Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Combination of Lacticaseibacillus paracasei BEPC22 and Lactiplantibacillus plantarum BELP53 attenuates fat accumulation and alters the metabolome and gut microbiota in mice with high-fat diet-induced obesity†
Na-Rae
Lee‡
 a,
Tae-Jun
Kwon‡
b,
Eui-Chun
Chung‡
c,
Jaewoong
Bae‡
c,
Song-Hui
Soung
d,
Hyun-Ji
Tak
d,
Jun-Young
Choi
b,
Young-Eun
Lee
e,
Nak
Won Hwang
c,
Jong Seo
Lee
c,
Kum-Joo
Shin
c,
Choong Hwan
Lee
ad,
KilSoo
Kim
*bf and
Seokjin
Kim
a,
Tae-Jun
Kwon‡
b,
Eui-Chun
Chung‡
c,
Jaewoong
Bae‡
c,
Song-Hui
Soung
d,
Hyun-Ji
Tak
d,
Jun-Young
Choi
b,
Young-Eun
Lee
e,
Nak
Won Hwang
c,
Jong Seo
Lee
c,
Kum-Joo
Shin
c,
Choong Hwan
Lee
ad,
KilSoo
Kim
*bf and
Seokjin
Kim
 *c
*c
aResearch Institute for Bioactive-Metabolome Network, Konkuk University, Seoul 05029, Republic of Korea
bPreclinical Research Center, Daegu-Gyeongbuk Medical Innovation Foundation (K-MEDI Hub), Daegu 41061, Republic of Korea. E-mail: kslac@kmedihub.re.kr
cR&D Center, Hecto Healthcare Co., Ltd, Seoul 06142, Republic of Korea. E-mail: jinkim@hetco.co.kr
dDepartment of Bioscience and Biotechnology, Konkuk University, Seoul 05209, Republic of Korea
eCognitive Science Research Group, Korea Brain Research Institute, Daegu 41062, Republic of Korea
fCollege of Veterinary Medicine, Kyungpook National University, 80 Daehakro, Buk-gu, Daegu 41566, Korea
First published on 15th December 2023
Abstract
This study evaluated the effects of formulations with Lacticaseibacillus paracasei BEPC22 and Lactiplantibacillus plantarum BELP53 on adiposity, the alteration of microbiota, and the metabolome in high-fat diet-fed mice. The strains were selected based on their fat and glucose absorption inhibitory activities and potential metabolic interactions. The optimal ratio of the two strains in the probiotic formulation was determined based on their adipocyte differentiation inhibitory activities. Treatment of formulations with BEPC22 and BELP53 for 10 weeks decreased body weight gain at 6 weeks; it also decreased the food efficiency ratio, white adipose tissue volume, and adipocyte size. Moreover, it decreased the expression of the lipogenic gene Ppar-γ in the liver, while significantly increasing the expression of the fat oxidation gene Ppar-α in the white adipose tissue. Notably, treatment with a combination of the two strains significantly reduced the plasma levels of the obesity hormone leptin and altered the microbiota and metabolome. The omics data also indicated the alteration of anti-obesity microbes and metabolites such as Akkermansia and indolelactic acid, respectively. These findings suggest that treatment with a combination of BEPC22 and BELP53 exerts synergistic beneficial effects against obesity.
Introduction
Obesity is a major global health issue that is associated with several diseases, including diabetes, and some types of cancer.1 Hormonal and medical treatments for obesity may lack long-term sustainability in terms of their effectiveness and potential adverse effects.2 Therefore, numerous research groups have endeavoured to develop effective anti-obesity treatments with few side effects.Probiotics are known to confer health benefits without adverse side effects when consumed in adequate amounts. Some probiotics exhibit anti-obesity effects by regulating glucose and lipid metabolism.3 In particular, Lactobacillus species (e.g. L. rhamnosus, L. brevis, L. plantarum, and L. paracasei) lower lipid levels, reduce body weight, and increase glucose and lipid metabolism by modulating the production of hormones and expression of genes related to obesity and inhibiting adipogenesis.4 Other anti-obesity mechanisms of probiotics include the augmentation of short-chain fatty acid production and modulation of bile acid metabolism.5 However, such functions of probiotics are strain specific; thus, combining multiple strains may result in symbiotic and synergistic effects.6 Many probiotics are composed of multiple strains because they are more effective than those composed of individual strains.2,7
The gut microbiota plays diverse roles in obesity, from influencing lipid metabolism to modulating appetite.8–11 The composition and biodiversity of the gut microbiome significantly differ between individuals with and without obesity.12–14 The Firmicutes/Bacteroidetes (F/B) ratio, which is positively correlated with the BMI, is high in patients with obesity, but treatment with Lacticaseibacillus probiotics can decrease the F/B ratio, as well as the lipoprotein cholesterol levels and body weight.15,16 The gut microbiome plays a vital role in obesity and is linked to alterations in constituent metabolites during obesity progression.17
Therefore, in this study, we paired two strains selected through in vitro assays and metabolic characterisation to maximise their anti-obesity effects. Then, we evaluated the anti-obesity activity of the probiotic pair in a high-fat diet (HFD)-induced obese mouse model and analysed its effect on the microbiome and metabolome.
Materials and methods
Isolation and identification of lactic acid bacteria (LAB)
This study was approved by the Public Institutional Review Board designated by the Ministry of Health and Welfare (IRB number: P01-201712-33-002). Human-derived sampling was conducted in accordance with the protocols approved by the Korea's Bioethics and Safety Act no. 16372. All subjects provided written informed consent. Samples of healthy lean adult and infant faeces, raw milk, and kimchi were collected to isolate LAB strains. The samples were mixed with 0.85% NaCl saline for 10 min and then streaked onto De Man–Rogosa–Sharpe (MRS) (Difco-BD, Sparks, MD, USA) agar. The agar plates were incubated anaerobically at 37 °C for 48–72 h. For identification, representative colonies were isolated from plates and inoculated into fresh MRS broth. The isolates were characterised through Gram staining and then subjected to biochemical identification using an API 50 CHL kit (BioMérieux, Marcy, Lyon, France) and 16S ribosomal RNA (rRNA) gene sequencing. For the latter, DNA was extracted using a commercial G-spin kit from each strain (Intron, Gyeonggi, Korea), and the genes were amplified by PCR using the following universal primers: 27F (5′-GAGTTGGATCCTGGCTCAG-3′) and 1492R (5′-AAGGAGGGGATCCAGCC-3′). The isolates were identified through 16S rRNA gene sequence homology analysis using the GenBank database (NCBI, Bethesda, MD, USA).Bacterial strains
Four initially selected strains, Lacticaseibacillus paracasei BEPC22 (BEPC22), Lactiplantibacillus plantarum BELP53 (BELP53), Lactobacillus helveticus BELH04 (BELH04), and Limosilactobacillus fermentum BELF01 (BELF01), were isolated from Korean faeces. The patents deposited in the Korean Collection for Type Cultures (KCTC) are as follows: Lacticaseibacillus paracasei BCC-LP-02 (KCTC 14808BP), Lactiplantibacillus plantarum BCC-LPL-53 (KCTC 14809BP), Lactobacillus helveticus BCC-LH-04 (KCTC 148010BP), and Limosilactobacillus fermentum BCC-LF-01 (KCTC14461BP). The NCBI GenBank accession numbers for these sequences are OL988622, OL988624, OL988623, and OL988625, respectively.Fatty acid absorption assay
Each strain was activated anaerobically in an MRS medium at 37 °C for 18 h. Subsequently, 1% of the culture was inoculated in MRS medium containing 0.5% (w/v) Brij 58 (Sigma-Aldrich, St Louis, MO, USA) and 0.25 mmol L−1 sodium palmitate (Tokyo Chemical Industry Co., Ltd, Tokyo, Kanagawa, Japan) and then incubated at 37 °C for 24 h. The concentrations of fatty acids in the culture media were measured using the EnzyChrom™ free fatty acid assay kit (Bio-Assay Systems, Hayward, CA, USA). The remaining palmitic acid (standard) in the culture medium was quantified to select fatty acid-absorbing bacteria.Metabolite analyses of the bacterial culture medium
Four strains (BELP53, BEPC22, BELF01, and BELH04) with potential anti-obesity effects were anaerobically cultured in MRS medium at 37 °C for 20 h. The growth of each strain was measured by obtaining the absorbance at 600 nm, and 1 mL of the supernatant was collected at different cell growth phases, including starting, mid-exponential, late-exponential, early stationary, and stationary, for metabolite analyses.The filtered supernatant (500 μL) was dried and then dissolved in 100% methanol to obtain a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm solution. The derivatisation for gas chromatography time-of-flight mass spectrometry (GC-TOF-MS) was conducted as follows: (1) incubation for 90 min at 30 °C after adding 50 μL of 20 mg mL−1 methoxyamine hydrochloride in pyridine and (2) incubation for 30 min at 37 °C after adding 50 μL of N-Methyl-N-(trimethylsilyl)trifluoroacetamide (Sigma-Aldrich). For instrumental analysis, an Agilent 7890A GC system (Agilent Technologies, Santa Clara, CA, USA) coupled with an Agilent 7693 auto-sampler and a Pegasus® HT TOF-MS (LECO Corp., St Joseph, MI, USA) was utilised. An Rtx-5MS (J&W Scientific, Folsom, CA, USA) column was used. The GC-TOF-MS analysis settings were modified based on previous studies.18,19 Each sample was analysed in triplicate.
000 ppm solution. The derivatisation for gas chromatography time-of-flight mass spectrometry (GC-TOF-MS) was conducted as follows: (1) incubation for 90 min at 30 °C after adding 50 μL of 20 mg mL−1 methoxyamine hydrochloride in pyridine and (2) incubation for 30 min at 37 °C after adding 50 μL of N-Methyl-N-(trimethylsilyl)trifluoroacetamide (Sigma-Aldrich). For instrumental analysis, an Agilent 7890A GC system (Agilent Technologies, Santa Clara, CA, USA) coupled with an Agilent 7693 auto-sampler and a Pegasus® HT TOF-MS (LECO Corp., St Joseph, MI, USA) was utilised. An Rtx-5MS (J&W Scientific, Folsom, CA, USA) column was used. The GC-TOF-MS analysis settings were modified based on previous studies.18,19 Each sample was analysed in triplicate.
Glucose uptake and pancreatic lipase activity assays
Each strain was co-cultured with Caco-2 cells obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) for glucose uptake analysis. Caco-2 cells were cultured in a 24-well plate for 15 days until a 3D cell surface layer was formed, with monitoring of the changes in the media every 2 days. LAB (1.0 × 107 CFU−1 mL) were co-cultured with Caco-2 cells for 6 h, and then the bacteria and medium were removed. Intracellular glucose concentrations in Caco-2 cells were measured using a Glucose Uptake-Glo Assay Kit (Promega, Madison, WI, USA). All experiments were independently performed in triplicate.Pancreatic lipase activity was examined following the method described by Han et al.20 Subsequently, 100 μL of the bacterial suspension in TES buffer and 50 μL of pancreatic lipase (10 units) were mixed with 100 μL of fat emulsion, and then the mixture was incubated at 37 °C for 30 min. The reaction was terminated by incubation in boiling water (100 °C) for at least 2 min. The blank sample was heated immediately after the addition of lipase. The amount of fatty acids in the mixture was measured using the EnzyChrom™ free fatty acid assay kit (Bio-Assay Systems).
Adipocyte differentiation assay
The selected strains were cultured as previously described for 18 h, sterilised at 121 °C, and then freeze dried. Adipocyte differentiation analysis was performed as previously described by Song et al.21 3T3-L1 pre-adipocytes (ATCC) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 0.5 mmol L−1 3-isobutyl-1-methylxanthine (Sigma-Aldrich) and 1 mol L−1 dexamethasone (Sigma-Aldrich) for 2 days. Then, the medium was replaced with DMEM containing 10% FBS and 1 g mL−1 insulin (Sigma-Aldrich) with or without lyophilised powder (1 mg mL−1) of the four LAB. After 4 days of treatment with the differentiation medium, fresh DMEM containing 10% FBS was added, and the medium was replaced every 2 days. The differentiated 3T3-L1 cells were fixed in 10% formaldehyde, and then stained with Oil Red O solution. After treating the cells with isopropanol, the lipid content was measured by obtaining the absorbance at 510 nm.Animal and experimental design
Specific pathogen-free male C57BL/6J mice (6-weeks old, 20–25 g, n = 30) (Charles River) were allowed ad libitum access to food and water. The animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Daegu-Gyeongbuk Medical Innovation Foundation (DGMIF) (approval no. DGMIF-21092702-00). All efforts were made to minimise pain, and the mice were euthanised in accordance with the endpoint criteria of an IACUC-approved protocol. All animal experiments were conducted in accordance with Korea's Animal Protection Act no. 18853 and Laboratory Animal Act no. 18969.The mice were randomly assigned to one of the following five groups (n = 6 per group): (1) normal diet (ND), (2) high fat diet (HFD), (3) HFD group treated with BEPC22 (HFDPC), (4) HFD group treated with BELP53 (HFDLP), and (5) HFD group treated with the formulation (HFDF). The mice in HFDPC and HFDLP groups were orally administered with 2 × 109 CFU per 200 μL of BEPC22 and BELP53, respectively, daily for 10 weeks. In the HFDF group, mice were orally administered with 2 × 109 CFU per 200 μL of BEPC22 and BELP53 at a ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The mice in the ND group were fed a standard diet (SAFE A40, SAFE Inc., Augy, France) containing 3.2% fat, whereas those in the HFD group were freely provided 60 kcal% fat rodent diet (D12492, Research Diets Inc., New Brunswick, NJ, USA) containing 60% fat, 20% protein, and 20% carbohydrates.
4. The mice in the ND group were fed a standard diet (SAFE A40, SAFE Inc., Augy, France) containing 3.2% fat, whereas those in the HFD group were freely provided 60 kcal% fat rodent diet (D12492, Research Diets Inc., New Brunswick, NJ, USA) containing 60% fat, 20% protein, and 20% carbohydrates.
The body weight and food intake of the mice were measured twice a week. The food efficiency ratio was calculated as the total body weight gained from the diet divided by the total diet consumed during the experiments.
After 10 weeks of treatment, the liver and white adipose tissues (epididymal, mesenteric, perirenal, and subcutaneous) were dissected and weighed, and then frozen in liquid nitrogen for qRT-PCR. The epididymal adipose tissue was fixed in 10% neutral buffered formalin (BBC Biochemical, Mount Vernon, WA, USA) for histological analysis. Blood samples were collected from the abdominal aortas of the mice under isoflurane (Hana Pharm, Co., Ltd, Seoul, Korea) anaesthesia and then centrifuged for 3 min at 8000g for plasma separation. Faecal samples were obtained from each of the 30 mice directly into sterile 1.5 mL Eppendorf tubes and stored at−80 °C until use.
Plasma biochemical analysis
Plasma levels of GIP, GLP-1, glucagon, insulin, leptin, PAI-1, and resistin were measured using a Bio-plex Pro Mouse Diabetes 8-plex Assay Kit (Bio-Rad Laboratories, Hercules, CA, USA). Adiponectin levels were measured using an adiponectin ELISA kit (Thermo Fisher Scientific, Waltham, MA, USA) in accordance with the manufacturer's instructions.Histological analysis
For histological analysis, epididymal adipose tissue was prepared using a tissue processor (Thermo Fisher Scientific). Sections (4 μm) were obtained from paraffin-embedded blocks and then stained with haematoxylin and eosin using an autostainer (Dako Coverstainer, Agilent, Santa Clara, CA, USA). Adipocyte size was measured using an IX83 microscope (Olympus Corporation, Tokyo, Kanagawa, Japan).Transcriptional analysis
Total RNA was extracted from the liver and epididymal adipose tissues by using a RNeasy Mini Kit and RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Nordrhein-Westfalen, Germany), respectively, in accordance with the manufacturer's instructions. The extracted RNA was reverse-transcribed using a SuperScript IV First-Strand Synthesis Kit (Thermo Fisher Scientific). Quantitative PCR (qPCR) was performed using an Exicycler 384 qPCR system (Bioneer, Daejeon, Korea) with the AccuPower 2X GreenStar qPCR Master Mix (Bioneer). The relative expression levels of the target genes (Ppar-α, Ppar-γ, Cpt1a, and Ucp2) were calculated using the 2−ΔΔCT method. Gapdh was used as the internal reference gene.Metabolite extraction
Metabolites were extracted from the plasma as follows. Mouse plasma (100 μL) was added with methanol (500 μL) containing 10 ppm of 2-chlorophenylalanine as an internal standard. The mixture was homogenised using a Retsch MM400 Mixer Mill (Retsch GmbH & Co., Haan, Nordrhein-Westfalen, Germany) at 30 s−1 for 10 min and then sonicated for 10 min (Hettich Zentrifugen Universal 320, Tuttlingen, Baden-württemberg, Germany). The samples were allowed to settle at −20 °C for 1 h. Subsequently, the mixtures were centrifuged at 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min at 4 °C.
000g for 10 min at 4 °C.
Metabolites were extracted from the faeces as follows. Faecal samples (100 mg) were added with 1 mL of methanol (containing the same amount of the internal standard). Subsequent procedures were the same as those used in plasma extraction but without resting in a freezer.
The supernatants were filtered into new Eppendorf tubes using Millex GP 0.22 μm filters (Merck Millipore, Billerica, MA, USA) and dried utilising a speed vacuum concentrator (Biotron, Seoul, Korea). The dried samples were re-dissolved in 100% methanol to obtain a final concentration of 5000 ppm.
Metabolome analysis
For GC-TOF-MS analysis, 100 μL of the re-dissolved samples was dried again using a speed vacuum concentrator. The dried samples were derivatised as described earlier. The analytical parameters were adapted from a previous study.19Ultra-high-performance liquid chromatography-linear trap quadrupole-orbitrap-tandem mass spectrometry (UHPLC-LTQ-Orbitrap-ESI-MS/MS) was performed using a Vanish binary pump H system (Thermo Fisher Scientific) coupled with an autosampler and column compartments. A Phenomenex KINETEX® C18 column (100 mm × 2.1 mm, 1.7 μm particle size; Torrance, CA, USA) was used to separate the sample analytes. The injection volume was 5 μL. The column temperature was maintained at 40 °C, and the flow rate was 0.3 mL min−1. The MS data were collected at 100–1500 m/z in negative and positive ion modes by using an Orbitrap Velos Pro™ system combined with an ion-trap mass spectrometer (Thermo Fisher Scientific) coupled with an HESI-II probe. The analytical parameters were adopted from a previous study.18
Raw data obtained from GC-TOF-MS and UHPLC-LTQ-Orbitrap-ESI-MS/MS were converted to NetCDF (*.cdf). Subsequently, the NetCDF files were processed using MetAlign software (https://www.metalign.nl, Wageningen, Netherlands) for data alignment in accordance with peak mass (m/z) and retention time (min).28 Metabolites were tentatively identified by comparison with various analysis data, such as molecular weight, formula, retention time, mass fragment patterns, and mass spectra of the standard compounds; previous publications; an in-house library; and commercial databases, such as the National Institutes of Standards and Technology Library (version 2.3, FairCom, Gaithersburg, MD, USA) and the Human Metabolome Database (https://www.hmdb.ca/ (accessed on Sep 2022)).
Extraction of genomic DNA from mouse faecal samples
Total genomic DNA was extracted from 200 mg of faecal samples using a Maxwell® RSC PureFood GMO and Authentication Kit (Promega) following the manufacturer's instructions. The concentration and quantity of DNA were determined using a UV-vis spectrophotometer (NanoDrop 2000c, Wilmington, DE, USA) and a QuantiFluor® ONE dsDNA System (Promega), respectively.Gut microbiota analysis using next-generation sequencing
The gut microbiota composition of the mice was analysed through 16S rRNA amplicon sequencing using an Illumina MiSeq platform (Illumina, San Diego, CA, USA) in accordance with the manufacturer's instructions. The V3–V4 region of the bacterial 16S rRNA gene was amplified using the primer set F319 and R806.The gut microbiota data were analysed using the QIIME 2 2022.02 pipeline.22 Paired-end sequence data were demultiplexed using MiSeq Reporter and joined using the 12-vsearch plugin. Merged sequences were filtered using the q2-quality-filter plugin and then denoised with Deblur (via q2-deblur).23 All amplicon sequence variants (ASVs) were aligned with mafft (via q2-alignment).24 Alpha–diversity metrices (Shannon and Simpson diversity), beta-diversity metrics (Bray–Curtis dissimilarity), and principal coordinate analysis (PCoA) were estimated using q2-diversity after the samples were rarefied (subsampled without replacement).25–27 Taxonomy was assigned to ASVs using the q2-feature–classifier classify–sklearn naïve Bayes taxonomy classifier against the SILVA database v138.28,29 Significant differences in the bacterial structure and taxonomy were determined using permutational multivariate analysis of variance and linear discriminant analysis (LDA) effect size (LEfSe) analysis (LDA score > 3.0), respectively.30,31 The correlation between variables was assessed using the ‘cor’ function of R, and the Spearman correlation coefficient was calculated using the ‘psych’ (v2.2.9) package to assess the correlation strength. The Benjamini–Hochberg correction was conducted to account for multiple testing. Heatmaps were generated using the ‘pheatmap’ (1.0.12) package to visualise the correlation patterns between variables.
Statistical analysis
Statistical analysis of in vivo data was performed using Prism 9 software (GraphPad Software Inc., San Diego, CA, USA). For comparisons of more than two groups, a one-way or two-way ANOVA was performed, followed by a post hoc test with Dunnett's correction for pairwise group differences. All data are expressed as the mean ± SD (standard deviation) or standard error of the mean (SEM), and p < 0.05 was considered statistically significant.Multivariate statistical analysis of the metabolome data was conducted using SIMCA-P + software (version 15.0.2, Umetrics, Umea, Sweden). Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were conducted to compare the metabolites among the samples. The discriminant metabolites were selected based on the variable importance in the projection (VIP) value > 0.7 and p-value < 0.05.
Results
Fatty acid absorption by Lactobacillus strains
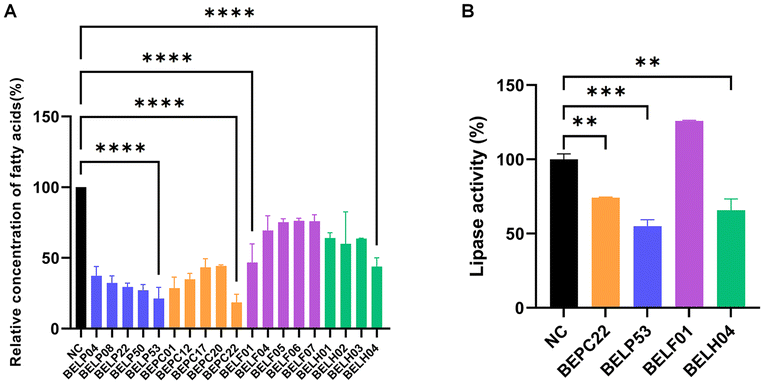
The fatty acid absorption capacities of more than 300 strains of Lactobacillus, Lactiplantibacillus, Lacticaseibacillus, Limosilactobacillus, Ligilactobacillus, Lactococcus, and Bifidobacterium species were evaluated by culturing them in MRS broth containing 0.25 mmol L−1 sodium palmitate for 24 h and measuring the fatty acid concentration in the culture broth. Interestingly, Lactobacillus spp. showed a higher free fatty acid (FFA) absorption capacity than Lactococcus and Bifidobacterium spp. (data not shown). Strains of Lactiplantibacillus plantarum (LPL), Lacticaseibacillus paracasei (LP), Limosilactobacillus fermentum (LF), and Lactobacillus helveticus (LH) showed notable decreases in total fatty acids in the medium (Fig. 1A). Specifically, LP and LPL strains absorbed 83.9%–86.6% (p < 0.01) of fatty acids in the medium. Thus, the strains of LPL, LP, LF, and LH with the highest FFA absorption capacity (BELP53, BEPC33, BELF01, and BELH04, respectively) were selected for further analyses.Pancreatic lipase inhibition by selected strains
Considering the key roles of pancreatic lipase in intestinal lipid metabolism and absorption, we investigated the inhibitory effects of the four selected strains on pancreatic lipase activity using fat emulsions.32 BEPC22, BELP53, and BELH04 inhibited pancreatic lipase activity by 25.8%, 45.0%, and 34.4%, respectively, compared with the negative control (strain-untreated), whereas BELF01 exerted no inhibitory effect on pancreatic lipase activity (Fig. 1B).Selection of the best pair of two strains
To identify the optimal pairs among the four strains, we compared their metabolic characteristics, including nutritional source utilisation and byproduct generation patterns, during cell growth. Metabolite analyses were conducted at the start, mid-exponential, late exponential, early stationary, and stationary growth phases of each strain (Fig. 2A). Primary metabolites essential for cell survival in the culture medium of the four selected anti-obesity candidates (BELH04, BEPC22, BELP53, and BELF01) were analysed using GC-TOF-MS. Significantly different metabolites were selected based on the VIP value (>0.7) using PLS-DA and p-value (<0.05) to investigate the metabolite changes of the four strains. We profiled 74 distinct metabolites, containing amino acids, organic acids, and carbohydrates, and analysed the metabolite consumption and production patterns of the strains (data not shown). Metabolites produced by all strains and/or fluctuating over the cell cultivation period were not of interest because they might not affect other strains in the microorganism formulation. Consequently, five metabolites (aconitic acid, malic acid, gluconic acid, orotic acid, and glyceric acid) were listed as potentially important for microorganism pairing based on the consumption and production patterns of each strain (Fig. 2B). Six pairs were formed from the four strains, but three of these pairs were excluded (BELH04–BELF01, BEPC22–BELP01, and BELP53–BELF01) because their same consumption patterns as the five abovementioned metabolites may cause competition between members and amensalism. Thus, three noncompeting pairs (BELH04–BEPC22, BELH04–BELP53 and BEPC22–BELP53) were used in the subsequent experiments. In the BELH04–BEPC22 pair, orotic acid and glyceric acid produced by BEPC22 can feed on BELH04, potentially forming commensalism. Intriguingly, between the BELH04 and BELP53 pair, the aconitic acid produced from BELH04 can be used by BELP53, while BELH04 can take up orotic acid which was excreted by BELP53. Similarly, in the BEPC22–BELP53 pair, glyceric acid produced by BEPC22 can be consumed by BELP53, whereas malic acid produced by BELP53 can be utilised by BEPC22. To select the best pair of the two pairs, we considered the distance of each metabolite from the central metabolism based on the hypothesis that the metabolite close to the central metabolism may be utilized more easily and more demanding by the microorganism. According to the hypothesis, orotic acid, produced in the pyrimidine metabolism, is distant from the central metabolism as compared to the malic acid and glyceric acid. Thus, we assumed that BEPC22 and BELP53 can form the best mutualism by cross-feeding glyceric acid and malic acid.Glucose uptake inhibition by the selected strains
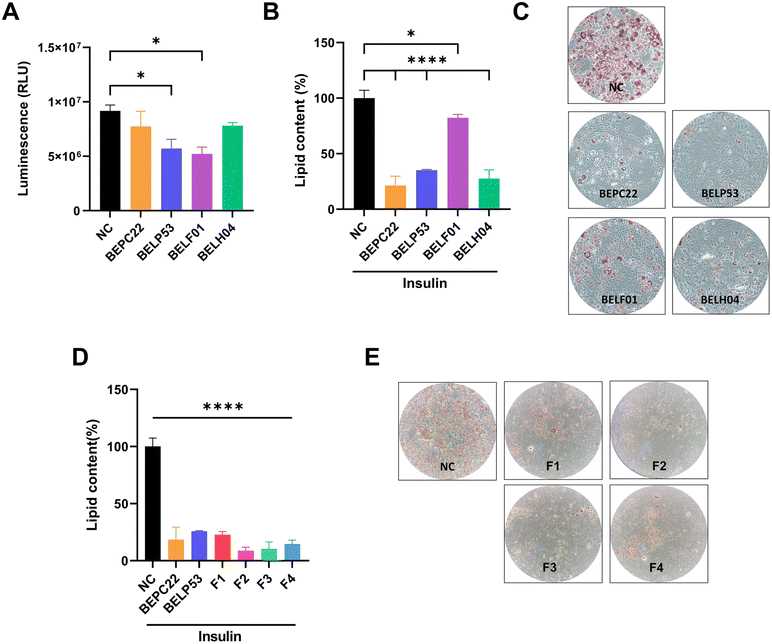
Fatty acids converted from excess sugar promote triglyceride synthesis and contribute to adipocyte accumulation.33 In the present study, the inhibitory effects of BEPC22, BELP53, BELF01 and BELH04 on glucose uptake were investigated in human colon epithelial Caco-2 cells. Treatment of Caco-2 cells with BELP53 and BELF01 decreased glucose uptake by 37.9% and 43.1%, respectively (Fig. 3A).Adipocyte differentiation inhibition by selected strains
Adipocyte differentiation is essential in obesity development. Therefore, inhibition of this process could be an anti-obesity mechanism of various LAB.4,34,35 In the present study, the inhibitory effects of BEPC22, BELP53, BELF01 and BELH04 on adipocyte differentiation were investigated in 3T3-L1 pre-adipocytes. Results showed that BEPC22, BELP53, BELF01 and BELH04 significantly decreased lipid contents in pre-adipocytes by 78.7%, 64.9%, 17.7% and 72.4%, respectively (p < 0.05; Fig. 3B). BELP22 showed the strongest inhibition of adipocyte differentiation. Therefore, we selected BEPC22 and BELP53 as the best probiotic pair for further analyses for their functionality properties.Formulation synergistic effect with BEPC22 and BELP53
In the present study, the inhibitory effects of BEPC22 and BELP53 on adipocyte differentiation were evaluated. Results showed that BEPC22 and BELP53 significantly decreased lipid contents in 3T3-L1 pre-adipocytes (p < 0.01; Fig. 3D). These two strains were not cytotoxic to 3T3-L1 cells (data not shown). To determine the optimum formulation (F) ratio of the probiotics with anti-obesity effects, we treated 3T3-L1 pre-adipocytes with a combination of BEPC22 and BELP53 at different ratios of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (F1), 6
2 (F1), 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (F2), 4
4 (F2), 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (F3), and 2
6 (F3), and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (F4). Each formulation-treated group exhibited a different degree of adipocyte differentiation (Fig. 3B and C). Interestingly, the 3T3-L1 cells treated with F2, F3, and F4 exhibited lower differentiation than those treated with the individual strains, suggesting a potential synergistic action between BEPC22 and BELP53. Among the formulations, F2 (6
8 (F4). Each formulation-treated group exhibited a different degree of adipocyte differentiation (Fig. 3B and C). Interestingly, the 3T3-L1 cells treated with F2, F3, and F4 exhibited lower differentiation than those treated with the individual strains, suggesting a potential synergistic action between BEPC22 and BELP53. Among the formulations, F2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) demonstrated the strongest inhibitory effect (91.3%) on adipogenesis.
4) demonstrated the strongest inhibitory effect (91.3%) on adipogenesis.
Inhibition of body weight gain and fat accumulation by probiotic administration in HFD-induced obese mice
We investigated the anti-obesity effects of the probiotics as individual strains or in combination on HFD-fed mice. As shown in Fig. 4A, the body weight gain of the mice in the HFD group was significantly higher than that of the mice in the ND group at 10 weeks after treatment (p < 0.0001). The mice in the HFDPC and HFDLP groups showed relatively lower body weights than those in the HFD group, but the differences were not statistically significant. At 38 days after treatment, the body weights of the mice in the HFDF group significantly decreased compared with those in the HFDPC and HFDLP groups. After 10 weeks, the body weight gain of the mice in the HFDF group was approximately 36.4% lower than that of the mice in the HFD group (p < 0.0001). Meanwhile, the food intake of the mice in the HFDPC and HFDLP groups was similar to that of the mice in the HFD group, whereas the mice in the HFDF group showed 1.8-fold higher food intake than those in the HFD group at 10 weeks. Surprisingly, the food efficiency ratio of the mice in the HFDF group was 50% lower than that of the mice in the HFD group, and it was similar to the that of the mice in the ND group (p < 0.0001, Fig. 4B). Taken together, formulation administration to the HFD-fed mice helped them gain much less weight even though their food intake increased by almost twofold.Compared with ND, HFD increased the weights of all white adipose tissues, including the subcutaneous, epididymal, mesenteric, and perirenal ones (Fig. 4C). The weights of all white adipose tissues showed negligible changes in the HFDLP group, whereas only the weight of the subcutaneous white adipose tissue significantly decreased in the HFDPC group. In contrast, the mice in the HFDF group had 48%, 66%, 42%, and 60% lower weights of the subcutaneous, epididymal, mesenteric, and perirenal adipose tissues, respectively, than the mice in the HFD group (p < 0.0001 for subcutaneous, p < 0.01 for mesenteric, epididymal, and perirenal). Additionally, haematoxylin and eosin (H&E) staining revealed that the average adipocyte size was dramatically larger in the HFD group than in the ND group, whereas the adipocyte sizes in the HFDPC, HFDLP, and HFDF groups were approximately 25%, 20%, and 40% smaller, respectively, than those in the HFD group (Fig. 4D).
Reduction in the plasma levels of obesity-related hormones by probiotics
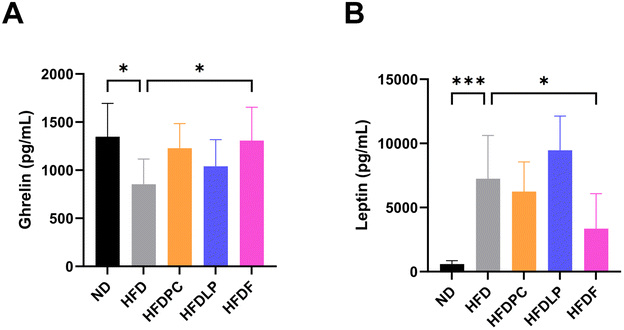
To understand the mechanisms underlying the anti-obesity effects of the formulation, we first measured the plasma levels of obesity-related hormones, such as adiponectin, ghrelin, GIP, insulin, leptin, PAI-1, resistin, and glucagon. The plasma levels of these hormones, except for PAI-1 and glucagon, showed opposite patterns in the HFD and ND groups (Fig. 5 and ESI Fig. 1†). Treatment with the probiotics significantly altered the plasma levels of ghrelin and leptin but not those of adiponectin, insulin, PAI-1, resistin, GIP, and glucagon. The plasma level of ghrelin was approximately 1.53-fold higher in the HFDF group than in the HFD group (p < 0.05, Fig. 5A). The plasma level of leptin was approximately 50% lower in the HFDF group than in the HFD group (p < 0.05, Fig. 5B).Effect of probiotics on the mRNA expression of obesity-related genes
We analysed the expression patterns of obesity-related genes in the liver and adipose tissue to understand the mechanisms by which probiotic administration reduces body weight gain. In the liver, the mRNA expression of Ppar-γ in the HFDF group was significantly lower than that in the HFD group (p < 0.05), whereas the mRNA expression of Ppar-γ in the HFD group was significantly higher than that in the ND group (Fig. 6A, p < 0.01). The mRNA expression of Ucp2 in the HFDPC, HFDLP, and HFDF groups was significantly lower than that in the HFD group (p < 0.05 for HFDPC and HFDLP, p < 0.001 for HFDF, Fig. 6B). However, the relative expression levels of Ppar-α and Cpt1a showed no significant changes in the groups, except for the ND group (ESI Fig. 2A and B†).In the epididymal adipose tissue, the mRNA expression of Ppar-α in the HFDF group was approximately 2.5-fold higher than that in the HFD group (p < 0.0001, Fig. 6C), whereas the mRNA expression of Ucp2 in the HFDF group was significantly lower than that in the HFD group (p < 0.001, Fig. 6D). In addition, the mRNA expression of Ppar-γ in the HFDF group was approximately 80% higher than that in the HFD group (p < 0.001, ESI Fig. 2C†), whereas the mRNA expression of Cpt1a in the HFD, HFDPC, HFDLP, and HFDF groups was higher than that in the ND group (ESI Fig. 2D†).
Changes of the metabolome by probiotics
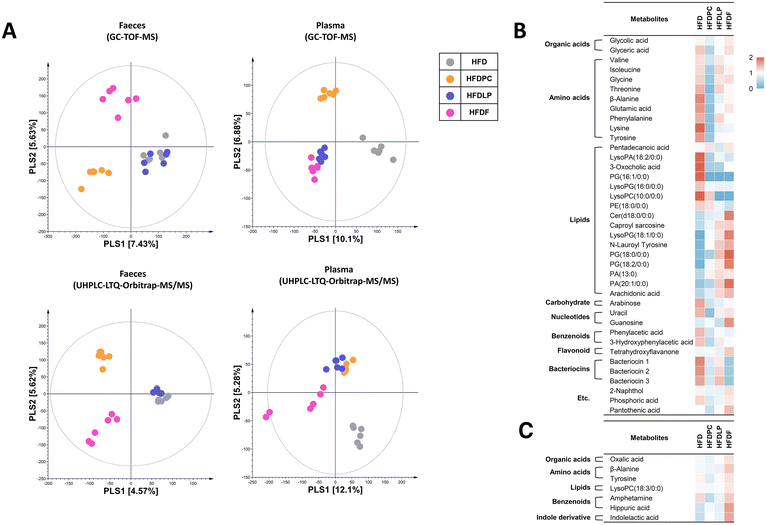
To identify the metabolites relevant to reduced weight gain, we performed non-targeted metabolomic analysis using mouse faeces and plasma. Multivariate statistical analysis revealed different patterns depending on the specimens and analytical instruments used (Fig. 7). PLS-DA of the faecal metabolomic data obtained using GC-TOF-MS showed a clear distinction between the HFDF and HFD groups (Fig. 7A). Interestingly, PLS-DA of the faecal metabolomic data obtained using UHPLC-LTQ-Orbitrap-MS/MS showed dissimilar patterns. The HFDF group clustered with the HFDLP group, and they were separate from the HFD and HFDPC groups (Fig. 7A). A total of 39 distinct metabolites were identified in the faeces, of which 20 and 19 were identified using GC-TOF-MS and UHPLC-LTQ-Orbitrap-MS/MS, respectively (ESI Tables 1 and 2†). The identified metabolites included 2 organic acids, 9 amino acids, 16 lipids (4 fatty acids and their derivatives, 10 glycerophospholipids, 1 sphingolipid, and 1 bile acid), 1 carbohydrate, 2 nucleotides, 2 benzenoids, 1 flavonoid, and 3 bacteriocins. Most amino acids, carbohydrates, benzenoids, bacteriocins, and some lipids were more abundant in the HFD group than in the other probiotic-treated groups. The HFDPC group showed significantly reduced levels of organic acids, amino acids, carbohydrates, nucleotides, benzenoids, bacteriocins, and some lipids in the faeces (Fig. 7B). In contrast, the HFDLP group showed lower reduction in the amounts of carbohydrates and some amino acids and alteration in lipid composition than the HFDPC group. The lipid composition in the HFDF group was similar to that in the HFDLP group. Notably, the HFDF group showed increased levels of guanosine and decreased levels of bacteriocins.The PLS-DA score plots of the plasma based on GC-TOF-MS and UHPLC-LTQ-Orbitrap-MS/MS analyses were dissimilar (Fig. 7A). Seven metabolites, including 1 organic acid, 2 amino acids, 1 lipid, 2 benzenoids, and 1 indole derivative, were identified in the plasma (ESI Tables 3 and 4†). Most of the identified metabolites were abundant in the HFDF group (Fig. 7C).
To evaluate the metabolites relevant to weight gain reduction, we conducted correlation analyses using discriminant metabolites in the faeces and plasma based on the mouse phenotype data. In the faeces, Cer(d18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/0
0/0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), guanosine, and tetrahydroxyflavone levels were negatively correlated with epididymal and perirenal fat weights (ESI Fig. 3†). Three metabolites, tentatively identified as bacteriocins, were negatively correlated with ghrelin levels. In the plasma, lysoPC(18
0), guanosine, and tetrahydroxyflavone levels were negatively correlated with epididymal and perirenal fat weights (ESI Fig. 3†). Three metabolites, tentatively identified as bacteriocins, were negatively correlated with ghrelin levels. In the plasma, lysoPC(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3/0
3/0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), hippuric acid, and indolelactic acid were negatively correlated with fat weight and positively correlated with Ppar-α expression (ESI Fig. 4†).
0), hippuric acid, and indolelactic acid were negatively correlated with fat weight and positively correlated with Ppar-α expression (ESI Fig. 4†).
Microbiome changes by probiotics
To investigate the effect of probiotics on the gut microbiota, we conducted a 16S metagenomic analysis of faecal samples. No difference in the Shannon index was found (ESI Fig. 5A†), but PCoA analysis showed a marked difference (p < 0.001) at 10 weeks between the HFD and probiotic-treated groups (ESI Fig. 5B†). At the phylum level, fewer Firmicutes species and more Bacteroidota species were observed in the HFDPC, HFDLP, and HFDF groups (Fig. 8A). Thus, the F/B ratio in the probiotic-treated groups was lower than that in the HFD group (p < 0.05, only in the HFDPC group, Fig. 8B). At the genus level, the abundance of Colidextribacter, Tuzzerella, Alistipes, Muribaculaceae, and Akkermansia significantly differed between the probiotic-administered groups. In particular, the abundance of Colidextribacter and Tuzzerella significantly decreased, whereas that of Alistipes significantly increased in the HFDPC and HFDF groups. The abundance of Muribaculaceae significantly increased only in the HFDPC group, whereas the abundance of Akkermansia significantly increased in the HFDF group compared with the HFDPC and HFDLP groups. In addition, LEfSe analysis showed that the abundance of Christensenellaceae, Paludicola, Ruminococcus, Butyricicoccus, Oscillospiraceae_UCG-005, Butyricicoccaceae_UCG-009, Oscillospirales_UCG-010, Muribaculaceae, and Alistipes significantly increased in the HFDPC group (Fig. 8D); Atopobiaceae, Ruminococcus, Coriobacteriaceae, Oscillospirales_UCG-010, Faecalibacterium, and Alistipes in the HFDLP group; and RF39, Ruminococcus, Butyricicoccaceae_UCG-009, Oscillospirales_UCG-010, Faecalibacterium, Akkermansia, and Alistipes in the HFDF group (Fig. 8D).Discussion
To develop an anti-obesity probiotic, we selected BEPC22 and BELP53 with synergistic inhibitory effects on fatty acid absorption, glucose uptake and metabolite producing/utilizing potential. Among the metabolites consumed/produced in the medium of the BEPC22–BELP53 pair, glyceric acid produced by BEPC22 can be consumed by BELP53, whereas malic acid produced by BELP53 can be utilised by BEPC22. Thus, we assumed that BEPC22 and BELP53 can form mutualism by cross-feeding glyceric acid and malic acid. Subsequently, an optimal ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 with BEPC22 and BELP53 was determined through in vitro anti-adipogenic assays.
4 with BEPC22 and BELP53 was determined through in vitro anti-adipogenic assays.
The effects of the single probiotic strains or formulation on HFD-induced obese mice were assessed. The mice in the HFDF group had the lowest body weight gain possibly because of the decreased weights of the subcutaneous and abdominal adipose tissues in this group, implying the combined treatment of the two strains exhibited a synergistic effect in ameliorating obesity when we compared with the single strain administered results (HFDPC and HFDLP). Within the gut, the administered probiotics affected the gut microbiome to restructure by engaging cross-feeding, which eventually caused reduction of body weight gain.36
Among obesity-related biomarkers, leptin is a key anti-obesity hormone that has the greatest impact on body fat weight and regulates appetite. This hormone is produced by adipocytes and secreted into the blood, and its production level correlates with adipocyte size.37,38 Previous studies have shown that the plasma levels of leptin decrease during weight loss.38–40 In the present study, the plasma level of leptin in the HFDF group was almost 50% lower than that in the HFD group, indicating that the decrease in body weight gain might be caused by the reduced adipose tissue weight resulting from the smaller adipocyte size. This result indicated that the probiotic treatment was responsible for the decrease in plasma leptin levels.
Leptin in the blood increases the expression of Ucp2, the gene which increases the thermogenic capacity of brown adipose tissue and helps reduce body fat.41,42 In the present study, the leptin level and Ucp2 expression decreased in the liver and epididymal adipose tissue in the HFDF group. This result suggested that the probiotic treatment decreased the leptin levels, which contributed to the decreased Ucp2 expression in the liver and epididymal adipose tissue. Promoting fatty acid oxidation and inhibiting fatty acid synthesis, which are mainly mediated by PPAR family members, are necessary to reduce fat weight.43 In the adipose tissue, Ppar-α plays an important role in reducing fat mass and body weight by promoting the oxidation of fatty acids, inhibiting the secretion of triglycerides, and increasing energy expenditure through the regulation of downstream genes, such as Acox and Cpt1.44–46 In the present study, we observed a relatively higher Ppar-α expression in the epididymal adipose tissue and, consequently, a lower fat weight in the HFDF group than in the HFD group. This result might be due to fatty acid oxidation and increased energy consumption. However, no significant difference in Cpt1a expression was found between the probiotic-treated groups and the HFD group, whereas Cpt1a expression showed significant differences between the HFD and ND groups. The decrease in body weight and fat weight in the formulation-treated group might have been caused by the relatively higher fatty acid oxidation in the adipose tissue than the reduction in fatty acid synthesis.
The liver takes up, synthesises, stores, and secretes fatty acids and triglycerides. Fatty acid synthesis is mainly contributed by Ppar-γ.47 In the present study, the mRNA expression of Ppar-γ in the liver was lower in the probiotic-treated groups than in the HFD group. Moreover, the HFDF group showed the lowest Ppar-γ expression. This result indicated that the administration of BEPC22 and BELP53 hindered Ppar-γ expression in the liver, which consequently inhibited fatty acid synthesis and decreased subcutaneous and abdominal fat weights.
The gut microbiome is associated with obesity and related metabolic disorders, and specific metabolites correlate with the structure of the gut microbiome.48,49 In the present study, the metabolome and microbiome analyses including the ND group showed a significantly distinct pattern between the ND and HFD groups. The PLS-DA (ESI Fig. 6A†) based on the metabolome data shows a clear separation between the ND and HFD (without and with the probiotic-treated groups) groups. Similarly, microbiome data showed a distinguished pattern of ND from HFD with the probiotic groups. Among alpha diversity (ESI Fig. 6B†), the observed features were significantly reduced in the HFD group compared to the ND group, and the alpha diversity in the formulation feeding group (HFDF) was recovered as much as in the ND group. In the beta diversity (ESI Fig. 6C†) analysis, the ND and HFD groups showed a significant difference, and the probiotic-treated groups were not close to the ND group, but the HFDF group was the closest to the ND group. The microbiome results indicate that the HFDF group is most similar to the ND group among the high fat diet-fed groups. In the faecal microbiome analysis excluding the ND group, the abundance of Colidextribacter and Tuzzerella decreased, whereas that of Alistipes, Akkermansia, and Muribaculaceae increased in the probiotic-fed groups compared with the HFD group. Previous studies have reported that the abundance of Colidextribacter increases in individuals with obesity, whereas the abundance of Akkermansia, a beneficial bacterium, increases in healthy lean individuals, but is depleted in those with obesity and metabolic diseases.50,51Alistipes is also more prevalent in healthy lean individuals and less prevalent in individuals with obesity, and these strains produce small amounts of SCFAs, which can inhibit Ppar-γ expression and promote fat oxidation.52 Furthermore, it was reported that Alistipes is capable of synthesizing membrane building blocks by utilizing medium- and long-chain fatty acids within the gut.53 However, our results represented reduction of some phospholipids with significant correlation with Alistipes. We guess the inconsistent result may be due to the different substrate specificity of the acyl-acyl carrier protein (ACP) synthetase in Alistipes, which form phospholipids.
We observed changes in lipids and specific metabolites in the faecal and plasma samples and correlated these changes with certain microbiomes. The HFDF group showed a different lipid composition in the faeces than the other groups. We assumed that the administered probiotics affected the lipid pool by modulating the gut microbiota, such as Faecalibaculum, Ruminococcus, Coriobacteriaceae, and Atopobiaceae. Among the identified lipids, Cer(d18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/0
0/0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) significantly correlated with body weight gain, fat weight, and Ppar-α mRNA expression in the adipose tissue, which is consistent with the results of a previous research.54 Additionally, as mentioned in previous research, an elevated level of Faecalibaculum in obesity mouse models was associated with weight loss.55,56 Our study showed that the level of PG(18
0) significantly correlated with body weight gain, fat weight, and Ppar-α mRNA expression in the adipose tissue, which is consistent with the results of a previous research.54 Additionally, as mentioned in previous research, an elevated level of Faecalibaculum in obesity mouse models was associated with weight loss.55,56 Our study showed that the level of PG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/0
2/0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) is increased along with the Faecalibaculum abundance in the faeces. As a result, we assumed that Alistipes and Faecalibaculum are important changers who change the gut lipid profile, potentially contributing to reduced weight gain.
0) is increased along with the Faecalibaculum abundance in the faeces. As a result, we assumed that Alistipes and Faecalibaculum are important changers who change the gut lipid profile, potentially contributing to reduced weight gain.
In addition, the amount of pantothenic acid, a key precursor of coenzyme A, significantly increased in the faeces of the HFDF group and was positively correlated with Akkermansia, Coriobacteriaceae, and Atopobiaceae (ESI Fig. 6†).
Furthermore, among the metabolites detected in the plasma, hippuric acid and indolelactic acid had comparatively higher levels in the HFDF group than in the other groups and were significantly correlated with anti-obesity indicators, such as fat weight and Ppar-α expression, in the epididymal adipose tissue.
Hippuric acid, possessing the protective effect against obesity, was relatively higher in the plasma of HFDF mice.57,58 Most studies revealed that the hippuric acid is originated from polyphenols in fruits and whole grains.58 However, in our study, we observed hippuric acid even though polyphenols were not supplied. Thus, we assumed that the hippuric acid might be produced from phenylalanine by the gut microbiota, and then, conjugated with glycine in the liver.59 Indole derivatives, which are tryptophan metabolites produced by the gut microbiota, play crucial roles in influencing gut homeostasis and immune responses. They are transported through the bloodstream to regulate lipid metabolism in the liver. Changes in dietary patterns can lead to metabolic disorders owing to gut dysbiosis, which can significantly affect indole metabolism.60 In the present study, indole-3-propionic acid and indolelactic acid were detected in the plasma of the HFD-fed mice, but only indolelactic acid showed a significantly higher level in the HFDF group, with a notable correlation with Coriobacteriaceae and Faecalibaculum (ESI Fig. 7†). Previous studies have shown that the indolelactic acid produced by L. plantarum modulates the gut environment, thus promoting the proliferation of Faecalibacterium and Bifidobacterium.61
Taken together, probiotic formulations with BEPC22 and BELP53 showed an anti-obesity effect not only by perturbation of lipid metabolism, but with assistance of anti-obesity metabolites such as hippuric acid and indole derivatives which might be synthesized by the changed gut microbiome. We guess that the altered gut microbial community was more capable of metabolizing aromatic amino acids (e.g. tryptophan and tyrosine) that eventually produced host beneficial metabolites (e.g. hippuric acid and indole derivatives). Therefore, we think it is imperative to construct gut microbial communities by harmonizing some microbes involved in lipid metabolism and other microorganisms responsible for producing metabolites with an anti-obesity effect for better efficacy. Even we observed an anti-obesity effect of the formulation in this study; the interaction of the abovementioned metabolites with specific colonic bacteria and the mechanisms underlying weight gain are needed to be understood in further studies.
Conclusions
The two-strain formulation effectively alleviated HFD-induced obesity in mice. The competitive lipid and carbohydrate metabolic characteristics of the two strains, which are expected to ameliorate obesity, may have resulted in changes in body weight, body fat mass, gut microbiota, and lipid profiles. Specifically, the administration of the two strains modulated the F/B ratio of the gut bacteria, which consequently decreased the abundance of certain genera associated with obesity. Furthermore, it increased blood indolelactic acid levels, which decreased during metabolic abnormalities, and enhanced intestinal lipid excretion. While our study successfully identified microbial changes and their corresponding metabolomic correlates, further investigation at the molecular pathway level is imperative to gain a deeper understanding of the mechanisms by which these two strains ameliorate obesity. Further research in this field will contribute to the development of obesity prevention strategies.Data availability
All data underlying the results are available as part of the article and no additional source data are required.Author contributions
Conceptualisation: S. J. Kim and K.-J. Sin; data curation: N.-R. Lee, E.-C. Chung, J. W. Bae, T.-J. Kwon and N. W. Hwang; formal analysis: N.-R. Lee, S.-H. Soung, H.-J. Tak, T.-J. Kwon, J.-Y. Choi, Y.-E. Lee and N. W. Hwang; investigation: N.-R. Lee, J. S. Lee, E.-C. Chung, T.-J. Kwon, S.-H. Soung and H.-J. Tak; methodology: N.-R. Lee, J. S. Lee and T.-J. Kwon; project administration: S. J. Kim, K.-J. Sin, J. S. Lee and K. S. Kim; resources: E.-C. Chung; software: J. W. Bae; supervision: C. H. Lee; visualization: N.-R. Lee, T.-J. Kwon and J. W. Bae; writing – original draft: N.-R. Lee, E.-C. Chung, J. W. Bae and T.-J. Kwon; and writing – review and editing: S.J. Kim and K.-J. Sin.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministry of Trade, Industry and Energy (MOTIE) and the Korea Evaluation Institute of Industrial Technology (KEIT) through the Research and Development Project for the Advanced Technology Center (ATC+) (Project No. 20009679, NTIS_1415179891).References
- M. Blüher, Obesity: global epidemiology and pathogenesis, Nat. Rev. Endocrinol., 2019, 15, 288–298 CrossRef PubMed.
- H. M. Timmerman, C. J. Koning, L. Mulder, F. M. Rombouts and A. C. Beynen, Monostrain, multistrain and multispecies probiotics—a comparison of functionality and efficacy, Int. J. Food Microbiol., 2004, 96, 219–233 CrossRef CAS PubMed.
- M. Miyoshi, A. Ogawa, S. Higurashi and Y. Kadooka, Anti-obesity effect of Lactobacillus gasseri SBT2055 accompanied by inhibition of pro-inflammatory gene expression in the visceral adipose tissue in diet-induced obese mice, Eur. J. Nutr., 2014, 53, 599–606 CrossRef PubMed.
- W. H. Jeung, J. J. Shim, S. W. Woo, J. H. Sim and J. L. Lee, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 Cell Extracts Inhibit Adipogenesis in 3T3-L1 and HepG2 Cells, J. Med. Food, 2018, 21, 876–886 CrossRef CAS PubMed.
- Y. Kang, X. Kang, H. Yang, H. Liu, X. Yang, Q. Liu, H. Tian, Y. Xue, P. Ren, X. Kuang, Y. Cai, M. Tong, L. Li and W. Fan, Lactobacillus acidophilus ameliorates obesity in mice through modulation of gut microbiota dysbiosis and intestinal permeability, Pharmacol. Res., 2022, 175, 106020 CrossRef CAS PubMed.
- S. Adamberg, I. Sumeri, R. Uusna, P. Ambalam, K. Kiran Kondepudi, K. Adamberg, T. Wadström, Å. Ljungh, T. Wadströ and A. Ljungh, Survival and synergistic growth of mixed cultures of bifidobacteria and lactobacilli combined with prebiotic oligosaccharides in a gastrointestinal tract simulator, Microb. Ecol. Health Dis., 2014, 25, 23062 Search PubMed.
- L. V. McFarland, Efficacy of single-strain probiotics versus multi-strain mixtures: systematic review of strain and disease specificity, Dig. Dis Sci., 2021, 66, 694–704 CrossRef CAS PubMed.
- P. D. Cani and N. M. Delzenne, Gut microflora as a target for energy and metabolic homeostasis, Curr. Opin. Clin. Nutr. Metab. Care, 2007, 10, 729–734 CrossRef PubMed.
- E. M. Hamad, M. Sato, K. Uzu, T. Yoshida, S. Higashi, H. Kawakami, Y. Kadooka, H. Matsuyama, I. A. Abd El-Gawad and K. Imaizumi, Milk fermented by Lactobacillus gasseri SBT2055 influences adipocyte size via inhibition of dietary fat absorption in Zucker rats, Br. J. Nutr., 2008, 101, 716–724 CrossRef PubMed.
- G. Karimi, M. R. Sabran, R. Jamaluddin, K. Parvaneh, N. Mohtarrudin, Z. Ahmad, H. Khazaai and A. Khodavandi, The anti-obesity effects of Lactobacillus casei strain Shirota versus Orlistat on high fat diet-induced obese rats, Food Nutr. Res., 2015, 59, 29273 CrossRef PubMed.
- Z. Cheng, L. Zhang, L. Yang and H. Chu, The critical role of gut microbiota in obesity, Front. Endocrinol., 2022, 13(1025706) Search PubMed.
- M. Million, E. Angelakis, M. Maraninchi, M. Henry, R. Giorgi, R. Valero, B. Vialettes and D. Raoult, Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli, Int. J. Obes., 2013, 37, 1460–1466 CrossRef CAS PubMed.
- A. Andoh, A. Nishida, K. Takahashi, O. Inatomi, H. Imaeda, S. Bamba, K. Kito, M. Sugimoto and T. Kobayashi, Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population, J. Clin. Biochem. Nutr., 2016, 59, 65–70 CrossRef CAS PubMed.
- S. M. Murga-Garrido, Y. C. Orbe-Orihuela, C. E. Díaz-Benítez, A. C. Castañeda-Márquez, F. Cornejo-Granados, A. Ochoa-Leyva, A. Sanchez-Flores, M. Cruz, A. I. Burguete-García and A. Lagunas-Martínez, Alterations of the gut microbiome associated to methane metabolism in Mexican children with obesity, Children, 2022, 9, 148 CrossRef PubMed.
- A. Koliada, G. Syzenko, V. Moseiko, L. Budovska, K. Puchkov, V. Perederiy, Y. Gavalko, A. Dorofeyev, M. Romanenko, S. Tkach, L. Sineok, O. Lushchak and A. Vaiserman, Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population, BMC Microbiol., 2017, 17, 1–6 CrossRef PubMed.
- P. Thiennimitr, S. Yasom, W. Tunapong, T. Chunchai, K. Wanchai, A. Pongchaidecha, A. Lungkaphin, S. Sirilun, C. Chaiyasut, N. Chattipakorn and S. C. Chattipakorn, Lactobacillus paracasei HII01, xylooligosaccharides, and synbiotics reduce gut disturbance in obese rats, Nutrition, 2018, 54, 40–47 CrossRef CAS PubMed.
- J. Geng, Q. Ni, W. Sun, L. Li and X. Feng, The links between gut microbiota and obesity and obesity related diseases, Biomed. Pharmacother., 2022, 147, 112678 CrossRef CAS PubMed.
- S. Y. Son, S. Lee, D. Singh, N.-R. Lee, D. Y. Lee and C. H. Lee, Comprehensive secondary metabolite profiling toward delineating the solid and submerged-state fermentation of Aspergillus oryzae KCCM 12698, Front. Microbiol., 2018, 9, 1076 CrossRef PubMed.
- Y. H. Lee, N.-R. Lee and C. H. Lee, Comprehensive metabolite profiling of four different beans fermented by Aspergillus oryzae, Molecules, 2022, 27, 7917 CrossRef CAS PubMed.
- L. Han, Y. Kimura and H. Okuda, Reduction in fat storage during chitin-chitosan treatment in mice fed a high-fat diet, Int. J. Obes., 1999, 23, 174–179 CrossRef CAS PubMed.
- N.-J. Song, H.-J. Yoon, K. H. Kim, S.-R. Jung, W.-S. Jang, C.-R. Seo, Y. M. Lee, D.-H. Kweon, J.-W. Hong, J.-S. Lee, K.-M. Park, K. R. Lee and K. W. Park, Butein is a novel anti-adipogenic compound, J. Lipid Res., 2013, 54, 1385–1396 CrossRef CAS PubMed.
- E. Bolyen, J. R. Rideout, M. R. Dillon, N. A. Bokulich, C. C. Abnet, G. A. Al-Ghalith, H. Alexander, E. J. Alm, M. Arumugam, F. Asnicar, Y. Bai, J. E. Bisanz, K. Bittinger, A. Brejnrod, C. J. Brislawn, C. T. Brown, B. J. Callahan, A. M. Caraballo-Rodríguez, J. Chase, E. K. Cope, R. Da Silva, C. Diener, P. C. Dorrestein, G. M. Douglas, D. M. Durall, C. Duvallet, C. F. Edwardson, M. Ernst, M. Estaki, J. Fouquier, J. M. Gauglitz, S. M. Gibbons, D. L. Gibson, A. Gonzalez, K. Gorlick, J. Guo, B. Hillmann, S. Holmes, H. Holste, C. Huttenhower, G. A. Huttley, S. Janssen, A. K. Jarmusch, L. Jiang, B. D. Kaehler, K. Bin Kang, C. R. Keefe, P. Keim, S. T. Kelley, D. Knights, I. Koester, T. Kosciolek, J. Kreps, M. G. I. Langille, J. Lee, R. Ley, Y.-X. Liu, E. Loftfield, C. Lozupone, M. Maher, C. Marotz, B. D. Martin, D. McDonald, L. J. McIver, A. V. Melnik, J. L. Metcalf, S. C. Morgan, J. T. Morton, A. T. Naimey, J. A. Navas-Molina, L. F. Nothias, S. B. Orchanian, T. Pearson, S. L. Peoples, D. Petras, M. L. Preuss, E. Pruesse, L. B. Rasmussen, A. Rivers, M. S. Robeson II, P. Rosenthal, N. Segata, M. Shaffer, A. Shiffer, R. Sinha, S. J. Song, J. R. Spear, A. D. Swafford, L. R. Thompson, P. J. Torres, P. Trinh, A. Tripathi, P. J. Turnbaugh, S. Ul-Hasan, J. J. J. Van der Hooft, F. Vargas, Y. Vázquez-Baeza, E. Vogtmann, M. von Hippel, W. Walters, Y. Wan, M. Wang, J. Warren, K. C. Weber, C. H. D. Williamson, A. D. Willis, Z. Z. Xu, J. R. Zaneveld, Y. Zhang, Q. Zhu, R. Knight and J. G. Caporaso, Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2, Nat. Biotechnol., 2019, 37, 852–857 CrossRef CAS PubMed.
- A. Amir, D. McDonald, J. A. Navas-Molina, E. Kopylova, J. T. Morton, Z. Zech Xu, E. P. Kightley, L. R. Thompson, E. R. Hyde, A. Gonzalez and R. Knight, Deblur Rapidly Resolves Single-Nucleotide Community Sequence Patterns, mSystems, 2017, 2, e00191–e00116 Search PubMed.
- K. Katoh, K. Misawa, K. Kuma and T. Miyata, MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform, Nucleic Acids Res., 2002, 30, 3059–3066 CrossRef CAS PubMed.
- D. P. Faith, Conservation evaluation and phylogenetic diversity, Biol. Conserv., 1992, 61, 1–10 CrossRef.
- C. A. Lozupone, M. Hamady, S. T. Kelley and R. Knight, Quantitative and qualitative β diversity measures lead to different insights into factors that structure microbial communities, Appl. Environ. Microbiol., 2007, 73, 1576–1585 CrossRef CAS PubMed.
- C. Lozupone and R. Knight, UniFrac: A new phylogenetic method for comparing microbial communities, Appl. Environ. Microbiol., 2005, 71, 8228–8235 CrossRef CAS PubMed.
- N. A. Bokulich, B. D. Kaehler, J. R. Rideout, M. Dillon, E. Bolyen, R. Knight, G. A. Huttley and J. Gregory Caporaso, Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2's q2-feature-classifier plugin, Microbiome, 2018, 6, 1–17 CrossRef PubMed.
- C. Quast, E. Pruesse, P. Yilmaz, J. Gerken, T. Schweer, P. Yarza, J. Peplies and F. O. Glöckner, The SILVA ribosomal RNA gene database project: improved data processing and web-based tools, Nucleic Acids Res., 2012, 41, D590–D596 CrossRef PubMed.
- M. J. Anderson, A new method for non–parametric multivariate analysis of variance, Aust. Ecol., 2008, 26, 32–46 CrossRef.
- N. Segata, J. Izard, L. Waldron, D. Gevers, L. Miropolsky, W. S. Garrett and C. Huttenhower, Metagenomic biomarker discovery and explanation, Genome Biol., 2011, 12, 1–18 CrossRef.
- M. Hirose, T. Ando, R. Shofiqur, K. Umeda, Y. Kodama, S. Van Nguyen, T. Goto, M. Shimada and S. Nagaoka, Anti-obesity activity of hen egg anti-lipase immunoglobulin yolk, a novel pancreatic lipase inhibitor, Nutr. Metab., 2013, 10, 1–6 CrossRef PubMed.
- J. K. Sethi and A. J. Vidal-Puig, Adipose tissue function and plasticity orchestrate nutritional adaptation, J. Lipid Res., 2007, 48, 1253–1262 CrossRef CAS PubMed.
- S. J. Kim, S.-I. Choi, M. Jang, Y. Jeong, C.-H. Kang and G.-H. Kim, Anti-adipogenic effect of Lactobacillus fermentum MG4231 and MG4244 through AMPK pathway in 3T3-L1 preadipocytes, Food Sci. Biotechnol., 2020, 29, 1541–1551 CrossRef CAS PubMed.
- Y.-C. Lin, Y.-T. Chen, K.-Y. Li and M.-J. Chen, Investigating the mechanistic differences of obesity-inducing Lactobacillus kefiranofaciens M1 and anti-obesity Lactobacillus mali APS1 by microbolomics and metabolomics, Front. Microbiol., 2020, 11, 1454 CrossRef PubMed.
- E. J. Culp and A. L. Goodman, Cross-feeding in the gut microbiome: Ecology and mechanisms, Cell Host Microbe, 2023, 31, 485–499 CrossRef CAS PubMed.
- C. Weyer, J. E. Foley, C. Bogardus, P. A. Tataranni and R. E. Pratley, Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts Type II diabetes independent of insulin resistance, Diabetologia, 2000, 43, 1498–1506 CrossRef CAS PubMed.
- R. V. Considine, M. K. Sinha, M. L. Heiman, A. Kriauciunas, T. W. Stephens, M. R. Nyce, J. P. Ohannesian, C. C. Marco, L. J. McKee, T. L. Bauer and J. F. Caro, Serum Immunoreactive-Leptin Concentrations in Normal-Weight and Obese Humans, N. Engl. J. Med., 1996, 334, 292–295 CrossRef CAS PubMed.
- F. Lönnqvist, P. Arner, L. Nordfors and M. Schalling, Overexpression of the obese (ob) gene in adipose tissue of human obese subjects, Nat. Med., 1995, 1, 950–953 CrossRef PubMed.
- B. S. Hamilton, D. Paglia, A. Y. M. Kwan and M. Deitel, Increased obese mRNA expression in omental fat cells from massively obese humans, Nat. Med., 1995, 1, 953–956 CrossRef CAS PubMed.
- P. J. Scarpace, M. Nicolson and M. Matheny, UCP2, UCP3 and leptin gene expression: modulation by food restriction and leptin, J. Endocrinol., 1998, 159, 349–357 CAS.
- M. Alivand, B. Alipour, S. Moradi, Y. Khaje-Bishak and M. Alipour, The review of the relationship between UCP2 and obesity: Focusing on inflammatory-obesity, New insight in Obesity: Genetics and Beyond, 2021, 5, 1–13 Search PubMed.
- I. Emilia, G. C. Farrell, G. Robertson, P. Hall, R. Kirsch and I. Leclercq, Central role of PPARα-dependent hepatic lipid turnover in dietary steatohepatitis in mice, Hepatology, 2003, 38, 123–132 Search PubMed.
- W. J. Lee, M. Kim, H.-S. Park, H. S. Kim, M. J. Jeon, K. S. Oh, E. H. Koh, J. C. Won, M.-S. Kim, G. T. Oh, M. Yoon, K.-U. Lee and J.-Y. Park, AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARα and PGC-1, Biochem. Biophys. Res. Commun., 2006, 1, 291–295 CrossRef PubMed.
- M. Vázquez, N. Roglans, Á. Cabrero, C. Rodríguez, T. Adzet, M. Alegret, R. M. Sánchez and J. C. Laguna, Bezafibrate induces acyl-CoA oxidase mRNA levels and fatty acid peroxisomal β-oxidation in rat white adipose tissue, Mol. Cell. Biochem., 2001, 216, 71–78 CrossRef PubMed.
- A. Cabrero, M. Alegret, R. M. Sánchez, T. Adzet, J. C. Laguna and M. Vázquez, Bezafibrate reduces mRNA levels of adipocyte markers and increases fatty acid oxidation in primary culture of adipocytes, Diabetes, 2001, 50, 1883–1890 CrossRef CAS PubMed.
- A. P. L. Jensen-Urstad and C. F. Semenkovich, Fatty acid synthase and liver triglyceride metabolism: housekeeper or messenger?, Biochim. Biophys. Acta, Bioenerg., 2012, 1821, 747–753 CrossRef CAS PubMed.
- H. S. Ejtahed, A. R. Soroush, P. Angoorani, B. Larijani and S. Hasani-Ranjbar, Gut Microbiota as a Target in the Pathogenesis of Metabolic Disorders: A New Approach to Novel Therapeutic Agents, Horm. Metab. Res., 2016, 48, 349–358 CrossRef CAS PubMed.
- G. Sharon, N. Garg, J. Debelius, R. Knight, P. C. Dorrestein and S. K. Mazmanian, Specialized metabolites from the microbiome in health and disease, Cell Metab., 2014, 20, 719–730 CrossRef CAS PubMed.
- Z. Yu, X. F. Yu, G. Kerem and P. G. Ren, Perturbation on gut microbiota impedes the onset of obesity in high fat diet-induced mice, Front. Endocrinol., 2022, 13, 795371 CrossRef PubMed.
- G. den Besten, A. Bleeker, A. Gerding, K. van Eunen, R. Havinga, T. H. van Dijk, M. H. Oosterveer, J. W. Jonker, A. K. Groen, D.-J. Reijngoud and B. M. Bakker, Short-chain fatty acids protect against high-fat diet–induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation, Diabetes, 2015, 64, 2398–2408 CrossRef CAS PubMed.
- Z. Xu, W. Jiang, W. Huang, Y. Lin, F. K. L. Chan and S. C. Ng, Gut microbiota in patients with obesity and metabolic disorders—a systematic review, Genes Nutr., 2022, 17, 1–18 CrossRef PubMed.
- C. D. Radka, M. W. Frank, C. O. Rock and J. Yao, Fatty acid activation and utilization by Alistipes finegoldii, a representative Bacteroidetes resident of the human gut microbiome, Mol. Microbiol., 2020, 113, 807–825 CrossRef CAS PubMed.
- H. Fang, Y. Cao, J. Zhang, X. Wang, M. Li, Z. Hong, Z. Wu and M. Fang, Lipidome remodeling activities of DPA-EA in palmitic acid-stimulated HepG2 cells and the in vivo anti-obesity effect of the DPA-EA and DHA-EA mixture prepared from algae oil, Front. Pharmacol., 2023, 14, 1146276 CrossRef CAS PubMed.
- S. Wang, M. Huang, X. You, J. Zhao, L. Chen, L. Wang, Y. Luo and Y. Chen, Gut microbiota mediates the anti-obesity effect of calorie restriction in mice, Sci. Rep., 2018, 8, 13037 CrossRef PubMed.
- G. H. Baek, K. M. Yoo, S.-Y. Kim, D. H. Lee, H. Chung, S.-C. Jung, S.-K. Park and J.-S. Kim, Collagen Peptide Exerts an Anti-Obesity Effect by Influencing the Firmicutes/Bacteroidetes Ratio in the Gut, Nutrients, 2023, 15, 2610 CrossRef CAS PubMed.
- K. M. Pruss, H. Chen, Y. Liu, W. Van Treuren, S. K. Higginbottom, J. B. Jarman, C. R. Fischer, J. Mak, B. Wong, T. M. Cowan, M. A. Fischbach, J. L. Sonnenburg and D. Dodd, Host-microbe co-metabolism via MCAD generates circulating metabolites including hippuric acid, Nat. Commun., 2023, 14, 512 CrossRef CAS PubMed.
- T. Pallister, M. A. Jackson, T. C. Martin, C. A. Glastonbury, A. Jennings, M. Beaumont, R. P. Mohney, K. S. Small, A. MacGregor, C. J. Steves, A. Cassidy, T. D. Spector, C. Menni and A. M. Valdes, Untangling the relationship between diet and visceral fat mass through blood metabolomics and gut microbiome profiling, Int. J. Obes., 2017, 41, 1106–1113 CrossRef CAS PubMed.
- A. Ticinesi, A. Guerra, A. Nouvenne, T. Meschi and S. Maggi, Disentangling the Complexity of Nutrition, Frailty and Gut Microbial Pathways during Aging: A Focus on Hippuric Acid, Nutrients, 2023, 15, 1138 CrossRef CAS PubMed.
- X. Li, B. Zhang, Y. Hu and Y. Zhao, New Insights Into Gut-Bacteria-Derived Indole and Its Derivatives in Intestinal and Liver Diseases, Front. Pharmacol., 2021, 12, 769501 CrossRef CAS PubMed.
- Q. Zhou, Z. Xie, D. Wu, L. Liu, Y. Shi, P. Li and Q. Gu, The Effect of Indole-3-Lactic Acid from Lactiplantibacillus plantarum ZJ316 on Human Intestinal Microbiota In Vitro, Foods, 2022, 11, 3302 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo03557c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |