRef CAS.
- C. M. B. Neves, S. L. H. Rebelo, M. A. F. Faustino, M. G. P. M. S. Neves and M. M. Q. Simões, Catalysts, 2019, 9, 967 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra02048k |
| This journal is © The Royal Society of Chemistry 2024 |