Open Access Article
Open Access ArticleModulating edible-oleogels physical and functional characteristics by controlling their microstructure†
Mohsen
Ramezani
 ab,
Laura
Salvia-Trujillo
ab,
Laura
Salvia-Trujillo
 ab and
Olga
Martín-Belloso
ab and
Olga
Martín-Belloso
 *ab
*ab
aDepartment of Food Technology, Engineering and Science. University of Lleida, Av. Alcalde Rovira Roure 191, 25198, Lleida, Spain. E-mail: mohsen.ramezani@udl.cat; laura.salvia@udl.cat; olga.martin@udl.cat; Tel: +34 973702593
bAgrotecnio Center, Av. Alcalde Rovira Roure 191, 25198, Lleida, Spain
First published on 13th December 2023
Abstract
The influence of co-oleogelators like lecithin or hydrogenated lecithin together with the addition of dispersed water droplets to modulate the microstructure and thus the physical properties of glyceryl stearate (GS)-corn oil oleogels was investigated by thermal profile, microstructure, hardness, and oil binding capacity (OBC). The addition of β-carotene (βC) was also assessed. With lecithin, crystallization and melting temperatures were reduced, resulting in less-ordered crystal networks with a lower hardness and OBC, while with hydrogenated lecithin, the opposite effect was observed. In the presence of water, oleogels became harder but more brittle. Finally, βC acted as a crystal modifier increasing the hardness and OBC in the presence of lecithin, but decreased these parameters in hydrogenated lecithin-containing and water-filled oleogels. This study provides a better understanding on how the composition of GS-based oleogels can affect their physical properties.
1. Introduction
Oleogelation is a relatively new method of structuring edible liquid oils. Recent attention has been drawn to oleogelation due to its versatility and ease of processing. Oleogels are a class of soft, solid or solid-like materials obtained by adding an oleogelator to a liquid oil under the appropriate processing conditions. It is known that oleogelators with a crystalline phase simultaneously assemble into supramolecular structures with a highly asymmetric morphology resembling fibers or platelets. The asymmetric morphology increases the contact area between self-assembled structures, eventually leading to oleogelation.1 Despite having a liquid oil bulk, oleogels are solid-like because the liquid oil is immobilized in a 3D network of self-assembling molecules.2 The glyceryl stearate (GS) is a monoglyceride (MAG) that has been used for the formation of oleogels and generally recognized as safe (GRAS). One of the objectives of developing such an oleogel is to use it as a fat substitute in food products3 because negative health impact, ethical issues and veganism have raised the concerns of consumers and food regulatory legislations towards finding fat substitutes.4 A key benefit of using GS in oleogels is that it is compatible with almost all vegetable oils, forms oleogels with low quantities and creates a smooth and homogeneous texture.5 This is because GS can act as an interfacial active agent, helping to disperse the oil phase throughout the solid network. This can result in a more uniform distribution of the oil phase, which can improve the sensory properties of the oleogel. The use of GS-oleogels as fat-replacers can result in sensory-acceptable food products.3 In a recent research, it has also been reported that adding GS to oleogels strengthened their gel network, enhancing the solubility of β-carotene (βC).6 However, replacing saturated fats by GS-oleogels remains a challenge due to their typical physical and technological characteristics that might lead to food products harder and brittler than those formulated with saturated fats, which limit their use in some food and medicinal applications despite their many advantages.As a result, researchers and food manufacturers are trying to control the micro- and thus macro-structure of oleogels, as well as the variables influencing their ultimate physicochemical characteristics. For modulating the techno-functional features of oleogels, some strategies can be followed such as the use co-oleogelators or the incorporation of water into the lipid substance. Co-oleogelators can act as crystal habit modifiers, regulating the oleogels’ macroscopic properties.7 Despite multiple studies utilizing co-oleogelators,2,7–9 altering the physicochemical properties of an oleogel using a plant-derived and economically efficient co-oleogelator has yet to be investigated. Lecithin from soya and its derivatives may be the most promising among those obtained from plants for meeting current consumerś demands. Lecithin is composed of biocompatible glycerophospholipid molecules with hydrophobic tails of fatty acids esterified to C1 and C2 of the glycerol backbone, and a polar head esterified to C3 of the glycerol backbone and is known as an interfacial active agent. Research on the self-assembly of lecithin in organic solvents in the presence of trace amounts of water has a long history.10,11 However, studies on its effect as a co-oleogelator are limited. Not only lecithin, but also its derivatives, exhibit interfacial active properties and could be used as co-oleogelators. When lecithin undergoes controlled hydrogenation, the double bonds in fatty acids are saturated, resulting in hydrogenated lecithin. Aside from a few studies on the phase behavior of hydrogenated lecithin in the presence of sitosteryl sulfate,12 and stability and release performance of bioactive-enriched liposomes with varied hydrogenated lecithin content,13 no study on the use of hydrogenated lecithin in oleogels has been undertaken.
Another strategy for modulating the techno-functional features of oleogels is the incorporation of water inside.14 Evidence exists that the macroscopic properties of oleogel can change significantly upon dispersion of water into the matrix. The crystals generated by the oleogelator can be hydrated and their crystalline state can change in the presence of water molecules. In an oleogel created by thermal treatment at elevated temperatures of MAGs and water mixtures, MAGs first form a lamellar phase, that is followed by the swelling of water molecules into the continuous lamellar phase. In this procedure, the MAG is hydrated to form a swollen, space-filling system of lamellar liquid-crystalline phase. When this lamellar liquid crystalline phase is cooled to undergo crystalline phase transformation, the lamellar structure involving the swelling water phase is preserved, generating a very viscous phase known as α-gel phase. Further cooling results in the formation of a rheologically hard phase, which is used in low-fat spreads.15 Nevertheless, water-filled oleogels, where water (1% w/w) is physically dispersed in the system, should not be confused with oleogel-emulsions generally with water content above 10% (w/w).2,16
Despite aforementioned, the use of oleogels in food systems does not end with the replacement of fats; they can also protect and transport lipophilic bioactive compounds into the gastrointestinal tract, where they can exert their benefits. The β-carotene (βC) is a lipophilic bioactive compound found in fruits and vegetables, which is a precursor of vitamin A,6 and has other beneficial properties like preventing certain cancers.17 By utilizing enriched oleogels, we may obtain not only the nutritional qualities of vegetable oils, but also the beneficial effects of bioactive compounds. There are two major advantages of employing oleogels as bioactive carriers: (i) oleogels can accommodate higher dosage (up to 10% w/w oleogel) of bioactive compounds than their oil or nanoemulsion counterparts, and (ii) due to their quasi-solid texture, they can hinder the degradation of the bioactive substances better than the other systems such as nanoemulsions. However, the addition of a lipophilic material such as bioactive compounds could lead to further changes in the microstructure of the oleogel network, thereby changing their physicochemical characteristics.18 Therefore, this should also be investigated in greater detail to understand how the incorporation of βC into oleogels impacts their ultimate physicochemical properties, which is largely unknown to this date. The obtained information may allow to increase the bioactive compound's shelf life while simultaneously boosting the nutritional and physicochemical properties of the final oleogel.
Thus, the aim of this research was to study the relationship between the oleogel microstructural characteristics and their technological and functional properties as influenced by their composition. Hence, oleogels with GS 20% (w/w), as main oleogelator, and incorporating lecithin or hydrogenated lecithin (0 to 2.5% w/w), as co-oleogelators, were formulated and their microstructural features were evaluated. In addition, water-filled oleogels were formed by dispersing water (1% w/w) into previously formulated oleogels and then characterized. Lastly, to study the feasibility of using oleogels as carriers of βC, the effect of adding βC (0.1% w/w) on their physical properties was also assessed.
2. Materials and methods
2.1. Materials
Corn oil (Koipe Asua, Spain) was obtained from local market and lecithin (soybean phospholipids with 70% phosphatidylcholine (non-genetically modified); phospholipid composition: phosphatidylcholine + lysophosphatidylcholine 68–73%, lysophosphatidylcholine <10%; fatty acid composition in % of total fatty acids: palmitic acid 12–17%, stearic acid 2–5%, oleic acid 8–12%, linoleic acid 58–65%, linolenic acid 5–9%), and hydrogenated lecithin (hydrogenated phospholipids from soybean about 70% phosphatidylcholine (non-genetically modified); phospholipid composition: hydrogenated phosphatidylcholine >62%, hydrogenated lysophosphatidylcholine <5%) from Lipoid GMBH (Germany). GS (purity >90%) was purchased from IOI Oleochemical (Germany) and βC from Sigma Aldrich (USA). All analyses were performed using ultrapure water attained by Synergy® UV, Millipore (France).2.2. Methods
GS concentration remained constant at 20% (w/w) based on preliminary test, and because it demonstrated the most effective preservative impact on βC stability,6 but lecithin and hydrogenated lecithin content varied up to 2.5% (w/w). The maximum concentration of both lecithin and hydrogenated lecithin was limited to 2.5% to ensure that it remains well below the threshold at which they function as oleogelators.20 The corn oil-oleogelators mixture was heated to 65 °C in an aluminum foil-covered beaker on a hot plate with magnetic stirring until the oleogelators were fully dissolved. After heating water to 65 °C, it was added to the oleogelators formula to form water-filled oleogels (1% w/w).2 Once cooling down to ambient temperature, oleogels readily formed and no flow was observed after inverting the containers after approximately 10 minutes. All oleogels were stored at 25 °C for 24 hours before analysis. A detailed description of the formulation of the oleogels is included in Table S1.†
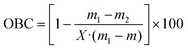 | (1) |
3. Results & discussion
The influence of the composition on the physical and functional properties of the oleogels was evaluated through their thermal characteristics, microstructure, visual properties, hardness, and OBC. For the sake of clarity, first, the results will be discussed for the (i) influence of adding lecithin or hydrogenated lecithin at different concentrations to the GS oleogels; (ii) influence of dispersing water into the oleogels to form water-filled oleogels; and (iii) influence of incorporating βC into the previously formed oleogels.3.1. Differential scanning calorimetry profile
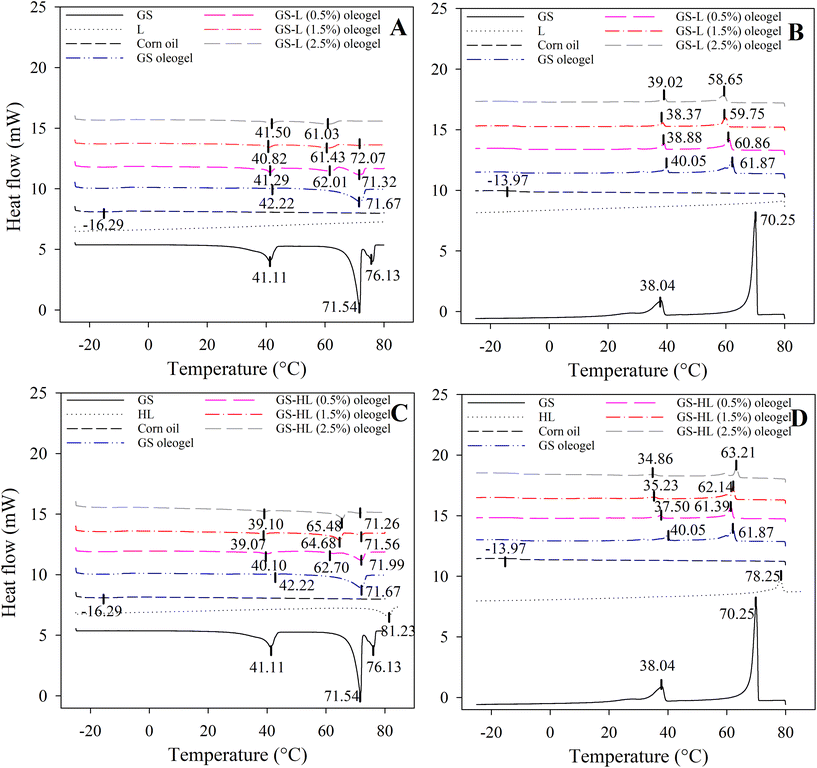
Thermograms revealed a melting peak of corn oil, which was found in all oleogels, between −15 °C and −19 °C (Table S3† and Fig. 1). The melting peak matched with the findings of Tan and Che Man21 where melting curve of corn oil consisted of an endotherm peak at −18.49 °C. In corn oil, unsaturated fatty acids (mono- and polyunsaturated fatty acids) account for around 84% of total fatty acid content, with saturated fatty acids accounting for the remaining 16%. Because of the large amounts of unsaturated fatty acids in corn oil, there were no melting or crystallization peaks above 0 °C.22 Lecithin did not exhibit any thermal peaks in the heating and cooling cycles ranging from −25 to 80 °C. In contrast, hydrogenated lecithin displayed a melting peak at 81.23 °C and a crystallization peak at 78.25 °C, aligning with the findings of Martínez-Ávila.11In the second heating cycle of GS (Fig. 1), three melting peaks exhibited at 41.11 °C, 71.54 °C, and 76.13 °C. Starting from the second cooling cycle onward, two crystallization peaks emerged at 70.25 °C and 38.04 °C. The DSC thermograms for the first heating cycle (Fig. S1, ESI†) of neat GS indicated a singular melting peak at 77.48 °C, with subsequent cooling displaying two peaks at 70.32 °C and 38.02 °C. This observation supports the conclusion that the peaks observed for GS corresponds to the GS polymorphism. In a study by López-Martínez et al., 2014,23 the crystallization thermogram for neat GS revealed two main melting peaks at 47 °C and 72 °C, corresponding to temperatures for the sub-α1 ⇔ Lα transitions and melting of the Lα (inverse lamellar α) state.
The melting points of GS-oleogel were −17.99 °C (Tm1), 42.22 °C (Tm3), and 71.67 °C (Tm5) where Tm1 denoted the existence of liquid corn oil in its structure, while Tm3 and Tm5 meant the presence of GS, associated with various GS polymorphs.24 Similarly, two melting peaks of GS at 55 °C (primary peak) and 31 °C (secondary peak) were observed in a study by Li et al., 2021.25 They inferred that in the GS-oil system, the primary peak was associated with the melting of the Lα phase, and the secondary peak with Lα ↔ sub-α. However, the melting and crystallization temperatures of GS crystals did not remain the same with the addition of lecithin or hydrogenated lecithin molecules.
In general, melting temperatures and enthalpies were reduced when lecithin molecules were added to GS-oleogel. In this context, Tm3 was lowered by lecithin addition to ≈41 °C (Fig. 1A), which was not directly proportional to lecithin concentration. Moreover, Tm5 (at 71.67 °C) began to diminish and Tm4 (at ≈62 °C) began to emerge when lecithin molecules were added to oleogels. As the Tm5 designates one of the key melting temperatures of GS crystals and Tm4 was not seen in GS-oleogels, this occurred most likely as a result of the effective co-assembly of lecithin molecules and GS. Nevertheless, Tm4 steadily decreased to 61.03 °C as lecithin content rose, demonstrating the softening effect of lecithin molecules. Ghan and colleagues20 also asserted that the melting point of GS-palm oil oleogel was decreased by the addition of lecithin. In fact, the lower melting temperatures point to a less ordered, easier to melt crystalline structure, which may be connected to the microstructural characteristics of the oleogels (discussed in the section 3.2). Further evidence can be found in the crystallization thermograms (Fig. 1B). The GS oleogels’ crystallization temperatures are lowered by the addition of lecithin, with the major peaks’ values falling between 38 and 60 °C. Moreover, the shoulder-less crystallization peak at ≈60 °C (Tc4) in the case of the lecithin containing oleogels suggest that crystallization was probably a one-step process since the lecithin molecules most likely attached GS molecules thanks to their polar moieties. According to Hu et al.,26 it is likely that oleogelators co-assemble during crystallization. The co-assembly of lecithin and GS in the oleogel carried out via hydrogen bonds and van der Waals forces,20 indicating that the lecithin molecules can affect the GS architecture. Moreover, compared to GS-oleogels, lecithin-containing oleogels showed lower intensity and broader exothermic peaks at their respective crystallization temperatures of 60 °C (Tc4), suggesting the formation of a weaker bilayer structure that may promote the ease of melting at lower temperatures. However, lecithin molecules had completely different effects than hydrogenated lecithin molecules on GS-oleogels’ thermal properties.
In the oleogel with GS and hydrogenated lecithin, crystals were melted at higher temperatures. The preservation of GS crystals with Tm5 (at 71 °C), as well as the existence of a Tm4 with steadily rising enthalpies and temperatures (from 62.7 °C to 65.48 °C) in response to hydrogenated lecithin concentration, demonstrated that the crystalline structure of GS-oleogels is preserved or even improved after the addition of hydrogenated lecithin (Fig. 1C). The melting of GS occurred at higher temperatures as the hydrogenated lecithin concentration increased. The results showed that hydrogenated lecithin was beneficial to the thermal stability of GS, resulting in a delayed commencement of the phase transition of GS. The results agreed with those of Martins et al.,18 who observed that increasing the concentration of oleogelator elevated the Tm and Tc. Moreover, the presence of a shoulder on the crystallization peaks of hydrogenated lecithin-containing oleogels suggests that the GS crystals were retained to a large extent that could be due to the lack of interactions with hydrogenated lecithin molecules. According to crystallization thermograms (Fig. 1D), the addition of hydrogenated lecithin raised the crystallization temperatures of GS oleogels from 61.87 °C to 63.21 °C (Tc4). As compared to GS-oleogels, hydrogenated lecithin-containing oleogels exhibited greater intensity and narrower exothermic peaks at their respective crystallization temperatures, implying that crystallization proceeded quicker and at higher temperatures.
According to the thermographs, adding lecithin resulted in less ordered and structured lipid molecules, whereas adding hydrogenated lecithin led to better structured and more crystalline oleogels.
3.2. Macro- and microstructure
All of the formed oleogels were self-standing, thermoreversible, and optically opaque (Fig. 2). Gel opacity is dictated by crystallinity, since diffracting units in the network contribute to light scattering.27 Oleogels containing lecithin were yellower, whereas those containing hydrogenated lecithin were whiter than GS-oleogels. Visual differences suggest that color is determined by the type and concentration of oleogelators, as well as other factors such as color and type of the oil used for the oleogel preparation.28The compositions of oleogels, on the other hand, had a considerable impact on their microstructures (Fig. 2). GS crystals were rod-like and homogenously distributed throughout the system, which was consistent with the findings of Kesselman and Shimoni.29 The rod-like shape of GS crystals was associated with their great efficiency in the formation of oleogels. For the same quantity of oleogelator mass, a rod-like shape has a substantially larger surface area than spheres or platelets. High surface areas enable more interactions between oleogelator molecules and the solvent, as well as greater contact between the microstructural elements that contribute to an oleogel's elastic and solid-like properties.30
The addition of lecithin changed the compactness and shape of the crystals dramatically, which differed from mono-component crystal structure (GS-oleogel), indicating co-crystallization of GS and lecithin. The acicular crystals in GS-lecithin oleogels were bigger than those in GS oleogels, but there were more void spaces between them. It is possible that the lecithin and GS molecules took longer to diffuse and were instead added preferentially to a nucleated crystallite surface. Crystal growth of already nucleated species was favoured over subsequent nucleation events, yielding fewer but bigger crystals. However, when lecithin concentration increased, the GS-lecithin crystal became smaller and the morphology altered to spherulites (e.g., fan-shaped).28 Nonetheless, when the concentration of lecithin molecules increased, the number of crystals multiplied as they became more tightly packed. This implies that the extra lecithin molecules acted as additional nuclei, increasing the total number of nuclei, therefore, crystals were abundant, albeit smaller.
When oleogels made with GS and hydrogenated lecithin were compared to oleogels made exclusively with GS, the crystal compactness and shape altered. They appeared to have smaller crystals than those found in GS-lecithin oleogels. In addition, GS-hydrogenated lecithin oleogels possessed a denser crystalline structure with less void spaces between them. This was consistent with the findings of Martins et al.,18 who observed that increasing the concentration of oleogelator boosted crystal compactness. Individual crystal structures were not clearly visible after hydrogenated lecithin addition due to crystal stacking, which was particularly true as concentrations rose. Higher hydrogenated lecithin concentrations resulted in a more linked and denser network, as well as a substantial bulk of crystals, which may be responsible for the gel structure's strengthening.
When the microscopic images of the oleogels were compared to those of their water-filled counterparts, no substantial variations were found. The only changes were the physically entrapped water droplets inside the matrix and the fluffier crystals.
3.3. Texture
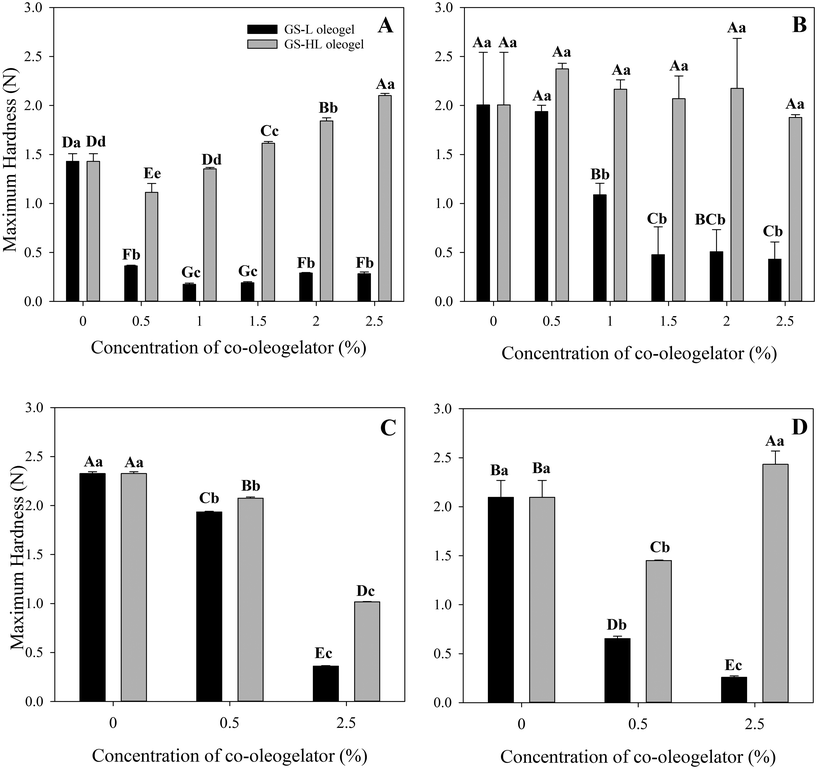
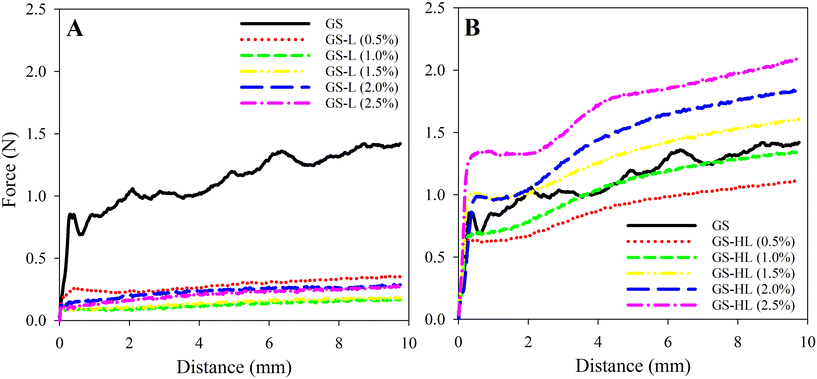
The inclusion of lecithin drastically reduced the hardness of oleogels by more than 75% when compared to oleogels made only with GS (Fig. 3A and 4A). Adding lecithin to the oleogel mixture resulted in a substantial drop in hardness values even at low lecithin concentrations (i.e., GS-lecithin 0.5% w/w). However, it should be noted that adding lecithin to oleogel enhanced their smoothness and effectively eliminated the oscillatory pattern found in the GS-based oleogel. The hardness of oleogel was not proportional to lecithin content. Increasing the lecithin content initially decreased but eventually enhanced oleogel hardness. As a consequence, the lowest measured oleogel hardness was 0.17 ± 0.01 N for 1% (w/w) lecithin.
The effect of lecithin as a co-oleogelator might be explained by several reasons. First, the presence of lecithin in a lipid matrix might increase its polarity33 thus favoring lecithin-oil interaction. If such interactions prevail, GS may have lesser interactions with oil, resulting in softer structures. Second, lecithin might modify the crystal morphology through hydrogen bonding with the main oleogelator, in this case GS.34 The development of new oleogelator-oleogelator interactions, as well as oil-oleogelator has been reported previously.33 Lončarević et al.35 found that adding lecithin from 0.3% to 0.5% (w/w) to a hard vegetable fat reduced its hardness significantly, while increasing its concentration to 0.7% (w/w), made the vegetable fat harder. In contrast, Pernetti et al.36 found that increasing lecithin concentration when sorbitan tri-stearate was added as a second oleogelator initially raised, but then decreased the oleogel's hardness. Besides its role as a crystal morphology modifier, lecithin also stimulated the formation of weak junctions between sorbitan tri-stearate crystals, which were responsible for structuring the network that entraps the oil. Nonetheless, after reaching a critical concentration, those weak junctions and self-aggregation from lecithin became dominant, reducing overall network strength.36 Generally speaking, once lecithin molecules reach the critical concentration, they align with each other to form micelles and lamellae (or multilayers),37 because the lipophilic ends of one monolayer lecithin can bind to the lipophilic ends of another layer,9 which contributes to the network's robustness. This can also explain their profile's lower oscillatory pattern, demonstrating that lecithin addition lowered brittleness through altering GS crystals. Adding lecithin to oleogel in combination with GS weakened and simultaneously smoothed it in this way.
In contrast to the softening effect observed in the lecithin-containing oleogels, the addition of hydrogenated lecithin to the oleogels gradually increased their hardness (Fig. 3A and 4B). In this regard, compared to GS-oleogels, lower concentrations of hydrogenated lecithin added to oleogels (e.g., 0.5% and 1% (w/w)) led to reduced oleogel hardness. Increasing the hydrogenated lecithin concentration gradually raised their hardness in a way that the maximum hydrogenated lecithin concentration resulted in the hardest oleogel (2.10 ± 0.02 N). All GS-hydrogenated lecithin-oleogels showed less oscillatory pattern thus less brittleness and more smoothness compared to GS- or GS-lecithin-oleogel systems. Aguilar-Zárate et al.33 observed similar pattern after adding hydrogenated lecithin or lecithin to cellulose-canola oil oleogels, hydrogenated lecithin has a higher lipophilicity of alkyl chains than lecithin, allowing it to interact with lipids more effectively. This may explain the superior hardness of hydrogenated lecithin-containing oleogels. Additionally, this may be due to the composition of hydrogenated lecithin, which contains abundant saturated triacylglycerides (TAGs). Saturated TAGs in hydrogenated lecithin lack double bonds, resulting in enhanced level of organization and hence strengthening of the oleogel structure.
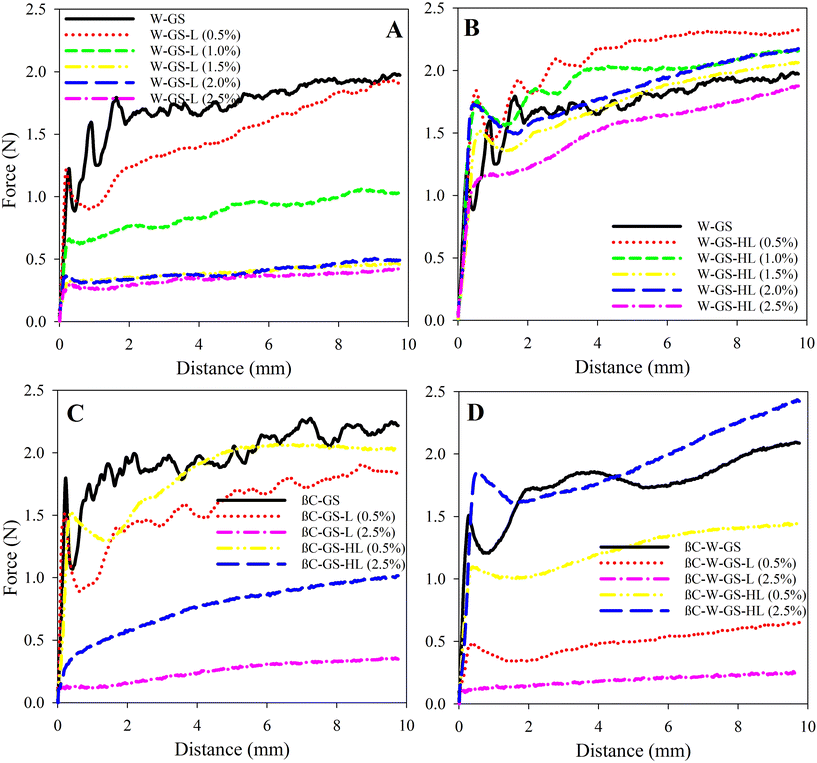
The hardness of lecithin-containing oleogels was markedly increased by water molecules as well as GS-based oleogels. Various water/lecithin molecular ratios (hereafter referred to as W0) have been found to affect the structural properties of lecithin in the oleogel systems.10 Scartazzini and Luisi10 found that deviating W0 by even one unit from its critical point prevents the gel from forming, indicating that water molecules play a critical role in the formation or modification of the lecithin-based structure. Despite this, Scartazzini and Luisi10 used lecithin as a sole oleogelator that can only form supramolecular structures, whereas, in this study, lecithin was used as a co-oleogelator in systems where the building blocks of the gel were already formed by GS. According to Mezzasalma et al.,38 when W0 = 4, the polymer-like micelles of lecithin form a temporal 3D network that can entrap the n-decane. Instead, when W0 = 3, the lecithin is in the form of reverse micelles. Accordingly, even trace amounts of water alter the physical properties of a system.9 Gaudino et al.2 also observed that adding 1% (w/w) water to various lecithin/stearic acid ratios resulted in the development of reverse micelles formed by lecithin molecules, which were able to interact with stearic acid via their nonpolar moieties and produce a hybrid oleogel. When water was introduced to GS-lecithin, the same scenario might occur; in the presence of water molecules, lecithin molecules may form reverse micelles, with GS molecules incorporated via their esterified stearic acids. It is also possible to assume that GS molecules established the foundation of a network in which lecithin molecules interacted with interior glycerol molecules and exterior esterified stearic acids via their polar heads and non-polar tails, respectively, therefore enhancing the complexity of the 3D network. In this circumstance, water molecules interacted with OH groups in GS molecules as well as polar moieties in lecithin molecules. Using lecithin and ceramide as oleogelators, Guo et al.39 found that adding water entirely changed the crystal shape of the oleogel. In the presence of water in the oleogel system, they noticed that the large 3D crystals transformed into 2D crystals with smaller sizes and larger surface area, resulting in a harder structure.
The 0.5% (w/w) lecithin concentration had the highest (p < 0.05) maximum hardness (1.93 ± 0.06 N), followed by the 1% (w/w) lecithin concentration (1.08 ± 0.11 N), and then the higher concentrations (1.5%, 2%, and 2.5% w/w) with no significant differences (p < 0.05). The elasticity and hardness of the system diminished as the concentration of lecithin rose. However, the effect of water molecules on network strengthening in oleogels with lower concentrations of lecithin plus GS is superior to that of single GS, which may be due to its significant effect on the degree of connectivity of lecithin molecules.
Hydrogenated lecithin-oleogels, on the other hand, showed a negative influence of hydrogenated lecithin content on water-filled oleogel hardness (Fig. 5B). Increasing the hydrogenated lecithin concentration resulted in a decrease in oleogel hardness, which might be attributed to the hydrophobic nature of hydrogenated lecithin. Water-filled oleogels had a higher oscillation in the penetration force–distance profiles, which was associated with a more brittle structure than the corresponding oleogels. This might be owing to the physical entrapment of water droplets within the oleogel structure. Water might physically interfere with the structure of the 3D network produced by GS and hydrogenated lecithin, but chemical interference has to be investigated further using other techniques.
The addition of βC to water-filled oleogels typically decreased hardness, except for βC-W-GS-HL (2.5%), which enhanced hardness (Fig. 3D and 5D). In this regard, βC-W-GS oleogel exhibited a lower value of 2.09 ± 0.16 N when compared to its counterpart. Similarly, βC-W-GS-L (0.5%), βC-W-GS-L (2.5%), and βC-W-GS-HL (0.5%), showed the reduced maximum hardness values of 0.65 ± 0.02 N, 0.25 ± 0.01 N, and 1.45 ± 0.00 N, respectively. However, βC-W-GS-HL (2.5%) oleogel had a higher maximum hardness value (i.e., 2.43 ± 0.13 N) than its counterpart. The performance of βC in the presence of water may be explained by its high hydrophobicity, which prevents water molecules from interfering with the crystal structure. Cirkel et al.40 also demonstrated that a trace quantity of βC in the presence of water (W0 = 1.5) had a negative influence on the viscosity of isooctane-based microemulsions.
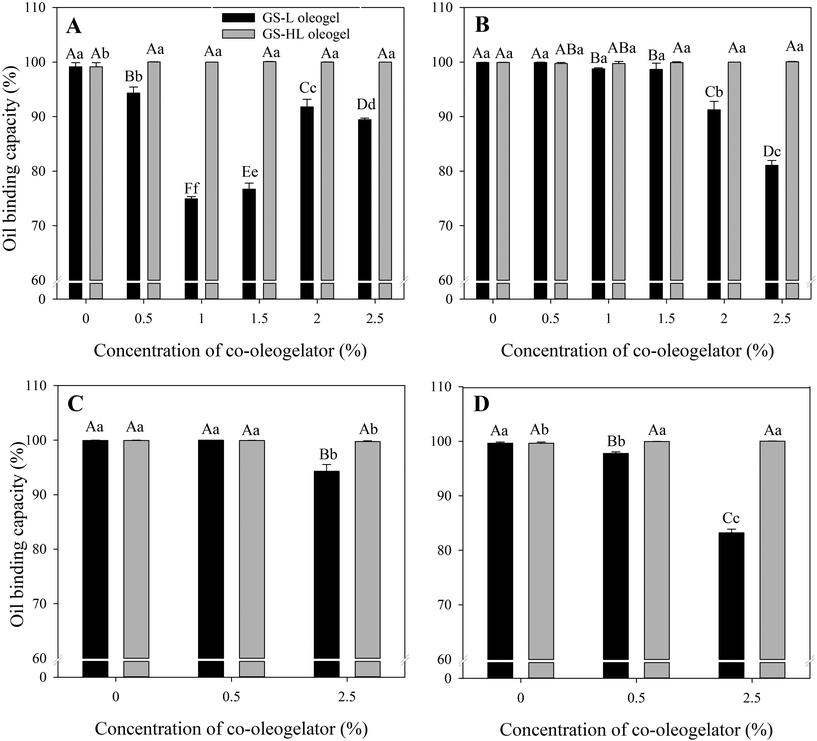
3.4. Oil binding capacity
OBC indicates oleogel's ability to retain oil.41 Overall, hydrogenated lecithin-containing oleogels had the highest OBC, followed by sole GS-oleogel, while lecithin-containing oleogels had the lowest OBC (Fig. 6 and Table S5†). The percentage of OBCs ranged from 74.91 ± 0.40% to 100.06 ± 0.04%, which was comparable to fat systems evaluated by Tavernier et al.42 (ranged from ≈70% to 97%), Blach et al.43 (ranged from ≈85% to 100%), Okuro et al.9 (ranged from 35% to 98%), Yang et al.28 (ranged from ≈85% to 96%), and Gaudino et al.2 (ranged from 40% to 100%).On the basis of OBC, lecithin-containing oleogels were the weakest (Fig. 6A), with OBC of GS-lecithin (1%), GS-lecithin (1.5%), and GS-lecithin (2.5%) having less than 90% OBC and therefore being considered as weak oleogels,43 whereas GS-lecithin (0.5%) and GS-lecithin (2%) oleogels expelled less oil. It is clear that the decline in OBC percentage was not proportional to the lecithin content. However, the results showed that lecithin had a detrimental effect on the OBC of oleogels, in contrast to Okuro et al.,9 who found that increasing lecithin concentration had a beneficial effect on OBC. The improved OBC was due to hydrogen bonding between lecithin and fruit wax (i.e., oleogelators), which boosted the strength and oil retention of the oleogel. As a result, it is possible to assume that lecithin has a favorable effect on oleogel strength and hence OBC when utilized as a co-oleogelator in systems where the main oleogelator does not construct the 3D network via hydrogen bonding. By promoting hydrogen bonding,9 lecithin strengthens the network of the resulting oleogel. In contrast, when lecithin is introduced to an oleogel system in which the main oleogelator (i.e., GS or stearic acid2) is capable of forming a robust network via hydrogen bonding, the detrimental effect of lecithin may be observed through interference with the hydrogen bonding of the building blocks.
Hydrogenated lecithin-containing oleogels efficiently held oil inside their matrix, which was consistent with their superior hardness (Fig. 3). It has also been reported that OBC is related with crystal size, i.e., when the crystal size decreases, the rate at which oil is released from the matrix reduces.30 The crystals in hydrogenated lecithin-containing oleogels were smaller and densely packed than those in lecithin-containing oleogels (Fig. 2), providing greater surface area for oil molecules to bind to. Simultaneously, stronger OBC could be obtained because hydrogenated lecithin-containing oleogels had tightly packed crystals with less void spaces for unbound oil to accumulate. The liquid corn oil was more strongly bound to the solid interfaces in hydrogenated lecithin-containing oleogels, but it was less bound (more physically entrapped) in lecithin-containing oleogels, resulting in a faster exudation from the matrix.
Regardless of water content, all hydrogenated lecithin-containing oleogels exhibited a maximum OBC, indicating that hydrogenated lecithin had a dominant effect on oil retention (Fig. 6B). As a result, hydrogenated lecithin-oleogels can be considered as firm gels with OBC more than 99%.43
In comparison to oleogels without βC, OBC values of βC-W-GS (99.65 ± 0.22%), βC-W-GS-L (0.5%) (97.78 ± 0.25%), and βC-W-GS-HL (2.5%) (100.03 ± 0.02%) increased, but βC-W-GS-L (2.5%) (83.22 ± 0.64%), and βC-W-GS-HL (0.5%) (99.96 ± 0.01%) decreased. Similar to water-free oleogels, the OBC gradually reduced as the lecithin content increased with the addition of βC. Enriching with βC, on the other hand, improved OBC by increasing hydrogenated lecithin content whenever water was added to hydrogenated lecithin-containing oleogels.
The result was highly correlated with the textural properties of the oleogels, see Fig. 3. The effect of βC on texture, which in turn affects OBC, is described by Martins et al.,18 who found that βC was effective in increasing the OBC of an oleogel. The authors attributed this to the fact that βC reduces the spacing between crystal arrangements, resulting in a more structured gel. In contrast, Ramírez-Carrasco et al.41 found that curcumin at varying concentrations had no effect on the OBC of oleogels and concluded that curcumin did not alter the structure of the crystal network.
4. Conclusions
The present study revealed the relationship between the microstructural characteristics of corn-oil oleogels and their physical and functional properties as affected by the oleogel composition. The addition of lecithin to the glyceryl stearate (GS)-corn oil-oleogels, rendered lipid materials with lower melting temperatures and enthalpies, which suggests the formation of loose structures. This was confirmed by optical microscopy, where the addition of lecithin to the oleogels led to the formation of less close-packed crystals thus with more open spaces. This was translated to oleogels with a dramatically softer texture, as well as with a reduced oil binding capacity (OBC) compared to the oleogels formulated only with GS. Oppositely, adding hydrogenated lecithin to the GS-corn oil formulation led to the formation of lipid materials with a compact and ordered crystalline structure as observed by elevated melting temperatures. Also, they presented a microstructure with a denser crystalline structure and with low void spaces. This explained that the hydrogenated lecithin-containing oleogels presented hard gels, with boosted OBC compared to the lecithin-containing ones. In general, water-filled oleogels presented harder gels compared to their counterparts without the addition of water. Similarly, the addition of β-carotene (βC) as an exemplary lipophilic bioactive compound within the oleogel structure had a significant impact on their physical properties, rendering harder gels in the case of the lecithin-containing ones and softer gels in the case of the hydrogenated lecithin-containing ones. Hence, βC plays a role as oleogelation modifier.In summary, using different strategies to formulate oloegels might greatly impact their microstructural properties, which in turn determine their physical and functional characteristics. Using this model, it is possible to tailor oleogels with desired physical properties by adding co-oleogelators (e.g., lecithin, hydrogenated lecithin), enriching them with bioactives, or decreasing the oil content in water-filled oleogels. However, the nutritional properties of these tailored oleogels have to be further explored.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This project has received funding from the MCIU, AEI; FEDER, UE [grant number RTI2018-094268-B-C21]; and the European Union's H2020 research and innovation programme under Marie Sklodowska-Curie [grant agreement No 801586].References
- E. D. Co and A. G. Marangoni, Oleogels: An Introduction, Elsevier Inc., 2nd edn, 2018 Search PubMed.
- N. Gaudino, S. M. Ghazani, S. Clark, A. G. Marangoni and N. C. Acevedo, Food Res. Int., 2019, 116, 79–89 CrossRef CAS PubMed.
- A. C. Ferro, C. de Souza Paglarini, M. A. Rodrigues Pollonio and R. Lopes Cunha, Meat Sci., 2021, 174, 108424 CrossRef CAS PubMed.
- F. C. Wang, A. J. Gravelle, A. I. Blake and A. G. Marangoni, Curr. Opin. Food Sci., 2016, 7, 27–34 CrossRef.
- A. C. Ferro, P. K. Okuro, A. P. Badan and R. L. Cunha, Food Res. Int., 2019, 120, 610–619 CrossRef PubMed.
- M. Cui, L. Mao, Y. Lu, F. Yuan and Y. Gao, LWT–Food Sci. Technol., 2019, 106, 83–91 CrossRef.
- A. P. B. Ribeiro, M. H. Masuchi, E. K. Miyasaki, M. A. F. Domingues, V. L. Z. Stroppa, G. M. de Oliveira and T. G. Kieckbusch, J. Food Sci. Technol., 2015, 52, 3925–3946 CrossRef PubMed.
- M. Davidovich-Pinhas, S. Barbut and A. G. Marangoni, Carbohydr. Polym., 2015, 127, 355–362 CrossRef PubMed.
- P. K. Okuro, I. Tavernier, M. D. Bin Sintang, A. G. Skirtach, A. A. Vicente, K. Dewettinck and R. L. Cunha, Food Funct., 2018, 9, 1755–1767 RSC.
- R. Scartazzini and P. L. Luisi, J. Phys. Chem., 1988, 92, 829–833 CrossRef.
- M. Martínez-Ávila, A. De la Peña-Gil, F. M. Álvarez-Mitre, M. A. Charó-Alonso and J. F. Toro-Vazquez, JAOCS, J. Am. Oil Chem. Soc., 2019, 96, 273–289 CrossRef.
- A. Kafle, M. Akamatsu, A. Bhadani, K. Sakai, K. Sakai, C. Kaise, C. Kaise, T. Kaneko, T. Kaneko, H. Sakai and H. Sakai, Langmuir, 2020, 36, 6025–6032 CrossRef PubMed.
- K. Tai, M. Rappolt, L. Mao, Y. Gao and F. Yuan, Food Chem., 2020, 326, 126973 CrossRef PubMed.
- T. L. T. da Silva and S. Danthine, Food Biophys., 2022, 17, 361–374 CrossRef.
- K. Larsson, P. Quinn, K. Sato and F. Tiberg, Lipids: Structure, physical properties and functionality, 2006 Search PubMed.
- K. Wijarnprecha, A. de Vries, S. Sonwai and D. Rousseau, Front. Sustain. Food Syst., 2021, 4, 1–6 Search PubMed.
- Y. Fan, L. Gao, J. Yi, Y. Zhang and W. Yokoyama, J. Agric. Food Chem., 2017, 65, 6188–6194 CrossRef PubMed.
- A. J. Martins, M. A. Cerqueira, R. L. Cunha and A. A. Vicente, Food Funct., 2017, 8, 4241–4250 RSC.
- A. Molet-Rodríguez, A. Torcello-Gómez, L. Salvia-Trujillo, O. Martín-Belloso and A. R. Mackie, Food Hydrocolloids, 2023, 135, 108121 CrossRef.
- S. Y. Ghan, L. F. Siow, C. P. Tan, K. W. Cheong and Y. Y. Thoo, Gels, 2022, 8, 30 CrossRef.
- C. P. Tan and Y. B. Che Man, JAOCS, J. Am. Oil Chem. Soc., 2000, 77, 143–155 CrossRef.
- J. F. Toro-Vazquez, R. Mauricio-Pérez, M. M. González-Chávez, M. Sánchez-Becerril, J. de J. Ornelas-Paz and J. D. Pérez-Martínez, Food Res. Int., 2013, 54, 1360–1368 CrossRef.
- A. López-Martínez, J. A. Morales-Rueda, E. Dibildox-Alvarado, M. A. Charó-Alonso, A. G. Marangoni and J. F. Toro-Vazquez, Food Res. Int., 2014, 64, 946–957 CrossRef PubMed.
- K. Wijarnprecha, A. de Vries, P. Santiwattana, S. Sonwai and D. Rousseau, LWT–Food Sci. Technol., 2019, 115, 108058 CrossRef.
- J. Li, R. Guo, Y. Bi, H. Zhang and X. Xu, LWT–Food Sci. Technol., 2021, 151, 112061 CrossRef.
- B. Hu, Q. Zheng, Z. Weng, J. Xiao, Y. Cao and Y. Lan, Food Chem., 2022, 389, 133123 CrossRef PubMed.
- P. Terech, D. Pasquier, V. Bordas and C. Rossat, Langmuir, 2000, 16, 4485–4494 CrossRef.
- S. Yang, M. Zhu, N. Wang, X. Cui, Q. Xu, A. S. M. Saleh, Y. Duan and Z. Xiao, Food Biophys., 2018, 13, 362–373 CrossRef.
- E. Kesselman and E. Shimoni, Food Biophys., 2007, 2, 117–123 CrossRef.
- A. I. Blake, E. D. Co and A. G. Marangoni, JAOCS, J. Am. Oil Chem. Soc., 2014, 91, 885–903 CrossRef.
- C. H. Chen, I. Van Damme and E. M. Terentjev, Soft Matter, 2009, 5, 432–439 RSC.
- A. J. Gravelle, M. Davidovich-Pinhas, S. Barbut and A. G. Marangoni, Food Res. Int., 2017, 91, 1–10 CrossRef.
- M. Aguilar-Zárate, B. A. Macias-Rodriguez, J. F. Toro-Vazquez and A. G. Marangoni, Carbohydr. Polym., 2019, 205, 98–105 CrossRef.
- T. Tamura and M. Ichikawa, JAOCS, J. Am. Oil Chem. Soc., 1997, 74, 491–495 CrossRef.
- I. Lončarević, B. Pajin, R. Omorjan, A. Torbica, D. Zarić, J. Maksimović and J. Švarc Gajić, J. Texture Stud., 2013, 44, 450–458 CrossRef.
- M. Pernetti, K. van Malssen, D. Kalnin and E. Flöter, Food Hydrocolloids, 2007, 21, 855–861 CrossRef.
- R. Gupta, H. S. Muralidhara and H. T. Davis, Langmuir, 2001, 17, 5176–5183 CrossRef CAS.
- S. A. Mezzasalma, G. J. M. Koper and Y. A. Shchipunov, Langmuir, 2000, 16, 10564–10565 CrossRef CAS.
- S. Guo, M. Song, X. He, F. Yang, Y. Cao, M. Rogers and Y. Lan, Food Funct., 2019, 10, 3923–3933 RSC.
- P. A. Cirkel, M. Fontana and G. J. M. Koper, Langmuir, 1999, 15, 3026–3028 CrossRef CAS.
- P. Ramírez-Carrasco, J. Paredes-Toledo, P. Romero-Hasler, E. Soto-Bustamante, P. Díaz-Calderón, P. Robert and B. Giménez, Antioxidants, 2020, 9, 1–18 CrossRef.
- I. Tavernier, C. D. Doan, P. Van der Meeren, B. Heyman and K. Dewettinck, Eur. J. Lipid Sci. Technol., 2018, 120, 1–13 CrossRef.
- C. Blach, A. J. Gravelle, F. Peyronel, J. Weiss, S. Barbut and A. G. Marangoni, RSC Adv., 2016, 6, 81151–81163 RSC.
- S. da Pieve, S. Calligaris, E. Co, M. C. Nicoli and A. G. Marangoni, Food Biophys., 2010, 5, 211–217 CrossRef.
- T. L. T. da Silva, D. B. Arellano and S. Martini, Food Biophys., 2019, 14, 30–40 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo03491g |
| This journal is © The Royal Society of Chemistry 2024 |