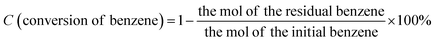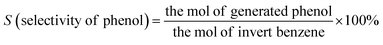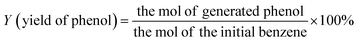Open Access Article
Open Access Articleg-C3N4-modified Zr-Fc MOFs as a novel photocatalysis-self-Fenton system toward the direct hydroxylation of benzene to phenol†
Xu Jiaa,
Cong Liua,
Xuetong Xua,
Fuying Wanga,
Weiwei Lia,
Liuxue Zhang *a,
Shuyan Jiao*a,
Genxing Zhua and
Xiulian Wangb
*a,
Shuyan Jiao*a,
Genxing Zhua and
Xiulian Wangb
aSchool of Materials and Chemical Engineering, Zhongyuan University of Technology, Zhengzhou, 450007, PR China. E-mail: zhanglx@zut.edu.cn; jiaosy@zut.edu.cn; Fax: +86-731-62506095; Tel: +86-731-62506699
bSchool of Energy and Environment, Zhongyuan University of Technology, Zhengzhou, 450007, PR China
First published on 23rd June 2023
Abstract
In order to explore a green, economic, and sustainable phenol production process, a heterojunction semiconductor materials g-C3N4/Zr-Fc MOF was synthesized via an in situ synthesis method. With the synergistic effect of photocatalysis and the Fenton effect, the composite could effectively catalyze the direct hydroxylation of benzene to phenol under visible light irradiation. The yield of phenol and the selectivity were 13.84% and 99.38% under the optimal conditions, respectively, and it could still maintain high photocatalytic activity after 5 photocatalytic cycles. Therefore, the designed photocatalysis-self-Fenton system has great potential in the field of the direct hydroxylation of benzene to phenol.
Introduction
Phenol is an important chemical intermediate in the industrial field. At present, it is mainly produced by the three-step isopropyl benzene process, which has serious limitations, such as dangerous intermediates and high energy consumption.1 Therefore, a green, economic, and sustainable phenol production process is urgently needed. In recent years, the direct hydroxylation of benzene has attracted increasing attention. This is a mild, economically green solution.2 Gu et al. achieved promising results with a phenol regimen using a light-induced benzene efficient hydroxylation, with high selectivity of up to 99% and good phenol yields.3 Owing to its cost-effectiveness and environmental friendliness, photocatalysis could be applied as a competitive method to achieve benzene hydroxylation into phenols.Recently, with the strong oxidation of hydroxyl radicals (·OH) the photocatalysis-self-Fenton process has been used by researchers to convert benzene into phenol.4 The process of photo-Fenton mainly involves the transformation between Fe(III) and Fe(II).5 Various iron-based MOFs have been used as photocatalyst for the direct hydroxylation of benzene to phenol. Xu proposed MIL-100(Fe) nanoparticles, which were synthesized by ethylene glycol with a uniform size and morphology to perform the photocatalytic direct hydroxylation of benzene experiments, and the result showed that the MIL-100(Fe) nanospheres possessed a good oxidation ability and high selectivity.6 The experiments into phenol production with iron-based MOFs reported by Wang et al. also confirmed the feasibility of benzene–phenol conversion using the Fenton effect of the Fe-based MOFs, whereby the high catalytic activity of iron-containing materials in the selective oxidation process enables the wide application of Fe-based MOFs for the direct hydroxylation of benzene to produce phenol.7 Owing to the tunable composition and structure of the iron-based MOF materials, they have great potential for the photocatalytic hydroxylation of benzene to produce phenol.8,9 To obtain better photocatalytic phenol performance, the iron-based MOF structure can be adjusted to extend the photoresponse range. Constructing heterojunctions is a promising strategy to improve the performance of the heterogeneous photo-Fenton reaction (PFR).10 As a photoresponse semiconductor, g-C3N4 has been often used to enhance the optical response range owing to its advantages of strong acid and alkali resistance, appropriate optical band range (2.76 eV), high thermal stability, and non-toxicity.11–13 Zehra Durmus et al. built a unique hybrid structure between Ce-MOFs and g-C3N4 to improve the photocatalytic performance.14 Devarayapalli et al. prepared g-C3N4/ZIF-67 heterojunction composites by a coupling cobalt–organic zeolite imidazole skeleton (ZIF-67) with g-C3N4 nanosheets synthesized by a simple microwave radiation method, which greatly improved the photocatalytic performance of the carbon nitride graphite.15 Reza Abazari synthesized Ti-MIL-125-NH2 modified with g-C3N4 by a simple-rapid ultrasonic method, which was then used as a visible-light photocatalyst with an improved photocatalytic performance.16 All these experimental protocols demonstrated that the combination of g-C3N4 and iron-based MOFs would enhance the photocatalytic performance.
In this investigation, we report an efficient photocatalysis-self-Fenton system, namely g-C3N4/Zr-Fc MOF, constructed by an in situ composite method. Thereinto, zirconium(IV), 1,1′-ferrocenedicarboxylic acid, and g-C3N4 were selected as the central metal ion, organic ligand, and support system, respectively. Due to the synergistic effect of the mixed metals in MOFs, Zr-Fc MOFs can provide more abundant electrons and holes.17–19 Besides, the heterojunction possesses a large specific surface area and stable structure. The hydroxyl radicals produced by the Fenton reaction between Fe(II) and hydrogen peroxide could have a synergistic effect with the presence of a bimetal to greatly improve the direct hydroxylation of benzene. At the same time, the band gap width of g-C3N4 is about 2.7 eV, which can effectively absorb sunlight and also it has a stable structure under light conditions.20 Therefore, the designed photocatalyst was expected to possess a better effect in the direct hydroxylation of benzene to phenol under visible light.
Experimental
Materials and chemicals
1,1′-Ferrocenedicarboxylic acid (98%) was purchased from Sun Chemical Technology (Shanghai) Co., Ltd. Zirconium tetrachloride (ZrCl4, 98%) was purchased from Guangdong Hachuring Chemical Reagent Co., Ltd. Melamine (99.5%) was purchased from Tianjin Guangfu Fine Chemical Research Institute. Acetic acid (AC) was purchased from Tianjin Tianli Chemical Reagent Co. Ltd. N-Dimethylformamide (AC) was purchased from Tianjin Fuyu Fine Chemical Co. Ltd. Benzene was purchased from Yantai Shuangshuang Chemical Co. Ltd. Ethanol (AC) was purchased from Tianjin Kermel Chemical Reagent Co., Ltd. Trifluoroacetic acid was purchased from Shanghai Macklin Biochemical Co., Ltd.The conversion rate of benzene, selectivity of phenol, and yield of phenol were calculated using the following three formulas:
Results and discussion
Sample structure characterization
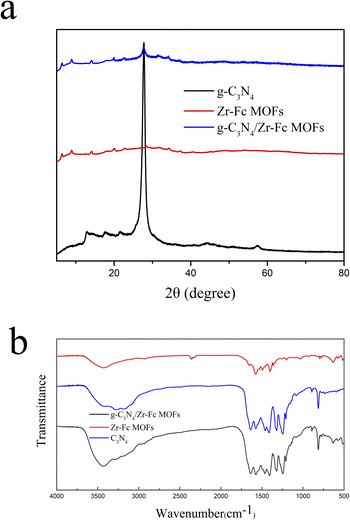
The morphologies of g-C3N4, Zr-Fc MOF, and g-C3N4/Zr-Fc MOF were observed by SEM. As shown in Fig. 1, the prepared g-C3N4 with a large specific surface area possessed a sheet stacked structure with an irregular appearance and different sizes.23 The Zr-Fc MOF possessed irregular sheet-like structures without agglomerates, which were consistent with previously reported SEM images.24 The g-C3N4/Zr-Fc MOF was combined based on the g-C3N4 and Zr-Fc MOF. The Zr-Fc MOF particles were evenly dispersed over the g-C3N4, basically maintaining the original morphologies of g-C3N4 and Zr-Fc MOF, which indicated the successful composition of the g-C3N4/Zr-Fc MOF material (Fig. 1c).The crystal structure of the sample as well as the crystallinity were investigated by X-ray diffraction, and the XRD profiles of g-C3N4, Zr-Fc MOF, and g-C3N4/Zr-Fc MOF are displayed in Fig. 2a. A strong diffraction peak occurring at 27.96° was the characteristic diffraction peak of the (002) crystal surface and corresponded to g-C3N4.25 The characteristic diffraction peaks of the Zr-Fc MOF were consistent with the previously reported literature. The diffraction peaks at 2θ = 6.35°, 8.93°, 14.01°, 19.94°, and 25.82° could be observed from the XRD pattern of Zr-Fc MOF, corresponding to the (100), (110), (210), (310), and (320) crystal faces of Zr-Fc MOF, respectively.26 The diffraction peaks of the g-C3N4/Zr-Fc MOF composites were consistent with the characteristic diffraction peaks of both g-C3N4 and Zr-Fc MOF, indicating that the g-C3N4/Zr-Fc MOF composites had been successfully prepared.
The structural information, such as chemical bonds and functional groups of g-C3N4, Zr-Fc MOF, and g-C3N4/Zr-Fc MOF, were acquired using the Fourier-transform infrared technique (Fig. 2b). For g-C3N4, the absorption peak at 813 cm−1 was attributed to the bending vibration peak of the triazine cycle in the g-C3N4 structure.27 The absorption peak at 487 cm−1 was assigned to the asymmetric expansion vibration peak of Zr-(OC),while the absorption peaks that appeared at 794, 1027, 1107, and 1492 cm−1 were attributed to the out-of-plane deformation vibration of C–H, C–H deformation vibration, C–C deformation vibration, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration on the cyclopentadienyl ring.28,29 This set of data suggested the formation of Zr-Fc MOF structures. The characteristic absorption peaks of ferrocene, Zr-(OC), and triazine cycle all appeared in the spectra of the composites, which indicated the successful preparation of the g-C3N4/Zr-Fc MOF.
C stretching vibration on the cyclopentadienyl ring.28,29 This set of data suggested the formation of Zr-Fc MOF structures. The characteristic absorption peaks of ferrocene, Zr-(OC), and triazine cycle all appeared in the spectra of the composites, which indicated the successful preparation of the g-C3N4/Zr-Fc MOF.
To explore the photoresponse range of the sample and calculate the band gap width of the catalyst, characterization analysis was performed by solid ultraviolet-visible diffuse reflectance spectroscopy. As shown in Fig. 3a, the photoresponse range of Zr-Fc MOF was 250–400 nm, with two absorption peaks noted at 260 and 380 nm, respectively. However, when the Zr-Fc MOF was modified with g-C3N4, the photoresponse range of the Zr-Fc MOF was 250–400 nm with a red-shift. The band gap of semiconductors can be calculated according to the equation (αhν)1/n = A(hν − Eg) (Fig. 3b). The band gap widths of g-C3N4, Zr-Fc MOF and g-C3N4/Zr-Fc MOF were 2.40, 2.13, and 2.03 eV, respectively. The band gap width of these semiconductors was gradually reduced, which indicated that the photocatalytic performance was gradually enhanced under visible light. Hence, the g-C3N4/Zr-Fc MOF might be a desired photocatalyst for benzene hydroxylation to produce phenol.
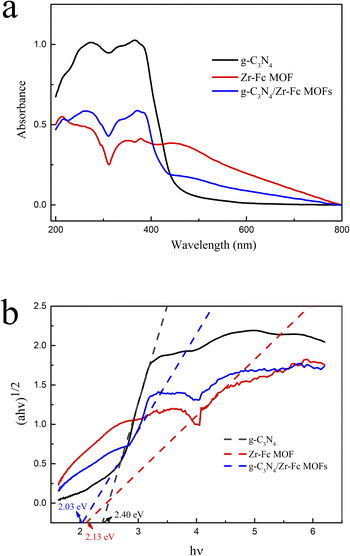 | ||
| Fig. 3 Solid UV diffuse reflection (a) and band gap diagram (c) of g-C3N4, Zr-Fc MOF, and g-C3N4/Zr-Fc MOF. | ||
The photogenerated hole–charge separation effect is a key factor in the photocatalytic organic oxidation reaction, whereby the higher the separation efficiency, the stronger the photocatalytic activity. Therefore, the photoluminescence performances of g-C3N4 and g-C3N4/Zr-Fc MOF were investigated at the emission wavelength of 375 nm (Fig. 4a). Compared to g-C3N4, the composite g-C3N4/Zr-Fc MOF displayed a lower fluorescence intensity at the wavelength of 440 nm. This phenomenon suggested that the g-C3N4/Zr-Fc MOF possessed a higher photogenerated hole–charge separation efficiency.
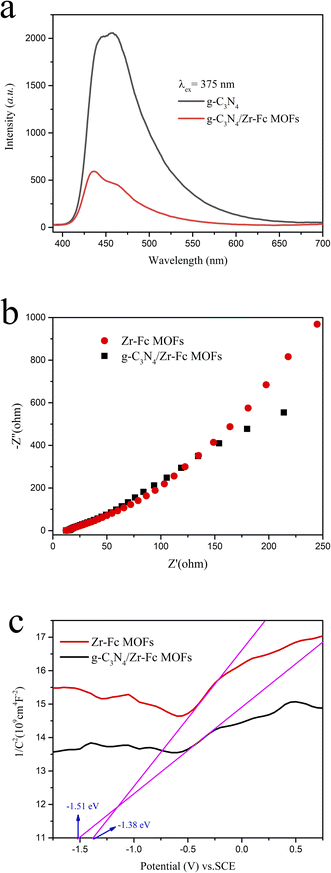 | ||
| Fig. 4 PL profiles (a) of g-C3N4 and g-C3N4/Zr-Fc MOF. Electrochemical impedance profiles (b) and Mott–Schottky diagram (c) of Zr-Fc MOF and g-C3N4/Zr-Fc MOF. | ||
The effect of photogenerated charge–hole recombination could be further observed by electrochemical impedance spectroscopy. The EIS Nyquist curves of Zr-Fc MOF and g-C3N4/Zr-Fc MOF electrodes were detected under light avoidance conditions (Fig. 4b). When g-C3N4 was introduced to Zr-Fc MOF, the g-C3N4/Zr-Fc MOF showed a smaller radius of the Nyquist circle and smaller transfer resistance, which implied the composite possessed a lower recombination rate of photogenerated carriers. By fitting calculations, the charge-transfer resistance (Rct) and solution resistance (Rs) of the g-C3N4/Zr-Fc MOF were calculated to be 6.97 and 9.39 Ω, respectively. The charge-transfer resistance (Rct) and solution resistance (Rs) of Zr-Fc MOF were 38.33 and 96.16 Ω, respectively. Hence, the g-C3N4/Zr-Fc MOF displayed a higher photocatalytic performance.
The information of the semiconductor type and flat band potential could be obtained via Mott–Schottky curves. In order to investigate the effect of g-C3N4 introduction to Zr-Fc MOF on the band structure, Mott–Schottky curves of Zr-Fc MOF and g-C3N4/Zr-Fc MOF semiconductors were obtained by the Mott–Schottky measurement method (Fig. 4c). The two curves revealed that the tangent line presented a positive slope at the frequency of 1000 Hz, which indicated that the Zr-Fc MOF and g-C3N4/Zr-Fc MOF were both typical N-type semiconductors. Further analysis showed that the flat band potential of the Zr-Fc MOF and g-C3N4/Zr-Fc MOF were about −1.38 and −1.51 V, respectively. Previous studies have reported that the conduction band (CB) potential of N-type semiconductors is negative 0.1 V compared with its flat band potential.30 It could be found that g-C3N4/Zr-Fc MOF had a more negative conduction band potential than Zr-Fc MOF, which demonstrated that the incorporation of g-C3N4 was valuable for the improvement of the photogenerated charge–hole separation efficiency. In conclusion, as the composite rate of the photocharged holes could be effectively suppressed, the photocarrier lifetime could be extended, and the photoelectrons could be effectively transferred to produce active free radicals, which could be applied for the photocatalytic benzene hydroxylation reaction.
Photocatalytic experiments
| Sample | Yield (%) | Conversion (%) | Selectivity (%) |
|---|---|---|---|
| a Reaction conditions: catalyst (1.5 mg mL−1), benzene (1.6 mL, 18.05 mmol), H2O2 (30 wt%) (0.6 mL), acetonitrile (11 mL), trifluoroacetic acid (0.1 g), time: 4 h, and T: 333 K. | |||
| g-C3N4 | 4.78 | 4.92 | 97.15 |
| Zr-Fc MOF | 8.80 | 8.95 | 98.32 |
| g-C3N4/Zr-Fc MOF | 13.84 | 13.93 | 99.38 |
| Entry | m (mg mL−1) | Yield (%) | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|
| a Reaction conditions: benzene (1.6 mL, 18.05 mmol), H2O2 (30 wt%) (0.6 mL), acetonitrile (11 mL), trifluoroacetic acid (0.1 g), time: 4 h, and T: 333 K. | ||||
| 1 | 0 | 4.87 | 5.03 | 96.81 |
| 2 | 0.75 | 7.69 | 7.96 | 96.61 |
| 3 | 1.50 | 13.84 | 13.93 | 99.38 |
| 4 | 2.25 | 8.48 | 8.85 | 95.82 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. However, when the amount of H2O2 was increased, the yield of phenol, the conversion rate of benzene, and the selectivity of phenol gradually decreased. This might be due to the H2O2 undergoing a self-decomposition during the reaction, and the self-decomposition rate was faster than that of benzene hydroxylation, which would cause the reaction solution to gradually become two phases, thus hindering the interaction between benzene and the catalyst.33 Furthermore, the effect of light on benzene hydroxylation to produce phenol was also explored under the same reaction conditions with the volume ratio of n(H2O2)/n(benzene) = 8
3. However, when the amount of H2O2 was increased, the yield of phenol, the conversion rate of benzene, and the selectivity of phenol gradually decreased. This might be due to the H2O2 undergoing a self-decomposition during the reaction, and the self-decomposition rate was faster than that of benzene hydroxylation, which would cause the reaction solution to gradually become two phases, thus hindering the interaction between benzene and the catalyst.33 Furthermore, the effect of light on benzene hydroxylation to produce phenol was also explored under the same reaction conditions with the volume ratio of n(H2O2)/n(benzene) = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. When there was no visible-light irradiation, the yield of phenol was 8.17%, which implied that only the Fenton reaction occurred under dark conditions. Hence, the Fenton reaction and photocatalysis cooperate to improve the benzene hydroxylation to phenol performance.
3. When there was no visible-light irradiation, the yield of phenol was 8.17%, which implied that only the Fenton reaction occurred under dark conditions. Hence, the Fenton reaction and photocatalysis cooperate to improve the benzene hydroxylation to phenol performance.
| Entry | n(H2O2)/n(benzene) | Yield (%) | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|
| a Reaction conditions: catalyst (1.5 mg mL−1), benzene (1.6 mL, 18.05 mmol), acetonitrile (11 mL), trifluoroacetic acid (0.1 g), time: 4 h, and T: 333 K.b No visible-light exposure. | ||||
| 1 | 2/8 | 7.39 | 7.57 | 97.62 |
| 2 | 3/8 | 13.84 | 13.93 | 99.38 |
| 3 | 4/8 | 9.85 | 9.98 | 98.70 |
| 4 | 5/8 | 8.66 | 8.80 | 98.40 |
| 5b | 3/8 | 8.17 | 8.28 | 98.67 |
| Entry | Mass of TFA (g) | Yield (%) | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|
| a Reaction conditions: catalyst (1.5 mg mL−1), benzene (1.6 mL, 18.05 mmol), H2O2 (30 wt%) (0.6 mL), acetonitrile (11 mL), time: 4 h, and T: 333 K. | ||||
| 1 | 0 | 6.27 | 6.52 | 96.16 |
| 2 | 0.05 | 7.45 | 7.79 | 95.64 |
| 3 | 0.10 | 13.84 | 13.93 | 99.38 |
| 4 | 0.15 | 10.86 | 11.23 | 96.71 |
| 5 | 0.20 | 7.96 | 8.03 | 99.13 |
| Entry | t (h) | Yield (%) | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|
| a Reaction conditions: catalyst (1.5 mg mL−1), benzene (1.6 mL, 18.05 mmol), H2O2 (30 wt%) (0.6 mL), acetonitrile (11 mL), trifluoroacetic acid (0.1 g), and T: 333 K. | ||||
| 1 | 3 | 9.53 | 10.06 | 94.73 |
| 2 | 4 | 13.84 | 13.93 | 99.38 |
| 3 | 5 | 10.61 | 11.37 | 93.31 |
The effect of different reaction temperatures on the selective catalytic oxidation of benzene by g-C3N4/Zr-Fc MOF is illustrated in Table 6. Initially the yield increased with the increase in reaction time, and then the yield decreased as the time was further increased. The ideal benzene conversion and phenol selectivity were obtained at 60 °C. The reason for this might be because the higher reaction temperature was conducive to the generation of active free radicals required in the photocatalytic process, which accelerated the photocatalytic reaction and increased the benzene conversion and yield of phenol to 13.84% and 13.93%, respectively. When the reaction temperature was raised close to the boiling point of benzene, the volatilization of the reactants was easier.35 Also, the self-decomposition of hydrogen peroxide and the deep oxidation of phenol might be caused by high temperature. Therefore, the optimum time for benzene hydroxylation was 4 h and the optimum temperature was 60 °C.
| Entry | T (°C) | Yield (%) | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|
| a Reaction conditions: catalyst (1.5 mg mL−1), benzene (1.6 mL, 18.05 mmol), H2O2 (30 wt%) (0.6 mL), acetonitrile (11 mL), trifluoroacetic acid (0.1 g), and time: 4 h. | ||||
| 1 | 50 | 9.30 | 9.64 | 96.47 |
| 2 | 60 | 13.84 | 13.93 | 99.38 |
| 3 | 70 | 5.22 | 5.39 | 96.85 |
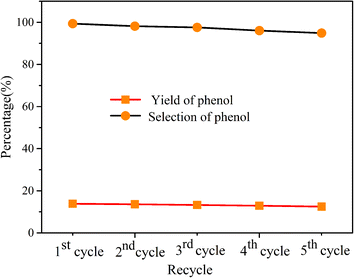
For the catalyst, its regeneration and reusability are also an important factor to be investigated. Thus here, after each photocatalytic reaction, the composite was collected by centrifugation and washed with ethanol, as shown in Fig. 5, the photocatalytic performance of g-C3N4/Zr-Fc MOF could be well maintained after five cycles, and the yield of phenol and the selectivity were 12.49% and 94.88%, respectively. This suggested that the semiconductor photocatalyst was relatively stable and could achieve the goal of being recycled and reused.
A comparative study of g-C3N4/Zr-Fc MOF and other photocatalysts previously reported for the photocatalytic hydroxylation of benzene to phenol was conducted, and the results are shown in Table 7. The phenol selectivity (99%) and yield (13.84%) obtained using the g-C3N4/Zr-Fc MOF photocatalyst were higher than or comparable with those obtained using other photocatalysts.
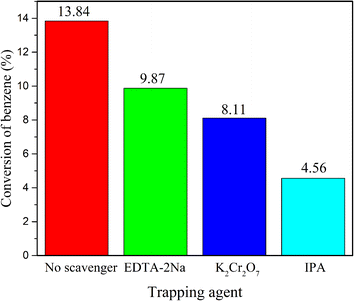
The h+, e−, and ·OH were the main active substances involved in the photocatalytic reaction.41 In order to illuminate the mechanism of g-C3N4/Zr-Fc MOF in the process of the direct hydroxylation of benzene to phenol under photocatalysis, the active substances that played a vital role in the reaction were determined by free-radical-capture experiments under the optimal conditions. Thereinto, isopropanol (IPA), potassium dichromate (K2Cr2O7), and disodium ethylenediaminetetraacetate (EDTA-2Na) were selected as scavengers of hydroxyl radicals (·OH), electrons (e−), and holes (h+), respectively. As shown in Fig. 6, when the capture agents were added into the reaction mixture, the yield of phenol decreased to some extent in all cases. Thereinto, the yield of phenol was greatly inhibited with the addition of IPA. This phenomenon implied that the direct hydroxylation of benzene to phenol was primarily driven by the participation of hydroxyl radicals.
Basic reaction mechanism
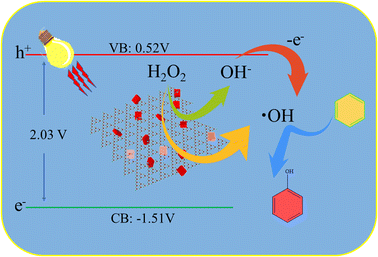
According to the aforementioned free-radical-trapping experiment results, a possible photocatalytic mechanism of benzene hydroxylation to phenol is proposed in Fig. 7. In the visible-irradiated Fenton system, electrons and holes are mainly generated from the semiconductor g-C3N4/Zr-Fc MOF under visible light, and hydroxyl radicals (·OH) are primarily produced by the Fenton reaction, in which hydrogen peroxide is reduced by Fe2+ in the photocatalyst. Furthermore, ·OH also could be generated by the interaction between hydrogen peroxide, electrons, and holes. First, the H2O2 reacts with electrons to produce OH−, and then, the generated OH− could be oxidized by h+ to give ·OH. All the obtained ·OH could then oxidize benzene molecules to phenol.42,43Conclusions
In summary, a novel photocatalytic semiconductor material g-C3N4/Zr-Fc MOFs was successfully prepared via an in situ synthesis method. Compared with g-C3N4 and Zr-Fc MOF, the photocatalytic performance of the composite was significantly improved, and it could effectively catalyze the direct hydroxylation of benzene to phenol under visible-light irradiation. The yield of phenol and selectivity were 13.84% and 99.38% under the optimal conditions, respectively. The performance and structure of the catalyst were relatively stable, and it could still maintain high photocatalytic activity after 5 photocatalytic cycles. Based on these excellent properties, the g-C3N4/Zr-Fc MOF can be expected to be widely used in the field of photocatalytic benzene direct hydroxylation to phenol.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was financially supported by 2018 Funding Plan for Young Core Teachers of Zhongyuan University of Technology, Discipline Strength Improvement Program of Zhongyuan University of Technology: Program for Cultivating Disciplinary Young Master Supervisor (SD202204), the Basic Scientific Research Program of Zhongyuan University of Technology (K2018QN018).Notes and references
- W. Han, W. Xiang and J. Shi, et al., Recent advances in the heterogeneous photocatalytic hydroxylation of benzene to phenol, Molecules, 2022, 27(17), 5457 CrossRef CAS PubMed.
- S. M. Hosseini, M. Ghiaci and S. A. Kulinich, et al., Au-Pd@g-C3N4 as an efficient photocatalyst for visible-light oxidation of benzene to phenol: experimental and mechanistic study, J. Phys. Chem. C, 2018, 122(48), 27477–27485 CrossRef CAS.
- Y. Gu, Q. Li and D. Zang, et al., Light-induced efficient hydroxylation of benzene to phenol by quinolinium and polyoxovanadate-based supramolecular catalysts, Angew. Chem., Int. Ed., 2021, 60(24), 13310–13316 CrossRef CAS PubMed.
- R. Liu, Y. Xu and B. Chen, Self-assembled nano-FeO(OH)/reduced graphene oxide aerogel as a reusable catalyst for photo-Fenton degradation of phenolic organics, Environ. Sci. Technol., 2018, 52(12), 7043–7053 CrossRef CAS PubMed.
- W. Q. Li, Y. X. Wang and J. Q. Chen, et al., Boosting photo-Fenton process enabled by ligand-to-cluster charge transfer excitations in iron-based metal organic framework, Appl. Catal., B, 2022, 302, 120882 CrossRef CAS.
- B. Xu, Z. Chen and B. Han, et al., Glycol assisted synthesis of MIL-100(Fe) nanospheres for photocatalytic oxidation of benzene to phenol, Catal. Commun., 2017, 98, 112–115 CrossRef CAS.
- D. Wang, M. Wang and Z. Li, Fe-based metal–organic frameworks for highly selective photocatalytic benzene hydroxylation to phenol, ACS Catal., 2015, 5(11), 6852–6857 CrossRef CAS.
- B. L. Xiang, L. Fu and Y. Li, et al., Preparation of Fe(II)/MOF-5 catalyst for highly selective catalytic hydroxylation of phenol by equivalent loading at room temperature, J. Chem., 2019, 2019, 1–10 Search PubMed.
- Q. Zhao, L. Zhang and M. Zhao, et al., Vanadium oxyacetylacetonate grated on metal organic framework as catalyst for the direct hydroxylation of benzene to phenol, ChemistrySelect, 2020, 5(22), 6818–6822 CrossRef CAS.
- D. Wang and Z. Li, Iron-based metal–organic frameworks (MOFs) for visible-light-induced photocatalysis, Res. Chem. Intermed., 2017, 43, 5169–5186 CrossRef CAS.
- X. Wang, S. Blechert and M. Antonietti, Polymeric graphitic carbon nitride for heterogeneous photocatalysis, ACS Catal., 2012, 2(8), 1596–1606 CrossRef CAS.
- S. Cao, J. Low and J. Yu, et al., Polymeric photocatalysts based on graphitic carbon nitride, Adv. Mater., 2015, 27(13), 2150–2176 CrossRef CAS PubMed.
- J. Liu, H. Wang and M. Antonietti, Graphitic carbon nitride “reloaded”: emerging applications beyond (photo) catalysis, Chem. Soc. Rev., 2016, 45(8), 2308–2326 RSC.
- Z. Durmus, R. Köferstein and T. Lindenberg, et al., Preparation and characterization of Ce-MOF/g-C3N4 composites and evaluation of their photocatalytic performance, Ceram. Int., 2023, 49, 24428–24441 CrossRef.
- K. C. Devarayapalli, S. V. P. Vattikuti and T. V. M. Sreekanth, et al., Hydrogen production and photocatalytic activity of g-C3N4/Co-MOF (ZIF-67) nanocomposite under visible light irradiation, Appl. Organomet. Chem., 2020, 34(3), e5376 CrossRef CAS.
- R. Abazari, A. R. Mahjoub and G. Salehi, Preparation of amine functionalized g-C3N4@ H/SMOF NCs with visible light photocatalytic characteristic for 4-nitrophenol degradation from aqueous solution, J. Hazard. Mater., 2019, 365, 921–931 CrossRef CAS PubMed.
- P. Xiao, R. Osuga and Y. Wang, et al., Bimetallic Fe–Cu/beta zeolite catalysts for direct hydroxylation of benzene to phenol: effect of the sequence of ion exchange for Fe and Cu cations, Catal. Sci. Technol., 2020, 10(20), 6977–6986 RSC.
- Y. Wu, X. Zhang and F. Wang, et al., Synergistic effect between Fe and Cu species on mesoporous silica for hydroxylation of benzene to phenol, Ind. Eng. Chem. Res., 2021, 60(23), 8386–8395 CrossRef CAS.
- L. Zeng, H. Liang and P. An, et al., Carbon encapsulated bimetallic FeCo nanoalloys for one-step hydroxylation of benzene to phenol, Appl. Catal., A, 2022, 633, 118499 CrossRef CAS.
- M. Garrido, M. Barrejón and J. A. Berrocal, et al., Polyaromatic cores for the exfoliation of popular 2D materials, Nanoscale, 2022, 14(25), 8986–8994 RSC.
- J. Du, S. Li and Z. Du, et al., Boron/oxygen-codoped graphitic carbon nitride nanomesh for efficient photocatalytic hydrogen evolution, Chem. Eng. J., 2021, 407, 127114 CrossRef CAS.
- X. Ma, Z. Deng and Z. Li, et al., A photothermal and Fenton active MOF-based membrane for high-efficiency solar water evaporation and clean water production, J. Mater. Chem. A, 2020, 8(43), 22728–22735 RSC.
- W. Yan, L. Yan and C. Jing, Impact of doped metals on urea-derived g-C3N4 for photocatalytic degradation of antibiotics: structure, photoactivity and degradation mechanisms, Appl. Catal., B, 2019, 244, 475–485 CrossRef CAS.
- Z. Deng, H. Yu and L. Wang, et al., Ferrocene-based metal-organic framework nanosheets loaded with palladium as a super-high active hydrogenation catalyst, J. Mater. Chem. A, 2019, 7(26), 15975–15980 RSC.
- X. Wang, F. Wang and C. Bo, et al., Promotion of phenol photodecomposition and the corresponding decomposition mechanism over g-C3N4/TiO2 nanocomposites, Appl. Surf. Sci., 2018, 453, 320–329 CrossRef CAS.
- Z. Deng, T. Wan and D. Chen, et al., Photothermal-Responsive Microporous Nanosheets Confined Ionic Liquid for Efficient CO2 Separation, Small, 2020, 16(34), 2002699 CrossRef CAS PubMed.
- M. Lu, Z. Pei and S. Weng, et al., Constructing atomic layer g-C3N4-CdS nanoheterojunctions with efficiently enhanced visible light photocatalytic activity, Phys. Chem. Chem. Phys., 2014, 16(39), 21280–21288 RSC.
- B. Van de Voorde, I. Stassen and B. Bueken, et al., Improving the mechanical stability of zirconium-based metal–organic frameworks by incorporation of acidic modulators, J. Mater. Chem. A, 2015, 3(4), 1737–1742 RSC.
- J. Shimei and W. Yue, Vibrational spectra of an open ferrocene and a half-open ferrocene, Spectrochim. Acta, Part A, 1999, 55(5), 1025–1033 CrossRef.
- S. Samanta, R. Yadav and A. Kumar, et al., Surface modified C, O co-doped polymeric g-C3N4 as an efficient photocatalyst for visible light assisted CO2 reduction and H2O2 production, Appl. Catal., B, 2019, 259, 118054 CrossRef CAS.
- X. Wei, N. Zhu and X. Huang, et al., Efficient degradation of sodium diclofenac via heterogeneous Fenton reaction boosted by Pd/Fe@Fe3O4 nanoparticles derived from bio-recovered palladium, J. Environ. Manage., 2020, 260, 110072 CrossRef CAS PubMed.
- P. P. Xu, L. Zhang and X. Jia, et al., A novel heterogeneous catalyst NH2-MIL-88/PMo10V2 for the photocatalytic activity enhancement of benzene hydroxylation, Catal. Sci. Technol., 2021, 11(19), 6507–6515 RSC.
- Y. Fang, L. Zhang and Q. Zhao, et al., Highly selective visible-light photocatalytic benzene hydroxylation to phenol using a new heterogeneous photocatalyst UiO-66-NH2-SA-V, Catal. Lett., 2019, 149, 2408–2414 CrossRef CAS.
- G. Grigoropoulou, J. H. Clark and J. A. Elings, Recent developments on the epoxidation of alkenes using hydrogen peroxide as an oxidant, Green Chem., 2003, 5(1), 1–7 RSC.
- S. Farahmand, M. Ghiaci and S. Asghari, Oxo-vanadium(IV) phthalocyanine implanted onto the modified SBA-15 as a catalyst for direct hydroxylation of benzene to phenol in acetonitrile-water medium: A kinetic study, Chem. Eng. Sci., 2021, 232, 116331 CrossRef CAS.
- P. Xiao, Y. Wang and J. N. Kondo, et al., Iron-and copper-exchanged Beta zeolite catalysts for hydroxylation of benzene to phenol with H2O2, Chem. Lett., 2018, 47(9), 1112–1115 CrossRef CAS.
- X. Chen, J. Zhang and X. Fu, et al., Fe-g-C3N4-catalyzed oxidation of benzene to phenol using hydrogen peroxide and visible light, J. Am. Chem. Soc., 2009, 131(33), 11658–11659 CrossRef CAS PubMed.
- Z. Ding, X. Chen and M. Antonietti, et al., Synthesis of transition metal-modified carbon nitride polymers for selective hydrocarbon oxidation, ChemSusChem, 2011, 4(2), 274–281 CAS.
- Y. Fang, L. Zhang and Q. Zhao, et al., Highly Selective Visible-Light Photocatalytic Benzene Hydroxylation to Phenol Using a New Heterogeneous Photocatalyst UiO-66-NH2-SA-V, Catal. Lett., 2019, 149, 2408–2414 CrossRef CAS.
- W. Han, W. Xiang and Z. Meng, et al., A novel Cr-doped CdS/ZnO nanocomposite for efficient photocatalytic hydroxylation of benzene to phenol, Colloids Surf., A, 2023, 670, 131529 CrossRef CAS.
- B. Han, X. Li and Z. Zhang, et al., A novel strategy to research the mechanism of rutile TiO2 with excellent photocatalytic performance, Nanotechnology, 2021, 33(3), 035704 CrossRef PubMed.
- F. Zhu, S. Zhou and M. Sun, et al., Heterogeneous activation of persulfate by Mg doped Ni(OH)2 for efficient degradation of phenol, Chemosphere, 2021, 131647 Search PubMed.
- S. J. Feng, B. Yue and Y. Wang, et al., Hydroxylation of benzene over V-HMS catalysts with the addition of Fe as the second metal component, Acta Phys.-Chim. Sin., 2011, 27(12), 2881–2886 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra03055e |
| This journal is © The Royal Society of Chemistry 2023 |