Open Access Article
Open Access ArticleA flexible CO2 capture system for backup power plants using Ca(OH)2/CaCO3 solid storage
Yolanda A.
Criado
 *,
Borja
Arias
*,
Borja
Arias
 and
J. Carlos
Abanades
and
J. Carlos
Abanades

CSIC-INCAR, C/Francisco Pintado Fe, 26, 33011, Oviedo, Spain. E-mail: yolanda.ac@incar.csic.es; Fax: +34 985297662; Tel: +34 985119090
First published on 28th November 2022
Abstract
CO2 capture technologies are required to address intermittent sources of CO2, such as the natural gas combined cycle (NGCC) used for backup power applications. The high reactivity of Ca(OH)2 powder facilitates the design of low cost carbonators to capture diluted CO2 (typically below 4%v in NGCC flue gases) as CaCO3. By storing CaCO3 and Ca(OH)2 it is possible to decouple the CO2 capture step in the carbonator from the oxy-calciner/hydrator block in which Ca(OH)2 is regenerated and CO2 extracted. This facilitates the integration of CO2 capture elements in backup power systems. Simulations of the completely integrated backup power plant with and without capture indicated that for a NGCC capacity factor (CF) of 0.1, the thermal capacity of the oxy-calciner was just 2% of the gross power output of the NGCC gas turbine. Capture efficiencies of 90% can be reached without modifying the operating conditions of the gas power plant, while achieving a global efficiency of 38% for the system with CO2 capture. A basic economic analysis indicated that the proposed scheme would lead to a cost of CO2 avoided of approximately 200 $ per tCO2, making it suitable for retrofitting natural gas-based power plants.
1. Introduction
To achieve zero emissions by 2050, carbon-free backup power systems and/or large-scale energy storage are required for those periods of time with low and/or intermittent renewable resources.1–3 Among them, natural gas combustion turbines could be used as backup systems owing to their rapid response to changes in load and demand, as well as their low thermal stress during start-up.4,5 However, CO2 produced during the operational periods of these backup turbines needs to be captured in order to minimize CO2 emissions.6,7Most of the developed CO2 capture technologies, generally complex and capital-intensive, present sub-systems originally designed only for base-load operation. Therefore, even if the technical complexities associated with their dynamic operation are resolved, they will face prohibitive costs when operated under low capacity factors (CF), as expected in backup systems based on natural gas (i.e., CF of 0.1–0.2). This has been recognized as a weakness of CO2 capture technologies, and recently there is growing interest in more flexible CO2 capture processes. In previous studies,8–11 calcium looping (CaL) was already investigated as a suitable capture technology to address the challenge of capturing CO2 from intermittent sources. In CaL systems, CaO reacts with the CO2 present in a flue gas to produce CaCO3 and a “free”-CO2 flue gas, and then the carbonated solids are regenerated via calcination for further cycles while producing a CO2 stream ready for storage.12 The use of inexpensive limestone as a CO2 sorbent precursor (CaO) allows the steps of the CO2 capture process (carbonation and sorbent regeneration) to be decoupled8–11 by integrating a CaO/CaCO3 solid storage system. Although this type of approach may be suitable for coal-fired power plants, where the CO2 content in the flue gas is 10–15%v, the CaL technology is less effective for CO2 capture from gas turbine flue gases with a typical CO2 content of 3–4%v.13 In these cases the capture efficiency is limited by the CaO + CO2 ↔ CaCO3 equilibrium to values below 80% when the carbonator operates at standard temperatures around 650 °C as the CO2 equilibrium concentration is 1.2%v.14 Although studies on the integration of CaL systems with natural gas combined cycle (NGCC) power plants are scarce, most propose reducing this carbonation temperature to avoid equilibrium restrictions and achieve higher CO2 capture efficiencies.15–18 However, when the carbonation reaction temperature is reduced, the CO2 carrying capacity of the CaO sorbent decreases drastically19–21 and the carbonation reaction rate declines.22 As a result, increased solid circulation rates between the carbonator and calciner are needed for a given CO2 capture target. Also, the lower carbonation conversion of the CaO solids will require increasingly large solid storage volumes in the flexible CO2 capture system.8
To address the above-mentioned rate and conversion limitations, the use of finely powdered Ca(OH)2 (with a particle size of a few microns) as a CO2 sorbent has been proposed as an alternative to CaO23–26 by including an additional hydrator reactor in the standard CaL system (comprising only the carbonator and calciner reactors). It is recognized that Ca(OH)2 presents much faster carbonation kinetics, higher conversion in the temperature range of 500–650 °C even under low CO2 concentrations and high stability maintained along cycling.23,24,27 As a result, the sorbent and make-up flow requirements are minimized and more compact carbonator reactors with reduced gas/solid contact times can be used.28 For this application, and considering the small particle size and poor fluidization properties of Ca(OH)2, entrained bed gas–solid reactor configurations, similar to those used commercially for dry flue gas desulfurization,29 and pre-calciners, as those used in cement plants,30 could be used for the CO2 capture step and the calcination of the carbonated sorbent, respectively. Moreover, other reactor configurations such as multiple cyclonic reactors31 could also be proposed. However, the use of Ca(OH)2 as a sorbent instead of CaO results in thermal penalties linked with its low carbonation enthalpy (+72 kJ mol−1 at 650 °C). Thus, the amount of heat that can be recovered from the carbonator is considerably lower than that of standard CaL systems using CaO as a sorbent. In addition to this, the flue gas needs to be introduced to the carbonator at temperatures above 500 °C to ensure high carbonation conversion within short reaction times.28
Most of the previous studies reported in the literature on Ca(OH)2-based CaL systems23,25,26 are aimed at capturing CO2 from fuel-fired power plants operated at base load with high capacity factors. For these processes, the carbonator, calciner, and hydrator reactors are directly interconnected and continuously operated to capture CO2 in steady state mode.23,25,26 So, in this work the integration of a flexible CaL system based on Ca(OH)2 to capture CO2 from a NGCC power plant, which is operated as a backup power system, is investigated. The proposed flexible Ca(OH)2-based CaL system uses an intermediate storage of CaCO3 and Ca(OH)2-rich solids to decouple the CO2 capture step from the sorbent regeneration step.32 The objective of the process integration study is to minimize the energy penalty during short periods when backup power is required while shifting the energy penalties to periods of low-power demand. A basic economic analysis is performed to estimate the cost of CO2 avoided of the proposed system.
2. Process description
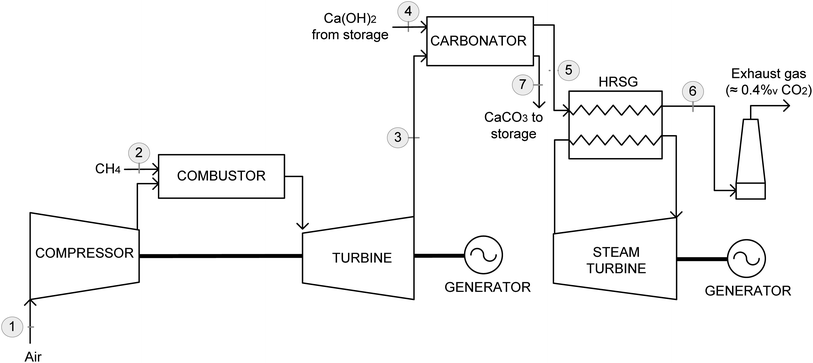
A general scheme of the CO2 capture process evaluated in this study is shown in Fig. 1, coupled with a state-of-the-art NGCC power plant. This process is similar to those of other CaL systems that are adapted to backup power plants based on CaO, including the carbonator and calciner reactors integrated with a sorbent storage system to decouple the CO2 capture and sorbent regeneration steps.8,9,11 Solid silos, similar to those commercially available on a large scale for the cement and lime industries,30 can be used as suitable sorbent storage systems. Moreover, an additional hydrator is included to produce Ca(OH)2 from the CaO solids leaving the oxy-calciner so the CaL system benefits from the advantages of Ca(OH)2 as an efficient and rapid CO2 sorbent.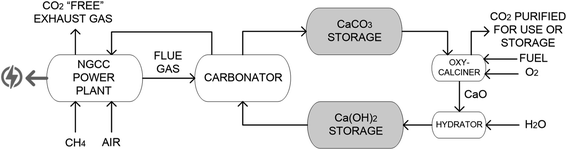 | ||
| Fig. 1 Schematic of the highly flexible backup NGCC power plant with CO2 capture using a CaO/Ca(OH)2/CaCO3 loop, including Ca(OH)2 and CaCO3 storage silos. | ||
As stated in the Introduction section, the use of Ca(OH)2 presents certain drawbacks due to the lower carbonation enthalpy and the higher temperature required for the gas entering the carbonator. In the proposed system, the first problem is compensated by the simplicity of the carbonation step. Thus, the carbonator is considered as an adiabatic reactor with no heat recovery in the steam cycle (i.e., without heat transfer surfaces to recover the heat released during the process). This facilitates the operation of the power plant and carbonator (especially during start-up and shut-down periods) and reduces the cost associated with the CO2 capture equipment, which typically results in a large economic penalty during operation under low CFs. The second problem is addressed by integrating the carbonator between the exit of the natural gas turbine and the inlet of the heat-recovery steam generator (HRSG), as discussed in the following section. This is similar to previous base-load steady-state NGCC-CaL systems proposed in the literature.16–18 Moreover, the temperature required for the gas entering the carbonator (i.e., above 500 °C) conveniently corresponds to the typical turbine outlet gas temperatures, which are in the range of 580–640 °C.33
In the proposed system, when the backup NGCC power plant enters into operation, the flue gas leaving the gas turbine is fed into the carbonator, where it is contacted with Ca(OH)2 from a storage silo to react with the CO2 present in the flue gas. At the carbonator exit, the carbonated solids are separated from the decarbonized flue gas and stored in another silo. Subsequently, the decarbonized flue gas is sent to the NGCC power plant to recover the heat contained in this stream in the HRSG section (not shown in Fig. 1 for simplicity) before being released to the atmosphere.
In contrast to the intermittent operation of the NGCC power plant and carbonator, the sorbent regeneration block (right-hand side of Fig. 1) is operated at a steady state. Thus, a continuous flow of CaCO3-rich solids from the storage silo is continuously calcined in the oxy-calciner, where fuel is burned using O2. Then, the CaO solids separated from the CO2 gas stream are fed into the hydrator where they react with liquid water to produce Ca(OH)2. The obtained Ca(OH)2 is stored in a silo for use during the next period of the NGCC backup power block operation. The heat available from the gas and solid streams leaving the oxy-calciner can be recovered for power generation in a small steam cycle, as in base-load operated CaL systems, or used to preheat the CaO and O2 streams to minimize the energy demand of the oxy-calciner block, as presented subsequently.
2.1. Reference natural gas combined cycle power plant
An NGCC based on a single-cycle gas turbine was used as the reference backup system. The NGCC power plant, including the gas turbine and HRSG, was modeled using the Aspen Hysys® software to solve the mass and heat balances in the steady-state mode during the operating periods, marked by step changes. The transient periods during the start-up and shut-down processes of the backup power plant were not considered in this study.This system is considered to operate with a CF of 0.1 during a maximum period of 5 h. The basic scheme of this power plant is shown in Fig. 2. The main operational parameters were selected based on the data available in the literature as inputs to the process model.34,35 The gas turbine produces 70 MWe and is assumed to operate at a compression pressure ratio of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.33 Moreover, it has a single efficiency of 36.8% and an isentropic efficiency of 90%.36 To simplify this example, it is assumed that the fuel is composed of CH4 with a lower heating value of 50 MJ kg−1. During operation, a fuel flow of 3.8 kg s−1 is burned producing 166.7 kg s−1 of flue gas with a composition of 4%v CO2, 8%v H2O, 12.2%v O2 and the rest is N2. The flue gas exits the turbine at 625 °C and 1.04 bar. A pressure loss on the hot side of the HRSG of 2.8% is assumed.37 The heat available from the flue gas is recovered in an HRSG, which includes a steam cycle operated with live steam at 565 °C and 166.5 bar. The main conditions of this cycle are shown in Fig. 3.34,35 This allows an additional power of 40.8 MWe to be produced, which results in a combined cycle efficiency of 58.3% (ηRef, being calculated as the ratio between the electrical power output, 110.8 MWe, and the thermal power input from the fuel, 190 MWth).
1.33 Moreover, it has a single efficiency of 36.8% and an isentropic efficiency of 90%.36 To simplify this example, it is assumed that the fuel is composed of CH4 with a lower heating value of 50 MJ kg−1. During operation, a fuel flow of 3.8 kg s−1 is burned producing 166.7 kg s−1 of flue gas with a composition of 4%v CO2, 8%v H2O, 12.2%v O2 and the rest is N2. The flue gas exits the turbine at 625 °C and 1.04 bar. A pressure loss on the hot side of the HRSG of 2.8% is assumed.37 The heat available from the flue gas is recovered in an HRSG, which includes a steam cycle operated with live steam at 565 °C and 166.5 bar. The main conditions of this cycle are shown in Fig. 3.34,35 This allows an additional power of 40.8 MWe to be produced, which results in a combined cycle efficiency of 58.3% (ηRef, being calculated as the ratio between the electrical power output, 110.8 MWe, and the thermal power input from the fuel, 190 MWth).
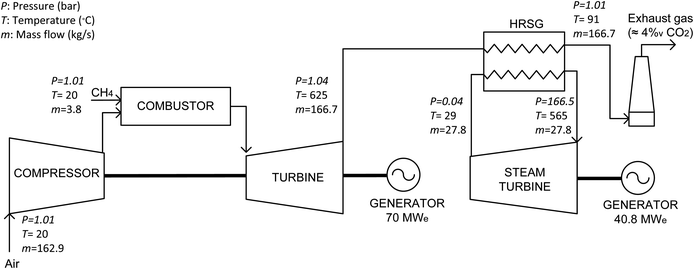 | ||
| Fig. 2 Simplified schematic of the reference NGCC. Reported pressure (P in bar), temperature (T in °C), and mass flow (m in kg s−1). | ||
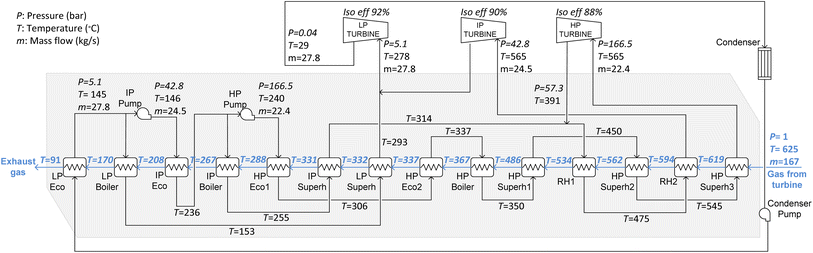 | ||
| Fig. 3 Reference HRSG. Reported pressure (P in bar), temperature (T in °C), and mass flow (m in kg s−1) corresponding to the reference NGCC presented in Fig. 2. “Iso eff” refers to the isentropic efficiency assumed for the turbines. | ||
3. Results and discussion
In this section, a case study is analyzed to demonstrate the operation and performance of the process shown in Fig. 1. As for the NGCC power plant, the carbonator, oxy-calciner, and hydrator reactors were modeled in Aspen Hysys®. Mass and heat balances were solved in the steady-state mode during the operating periods. For the power plant with CO2 capture, the start-up time was considered similar to those of standard NGCCs38 because carbonator preheating is not required to achieve the reaction conditions.3.1. Integration of the backup CO2 capture process
Fig. 4 and 5 show the integration of the CO2 capture system and regeneration of the sorbent in the backup power plant. As presented above, the carbonator is located at the exit of the gas turbine, and the decarbonized flue gas is fed into the HRSG (Fig. 4) to capture 90% of the CO2 produced in the NGCC power plant. The data for the gas and solid streams of the integrated process are listed in Table 1. For simplicity, it was assumed that an ideal separation of the solids and gases occurred at the exit of the carbonator, oxy-calciner, and hydrator reactors by using high efficiency cyclones, aided by the agglomeration tendency of the very fine powder used. In a non-ideal case, a make-up flow of limestone should be fed into the calciner to maintain the inventory of sorbent and compensate for solid losses.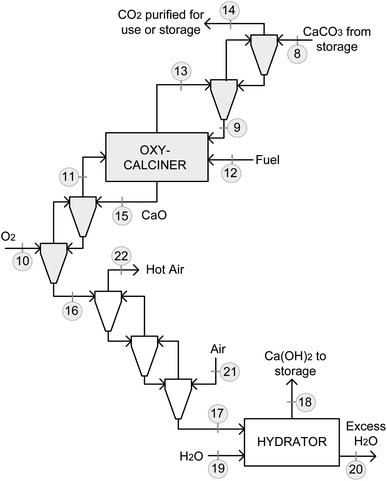 | ||
| Fig. 5 Sorbent regeneration proposed for the backup CO2 capture system including the oxy-calcination of the carbonated sorbent (in grey) and the hydration of the CaO (in white). | ||
| No. (Fig. 4 and 5) | Description | Temperature (°C) | Mass flow (kg s−1) | Composition (%wt for solids and %v for gases/liquids) |
|---|---|---|---|---|
| 1 | Air to gas turbine | 20 | 162.9 | 21%v O2, 79%v N2 |
| 2 | Fuel to combustor | 20 | 3.8 | 100%v CH4 |
| 3 | Gas from turbine to carbonator | 650 | 166.7 | 12.2%v O2, 75.8%v N2, 4.0%v CO2, 8.0%v H2O |
| 4 | Ca(OH)2 from storage to carbonator | 100 | 22.4 | 100%wt Ca(OH)2 |
| 5 | Gas from carbonator to HRSG | 602 | 162.8 | 12.0%v O2, 74.7%v N2, 0.4%v CO2, 12.9%v H2O |
| 6 | Gas outlet HRSG | 95 | ||
| 7 | CaCO3 from carbonator to storage | 602 | 26.2 | 80.6%wt CaCO3, 19.4%wt CaO |
| 8 | CaCO3 from storage to oxy-calciner | 602 | 2.6 | |
| 9 | Preheated CaCO3 to oxy-calciner | 765 | ||
| 10 | O2 to oxy-calciner | 20 | 0.4 | 100%v O2 |
| 11 | Preheated O2 to oxy-calciner | 875 | ||
| 12 | Fuel to oxy-calciner | 20 | 0.1 | 100%v CH4 |
| 13 | CO2 from oxy-calciner | 920 | 1.4 | 3.4%v O2, 68.5%v CO2, 28.1%v H2O |
| 14 | CO2 purified to use or storage | 663 | ||
| 15 | CaO from oxy-calciner | 920 | 1.7 | 100%wt CaO |
| 16 | CaO to hydrator block | 709 | ||
| 17 | CaO to hydrator | 80 | ||
| 18 | Ca(OH)2 to storage | 100 | 2.2 | 100%wt Ca(OH)2 |
| 19 | H2O(l) to hydrator | 20 | 1.3 | 100%v H2O |
| 20 | H2O excess exit hydrator | 100 | 0.7 | |
| 21 | Air for cooling CaO | 20 | 2.6 | 21.0%v O2, 79.0%v N2 |
| 22 | Hot air after cooling CaO | 376 |
In the integration scheme shown in Fig. 4, the carbonator induces a certain pressure drop in the system, affecting the conditions of the flue gas and thus the turbine efficiency. A conservative pressure drop in the carbonator reactor of 10% was assumed.39,40 This increases the outlet turbine pressure up to 1.16 bar, resulting in an isentropic temperature of 652 °C at a heat capacity ratio of 1.37. Consequently, the temperature of the flue gas leaving the turbine is approximately 650 °C. As a result, the turbine power generation is reduced from 70 MWe (as in the configuration of Fig. 2) to 65 MWe. Under these new operating conditions, a single efficiency of 34.2% is obtained for the gas turbine shown in Fig. 4.
Owing to the lower carbonation enthalpy of Ca(OH)2, the heat balance in the adiabatic carbonator is governed by the temperature of the gas and solids entering the reactor.28 The possibility of integrating residual heat from the power plant (i.e., that is contained in the exhausted flue gas from the HRSG) to preheat the sorbent before entering the carbonator is limited. Therefore, the temperature at which the sorbent enters the carbonator will be driven by the Ca(OH)2 storage conditions and, therefore, by the CaO hydration conditions. In the typical Ca(OH)2 production processes the hydrator consists of, for example, rotating paddles which agitate the lime in the presence of water.30 The strong exothermic reaction between water and CaO takes place in these reactors at an average temperature of approximately 100 °C, with the reaction heat (+104 kJ mol−1 of CaO) moderated by the addition of excess water (typically at a water-to-lime ratio in the range of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ref. 41)). Based on this, Ca(OH)2 solids are stored and fed to the carbonator reactor at 100 °C. This temperature also prevents the dehydration of the bulk of the solids as the equilibrium partial steam pressure is just 7 × 10−8 bar.42
1 (ref. 41)). Based on this, Ca(OH)2 solids are stored and fed to the carbonator reactor at 100 °C. This temperature also prevents the dehydration of the bulk of the solids as the equilibrium partial steam pressure is just 7 × 10−8 bar.42
When feeding the gas and solids to the carbonator at temperatures of 650 °C and 100 °C respectively, the temperature at the exit of the carbonator reactor was calculated as 602 °C. At such temperature the molar conversion of Ca to CaCO3 can be up to 0.7 for a gas–solid contact time of just 4 s.28 Therefore, for a capture efficiency of 90%, a Ca(OH)2 flow of 22.4 kg s−1 is required. After the carbonation reaction the gas and solid streams are separated. Due to the moderate temperature of such streams, the most favorable option to separate the solids from the gas before entering the HRSG is high efficiency cyclones as mentioned above. The tendency of solids to agglomerate also facilitates their separation from the gas phase by enhancing the cyclone efficiency.23 If required, a filter or electrostatic precipitator could be as well added before the stack after the HRSG, to further recover possible particles dragged by the gas flow, taking into account that contents about 30 g m−3 are common in heat recovery systems.43 Then, the carbonated solids are stored and the decarbonized flue gas (162.8 kg s−1) is transferred to the HRSG for heat recovery. An integration scheme similar to that in the reference power plant (Fig. 3) was used to recover heat from the flue gas, in this case at 602 °C. This scheme allows the flue gas to cool down to a temperature of 95 °C before being exhausted, producing an additional power of 38.5 MWe in the steam cycle. During the power-production periods a total of 103.5 MWe is generated in the backup NGCC power plant with CO2 capture. When compared with the reference NGCC without capture, the combined cycle efficiency is reduced to 54.5% (the reference being 58.3%).
As shown in Fig. 4, the CaCO3-rich solids leaving the carbonator are directly transferred to the CaCO3 storage at the outlet carbonator temperature. This will help to minimize the energy consumption in the oxy-calciner. For applications aimed at long-term storage and/or with larger sorbent requirements, the carbonated sorbent can be cooled down to facilitate storage operations. In such a case, the heat available from the solids leaving the carbonator can be integrated into the steam cycle of the HRSG to minimize the energy penalties.
To operate the backup power plant for a maximum of 5 h, the total amount of stored Ca(OH)2 is 403 ton (and 472 ton of CaCO3-rich solids). To achieve this requirement, a continuous flow of CaCO3-rich solids (2.6 kg s−1, an order of magnitude smaller than the equivalent molar flow of Ca(OH)2 fed into the carbonator) is fed from the CaCO3 silo into the oxy-calciner (Fig. 5). For simplicity, this reactor uses the same fuel as that in the NGCC power plant and is burnt with O2. The oxygen used in the calciner is assumed to be produced in an air separation unit. However, in the future, low-cost hydrogen produced by water electrolysis could be available extensively, and the oxygen obtained as a sub-product may be used to minimize the energy penalty associated with oxy-calcination. Moreover, alternative fuels, such as biomass (to achieve negative emissions) or electrolytic green-H2 obtained from renewable electricity (to electrify the CO2 capture process), may be used in the oxy-calciner, especially considering its reduced thermal capacity, as discussed below.
In standard CaL systems, the heat available in the CO2-rich flue gas and CaO solids leaving the oxy-calciner is used to produce additional power in a dedicated steam cycle. However, because of the low thermal capacity of the oxy-calciner in this application, the heat available is used to preheat the carbonated sorbent coming from the CaCO3 storage and the oxygen fed into the calciner. The integration scheme for preheating these streams is shown in Fig. 5. Thus, the CO2-rich flue gas is contacted with the carbonated solids in a 2-step cyclonic preheater, similar to those used in cement plants to increase the temperature of the raw meal. Similarly, oxygen fed into the calciner is preheated using CaO solids in a 2-step cyclonic preheater. The calciner is operated at a temperature of 920 °C to ensure complete calcination of the sorbent. Therefore, the integration scheme shown in Fig. 5 allows the preheating of the carbonated solids and oxygen up to temperatures of 765 and 875 °C, respectively, before entering the calciner. Consequently, a thermal input into the oxy-calciner of 4.4 MWth is calculated, which is produced by combusting 0.1 kg s−1 of CH4 with 0.4 kg s−1 of O2. The CO2-rich flue gas with a flow of 1.4 kg s−1 is separated from the calcined solids and then transferred to a compression and purification unit (not shown in Fig. 5 for simplicity). To reduce the energy demand during calcination or prevent the potential problems associated with the preheating steps for pure O2 there are additional possibilities for thermal integration. For example, Robin et al.44 developed a similar oxy-fired calciner in which hot air produced during the cooling of CaO was used to preheat part of the CaCO3 feed to the calciner. However, it is noteworthy that in the system here proposed, the energy cost can be very low during the regeneration stages. Therefore, intensive integration efforts to achieve adequate economics may not be required.
As presented above, the CaO produced during calcination is used to obtain Ca(OH)2via the standard hydration method. In the case of the proposed scheme shown in Fig. 5, the flow of hot CaO solids must be cooled before being fed into the hydrator. This is achieved by contacting the solids with an air flow of 2.6 kg s−1 at 20 °C in a 3-step cyclonic cooler, which reduces the temperature of CaO to 80 °C. In the hydrator, liquid water is added in excess of 2.3 relative to the stoichiometric value to control the temperature and operate the hydrator at a typical temperature of 100 °C.30,41 This results in a water flow consumption of 1.3 kg s−1 and an excess of 0.7 kg s−1, which is discharged as steam into the atmosphere. After hydration, the Ca(OH)2 flow produced (2.2 kg s−1) is transferred to the storage silo.
To estimate the energy penalty associated with sorbent regeneration, specific power consumptions of 200 kW he per tO2 and 120 kW he per tCO2 were assumed for the air separation and CO2 compression and purification units, respectively.45 Additionally, a power consumption of 0.15 kWe relative to the kWth fed into the calciner was considered for the auxiliaries used in the sorbent regeneration block.46 Based on these assumptions, a global efficiency (ηGlobal) of 38.2% was calculated using eqn (1), where Pth, CC and Pth, oxy are the thermal inputs to the gas turbine and oxy-calciner blocks (190 and 4.4 MWth, respectively), Pe, CC is the electric power output from the NGCC in Fig. 4 (103.5 MWe), and Pe, O2, Pe, CO2, and Pe, aux are the electrical consumption (1.4 MWe) for the air separation, CO2 compression and purification, and auxiliaries, respectively.
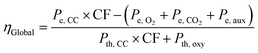 | (1) |
4. Cost analysis
A basic economic analysis was performed to estimate the cost of CO2 avoided (AC). A NGCC power plant with an emission factor of 336 kgCO2 kW−1 he−1 was used as the reference system, and the AC was calculated using eqn (2):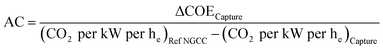 | (2) |
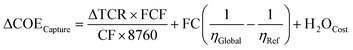 | (3) |
| ΔTCR = (TCRCarbo + TCRCPUCF + 2TCRStoragetStorage)fNGCC + (TCROxy + TCRHyd + TCRASU)fOxy | (4) |
| Parameter | Units | NGCC + CaO/Ca(OH)2/CaCO3 CaL |
|---|---|---|
| Increment of total capital requirements, ΔTCR | $ per kWth ($ per kWe) | 94 (245) |
| Fraction of power to NGCC, fNGCC | — | 0.81 |
| Fraction of power to oxy-calciner/hydrator, fOxy | — | 0.19 |
| Fixed charge factor, FCF | Per year | 0.1 |
| Fuel cost, FC | $ per GJ | 10 |
| Water cost, H2OCost | $ per m3 | 1.5 |
| Global efficiency, ηGlobal | kWe kWth−1 | 0.382 |
| Reference NGCC efficiency, ηRef | kWe kWth−1 | 0.583 |
| Storage time, tStorage | h | 5 |
| CO2 emission factor for the reference NGCC, (CO2 per kW per he)Ref NGCC | kg CO2 per MW per he | 336 |
| CO2 emission factor, (CO2 per kW per he)Capture | kg CO2 per MW per he | 36 |
| ΔCOECapture (CF = 0.1) | $ per kW per he | 0.061 |
| AC (CF = 0.1) | $ per tCO2 | 204 |
The cost of the oxy-calciner and gas/solid preheating system (TCROxy) was assumed to be 125 $ per kWth, which is equivalent to a cement plant pre-calciner with cyclone suspension preheaters, where approximately 90% of the cost is attributed to the preheater system.50 The cost associated with the adiabatic carbonator (that is, a refractory bed) was considered to be identical to that of a pre-calciner (TCRCarbo = 15 $ per kWth). The cost associated with the Ca(OH)2 and CaCO3 storage silos and their corresponding handling equipment was calculated based on a limestone cost of 10 $ per t (ref. 51) and the silos were dimensioned to operate for a maximum NGCC operation period (tStorage) of 5 h, resulting in a TCRStorage of 0.2 $ per kW per hth. The costs associated with the CPU and ASU (TCRCPU and TCRASU) were 80 and 110 $ per kWth, respectively.45 Finally, the cost of CaO hydration (TCRHyd) was estimated based on the cost of the cyclone suspension preheaters (115 $ per kWth)50 that are required to cool down the calcined solids plus the cost of the hydrator (40 $ per kWth),52 resulting in a TCRHyd of 155 $ per kWth.
As listed in Table 2, the calculated increment in the cost of electricity is 0.061 $ per kW per he, which is a reasonable value considering the extremely low capacity factor assumed (CF = 0.1). This results in a cost of CO2 avoided of 204 $ per tCO2 according to eqn (2). The flexible CO2 capture system evaluated in this study is a feasible option for retrofitting amortized natural gas power plants in future scenarios with high carbon prices and a large portion of renewables. Although the carbon prices required to make these CO2 capture systems economically feasible exceed the current prices, this technology may serve as a complementary system for low-carbon power mixes.53 Moreover, it may be considered a reasonable compromise to “close the carbon loop” in power-to-gas-to-power systems involving the manufacture of synthetic fuels from CO2, which otherwise leak CO2 into the atmosphere during the gas-to-power step.
5. Conclusions
CaL systems using Ca(OH)2 as a sorbent can be integrated into NGCC power plants operating at an extremely low capacity factor, benefiting from the favorable kinetics and carrying capacities of the sorbent. For this purpose, intermediate storage of the solids was used to decouple CO2 capture and sorbent regeneration. This reduces the capacity of elements related to sorbent regeneration and the investment cost while minimizing the energy penalty during power-production periods.A case for an NGCC power plant with a thermal input of 190 MWth and operating at a CF of 0.1 was evaluated. The CO2 capture process was integrated with minor modifications to the operating conditions of the gas turbine and heat-recovery steam generator. The power delivered by the NGCC power plant was reduced by 6.6% relative to the conventional system without CO2 capture during power-production periods. The thermal input of the oxy-calciner required to regenerate the sorbent was just 4.4 MWth resulting in a global efficiency of 38.2%. Storage capacities of 403 and 472 tons of Ca(OH)2 and CaCO3-rich solids, respectively, were required to operate the gas turbine for maximum periods of 5 h. To minimize thermal input into the calciner, the carbonated sorbent was stored at the carbonator outlet temperature (602 °C). A basic economic analysis was performed, indicating a cost of CO2 avoided of approximately 200 $ per tCO2. This suggests that the proposed system is a feasible option for capturing CO2 from natural gas power plants in future scenarios with high carbon prices and a large share of renewables in the global energy mix, or for closing the carbon loop in emerging power-to-gas-to-power energy storage systems.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge the financial support provided by the European Union under the Research Fund for Coal and Steel (RFCS) Program (BackCap Project, GA 10103400) and the Spanish Ministry of Science and Innovation under the R&D Program Oriented to Challenges of Society (Grant number: RTI2018-097224-B-I00).References
- IPCC, Climate change 2022 – mitigation of climate change, summary for policymakers, Working Group III contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 2022 Search PubMed.
- IEA, European Union 2020: energy policy review, International Energy Agency, Paris, France, 2020 Search PubMed.
- I. Tsiropoulos, W. Nijs, D. Tarvydas and P. Ruiz Castello, Towards net-zero emissions in the EU energy system by 2050 – insights from scenarios in line with the 2030 and 2050 ambitions of the European Green Deal, EUR 29981 EN, Luxembourg, 2020 Search PubMed.
- M. A. Gonzalez-Salazar, T. Kirsten and L. Prchlik, Renewable Sustainable Energy Rev., 2018, 82, 1497–1513 CrossRef CAS.
- IRENA, Flexibility in conventional power plants: innovation landscape brief, International Renewable Energy Agency, Abu Dhabi, 2019 Search PubMed.
- A. S. Brouwer, M. van den Broek, A. Seebregts and A. Faaij, Appl. Energy, 2015, 156, 107–128 CrossRef CAS.
- A. M. Abdilahi, M. W. Mustafa, S. Y. Abujarad and M. Mustapha, Renewable Sustainable Energy Rev., 2018, 81, 3101–3110 CrossRef CAS.
- Y. A. Criado, B. Arias and J. C. Abanades, Energy Environ. Sci., 2017, 10, 1994–2004 RSC.
- M. Astolfi, E. De Lena and M. C. Romano, Int. J. Greenhouse Gas Control, 2019, 83, 140–155 CrossRef CAS.
- M. Astolfi, E. De Lena, F. Casella and M. C. Romano, Appl. Therm. Eng., 2021, 194, 117048 CrossRef CAS.
- B. Arias, Y. A. Criado and J. C. Abanades, ACS Omega, 2020, 5, 4844–4852 CrossRef CAS.
- J. C. Abanades, B. Arias, A. Lyngfelt, T. Mattisson, D. E. Wiley, H. Li, M. T. Ho, E. Mangano and S. Brandani, Int. J. Greenhouse Gas Control, 2015, 40, 126–166 CrossRef CAS.
- NETL, Cost and performance baseline for fossil energy plants volume 1: bituminous coal and natural gas to electricity, National Energy Technology Laboratory, United States, 2019 Search PubMed.
- A. W. D. Hills, Inst. Min. Metall., 1967, 76, C241–C245 Search PubMed.
- Y. Hu and H. Ahn, Appl. Energy, 2017, 187, 480–488 CrossRef CAS.
- M. Erans, D. Hanak, J. Mir, E. Anthony and V. Manovic, Therm. Sci., 2016, 20, 59–67 CrossRef.
- C. Fu, S. Roussanaly, K. Jordal and R. Anantharaman, Front. Chem. Eng., 2021, 2, 28 Search PubMed.
- D. Berstad, R. Anantharaman, R. Blom, K. Jordal and B. Arstad, Int. J. Greenhouse Gas Control, 2014, 24, 43–53 CrossRef CAS.
- Y. A. Criado, B. Arias and J. C. Abanades, Ind. Eng. Chem. Res., 2018, 57, 12595–12599 CrossRef CAS.
- Z. Li, F. Fang, X. Tang and N. Cai, Energy Fuels, 2012, 26, 2473–2482 CrossRef CAS.
- V. Manovic and E. J. Anthony, Ind. Eng. Chem. Res., 2010, 49, 9105–9110 CrossRef CAS.
- S. K. Bhatia and D. D. Perlmutter, AIChE J., 1983, 29, 79–86 CrossRef CAS.
- N. Phalak, W. Wang and L.-S. Fan, Chem. Eng. Technol., 2013, 36, 1451–1459 CrossRef CAS.
- L. S. Fan, S. Ramkumar, W. Wang and R. Statnick, US Pat., US 8512611 B2, 2013 Search PubMed.
- W. Wang, S. Ramkumar, D. Wong and L. S. Fan, Fuel, 2012, 92, 94–106 CrossRef CAS.
- F. Zeman, Int. J. Greenhouse Gas Control, 2008, 2, 203–209 CrossRef CAS.
- B. Arias, Y. A. Criado, B. Pañeda and J. C. Abanades, Ind. Eng. Chem. Res., 2022, 61, 3272–3277 CrossRef CAS PubMed.
- Y. A. Criado and B. Arias, ACS Omega, 2022, 7, 28093–28100 CrossRef.
- A. M. Carpenter, Low water FGD technologies, IEA Clean Coal Centre, 2012 Search PubMed.
- F. Schorcht, I. Kourti, B. M. Scalet, S. Roudier and L. Delgado Sancho, Best Available Techniques (BAT) reference document for the production of cement, lime and magnesium oxide, Publications Office of the European Union, Luxembourg, 2013 Search PubMed.
- W.-C. Chen, S. Ouyang, C.-M. Huang, C.-H. Shen and H.-W. Hsu, US Pat., US 9610537 B2, 2017 Search PubMed.
- J. C. Abanades, B. Arias and Y. A. Criado, EP20383113, European Patent Office, 2020.
- Siemens, Reliable gas turbines, https://www.siemens-energy.com/global/en/offerings/power-generation/gas-turbines.html Search PubMed.
- DOE/NETL, Cost and performance baseline for fossil energy plants volume 1a: bituminous coal (PC) and natural gas to electricity, Revision 3., US Department of Energy, National Energy Technology Laboratory, 2015 Search PubMed.
- IEAGHG, CO2 capture at gas fired power plants, 2012/8, International Energy Agency Greenhouse Gas R&D Programme, 2012 Search PubMed.
- M. M. Rahman, T. K. Ibrahim and A. N. Abdalla, Int. J. Phys. Sci., 2011, 6, 3539–3550 Search PubMed.
- S. Dybe, M. Bartlett, J. Pålsson and P. Stathopoulos, Sustainability, 2021, 13, 651 CrossRef.
- DOE/NETL, Impact of load following on power plant cost and performance: literature review and industry interviews, US Department of Energy, National Energy Technology Laboratory, 2012 Search PubMed.
- M. Spinelli, I. Martínez, E. De Lena, G. Cinti, M. Hornberger, R. Spörl, J. C. Abanades, S. Becker, R. Mathai, K. Fleiger, V. Hoenig, M. Gatti, R. Scaccabarozzi, S. Campanari, S. Consonni and M. C. Romano, Energy Procedia, 2017, 114, 6206–6214 CrossRef CAS.
- E. De Lena, M. Spinelli, M. Gatti, R. Scaccabarozzi, S. Campanari, S. Consonni, G. Cinti and M. C. Romano, Int. J. Greenhouse Gas Control, 2019, 82, 244–260 CrossRef CAS.
- R. S. Boynton, Chemistry and technology of lime and limestone, John Wiley & Sons, New York, 2nd edn, 1980 Search PubMed.
- I. Barin, Thermochemical data of pure substances, VCH Verlagsgesellschaft Weinheim, Germany, 1989 Search PubMed.
- M. Pronobis, Environmentally oriented modernization of power boilers, 1st edn, 2020 Search PubMed.
- C. Robin, C. Pierre-Olivier, S. B. Guthrie and J. C. Abanades, WO2022/049137A1, World Intellectual Property Organization, 2022.
- A. Darde, R. Prabhakar, J.-P. Tranier and N. Perrin, Energy Procedia, 2009, 1, 527–534 CrossRef CAS.
- L. M. Romeo, J. C. Abanades, J. M. Escosa, J. Paño, A. Giménez, A. Sánchez-Biezma and J. C. Ballesteros, Energy Convers. Manage., 2008, 49, 2809–2814 CrossRef CAS.
- NREL, Cost and performance data for power generation technologies, National Renewable Energy Laboratory, USA, 2012 Search PubMed.
- IEA-ETSAP, Technology brief E02: gas-fired power, International Energy Agency – Energy Technology Systems Analysis Programme, Paris, France, 2010 Search PubMed.
- G. Guandalini, M. C. Romano, M. Ho, D. Wiley, E. S. Rubin and J. C. Abanades, Int. J. Greenhouse Gas Control, 2019, 84, 219–231 CrossRef CAS.
- IEAGHG, CO2 capture in the cement industry, International Energy Agency Greenhouse Gas R&D Programme, 2008 Search PubMed.
- USGS, Crushed stone statistics and information, https://www.usgs.gov/centers/nmic/crushed-stone-statistics-and-information Search PubMed.
- P. Renforth, B. G. Jenkins and T. Kruger, Energy, 2013, 60, 442–452 CrossRef CAS.
- IEA, Projected costs of generating electricity 2020, International Energy Agency, Paris, France, 2020 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |