Open Access Article
Open Access ArticleUsing deep eutectic solvent dissolved low-value cotton linter based efficient magnetic adsorbents for heavy metal removal
Sihong Ye†
a,
Mingli Xu†b,
Hui Suna,
Ying Nia,
Rui Wanga,
Runping Ye *c,
Lingzhong Wana,
Fangzhi Liua,
Xiaonan Deng*a and
Juan Wu*a
*c,
Lingzhong Wana,
Fangzhi Liua,
Xiaonan Deng*a and
Juan Wu*a
aInstitute of Cotton, Anhui Academy of Agricultural Sciences, Hefei, China. E-mail: xn_deng@foxmail.com; ecjtutmtm@163.com
bDepartment of Life Sciences, Anhui Agricultural University, Hefei, China
cKey Laboratory of Jiangxi Province for Environment and Energy Catalysis, Institute of Applied Chemistry, School of Chemistry and Chemical Engineering, Nanchang University, Nanchang, China. E-mail: rye@ncu.edu.cn
First published on 3rd May 2023
Abstract
In this study, a novel magnetic bio-adsorbent was synthesized by modifying cotton linter (CL) cellulose with deep eutectic solvents (DESs) and Fe3O4 magnetic nanoparticles. The adsorption capacity of CL, Fe3O4/CL, Fe3O4/CL-oxidation, and Fe3O4/CL-DES for Cu2+ was 11.0, 66.1, 85.7, and 93.1 mg g−1, respectively, under the optimal adsorption conditions of an initial pH value of 6.0, stirring rate of 300 rpm, and a temperature of 30 °C. The presence of Fe3O4 nanoparticles increased the proportion of hydroxyl groups and thus improved the ion-exchange ability of Cu2+. The dissolution of DES significantly decreased fiber crystallinity and increased the number of hydroxyl group (amorphous regions increased), thus improving the chelation reaction of Cu2+, which was favorable for surface adsorption. In addition, we used the Langmuir and Freundlich isothermal models to simulate the adsorption behavior of Fe3O4/CL-DES, and the results indicated that Cu2+ follows a Freundlich isotherm model of multilayer adsorption. The fitting of the adsorption kinetics model indicated that the adsorption process involves multiple adsorption mechanisms and can be described by a quasi-second-order model. These results provide a potential method for the preparation of high-efficiency adsorbents from low-value cotton linter, which has broad application prospects in wastewater treatment.
1 Introduction
In recent years, heavy metal pollution in soil, sediment, and water environments has garnered worldwide attention due to the high toxicity and ability of heavy metals to accumulate in living organisms.1–4 Water pollution, in particular, poses a significant threat to ecosystems and human health through the food chain.5,6 As a result, the safe, cost-effective, and efficient treatment of toxic metal ions in wastewater is a critical area of research. Various physical, chemical, and biological methods are currently employed to remove heavy metals from water, including adsorption, chemical precipitation, electrocatalysis, membranes, and bioremediation.7–9 Among these, adsorption and photocatalysis are widely used for the removal of different pollutants in wastewater.10 Adsorption relies on the physical or chemical binding of pollutants to a solid surface, which can be highly effective for certain types of pollutants but may require frequent replacement or regeneration of the adsorbent material.11 On the other hand, photocatalysis involves the use of catalysts and light energy to initiate chemical reactions that decompose pollutants, which can be effective for a wider range of pollutants but may require more complex and expensive setups.12 Therefore, adsorption is typically used to remove heavy metals or organic pollutants from industrial wastewater, while photocatalysis may be more suitable for removing emerging pollutants such as pharmaceuticals or personal care products.13Various adsorbent materials have been developed for removing heavy metals from wastewater, including inorganic substances like clay and metal oxides, organic polymers like cellulose and chitosan, carbon-based materials such as activated carbon and graphene, and porous skeleton materials such as MOFs and COFs.14–18 Biomass-based adsorbents incorporating a cellulose matrix have attracted attention due to their lower cost and improved mass transfer performance.19–21 Cotton linter (CL) is a staple fiber that remains on the surface of cotton seed after the process of ginning.22 It can be separated by a lint stripping machine, and the resulting product can be equivalent to 15% ∼20% of the total lint.23 In the 1980s, cotton seeds were typically directly used for oil pressing after refined cotton ginning due to outdated technology, leading to a significant waste of CL. However, with advancements in science and technology, numerous high-value applications for CL have been identified, such as its use as a raw material for the production of high-grade paper and the industrial extraction of cellulose.24–26 Cellulose, which is the main component of CL (Fig. 1), is characterized by a large number of hydroxyl groups, intramolecular and intermolecular hydrogen bonds, and multi-level cavities.27–29 These structural features make CL suitable for use as an adsorbent through surface adsorption and hydrogen bonding. However, the high crystallinity of cellulose can affect the accessibility of the hydroxyl groups, limiting the ability of contaminant molecules to penetrate the crystalline region of cellulose for reaction and leading to a low adsorption rate of pristine CL.30,31 Therefore, decreasing the crystallinity of cellulose and increasing the accessibility of the hydroxyl groups are key challenges for improving the adsorption rate of CL.
Ball milling is an effective method to decrease the crystallinity of cellulose and increase its amorphous content. Ling et al. used vibratory ball milling to break cellulose crystals, leading to a decrease in molecular weight, an increase in carbonyl groups, and a higher surface area.32 In contrast to the physical method of ball milling, cellulose can also be dissolved chemically. However, traditional solvents like CS2 and NMMO have limitations due to high pollution, energy consumption, and instability.33–35 A new green ionic liquid solvent called 1-butyl-3-methylimidazolide was discovered, but it is expensive and has high viscosity.36–38 To overcome this, a new type of ionic liquid analogue called deep eutectic solvents (DESs) has emerged, which are low-cost, easy to prepare, non-toxic, and biodegradable.39–41 Using DESs to dissolve cellulose can increase its reactive form and facilitate subsequent modifications.
Fe3O4, a magnetic material, has been widely utilized for the removal of heavy metal pollution from water due to its high affinity towards inorganic metal salt species (such as Cu2+, Pb2+, and Hg2+), resulting in selective adsorption.42,43 However, most bare Fe3O4 materials exhibit low Cu2+ adsorption capacities, and the adsorption process is slow, leading to reduced effectiveness and applicability. Against this backdrop, this study investigates the use of Fe3O4-loaded treated CL in Cu2+ adsorption, and extensively characterizes the structural properties of the adsorbents. Furthermore, the study focuses on the impact of different treatment methods on the crystallinity and hydroxyl group content of CL, and evaluates the adsorption properties in Cu2+ removal from aqueous solution under diverse experimental conditions, including the pH and temperature, reusability, and simulation of the adsorption isotherm and kinetic model.
2 Experimental section
2.1 Adsorbent synthesis
Commercially available reagents and solvents were used without further purification. Cotton linter (CL) was obtained from a pure natural experimental base. Cu(NO3)2, FeCl3, FeSO4·7H2O, NaIO4, NaOH, oxalic acid dihydrate (C2H2O4·2H2O), choline chloride, glycerol, and ethanol (C2H5OH) were employed as chemical reagents. To compare the efficacy of two modification methods for CL, Fe3O4 weight loading was fixed at approximately 20 wt%. The samples obtained through oxidation and deep eutectic solvent modification methods were denoted as Fe3O4/CL-O and Fe3O4/CL-D, respectively. A typical Fe3O4 loading procedure was as follows: 1.6 g of treated CL was added to a mixed salt solution (bath ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) containing 0.56 g of FeCl3 and 0.48 g of FeSO4·7H2O. The solution was heated to 70 °C at a rotational speed of 400 rpm, and 1 M NaOH was added to adjust the pH to >9. The sample was then matured for 2 h and washed until the filtrate was completely transparent. The resulting sample was dried at 45 °C for 12 h in air to obtain the Fe3O4/CL adsorbent. Fe3O4/CL-O and Fe3O4/CL-D differ in the pretreatment process of CL. The oxidation pretreatment of CL involved adding 5 g of untreated CL to 250 ml of 2% NaOH solution and boiling for 30 min. The fiber was then filtered and transferred to 18% NaOH solution (bath ratio of 1
50) containing 0.56 g of FeCl3 and 0.48 g of FeSO4·7H2O. The solution was heated to 70 °C at a rotational speed of 400 rpm, and 1 M NaOH was added to adjust the pH to >9. The sample was then matured for 2 h and washed until the filtrate was completely transparent. The resulting sample was dried at 45 °C for 12 h in air to obtain the Fe3O4/CL adsorbent. Fe3O4/CL-O and Fe3O4/CL-D differ in the pretreatment process of CL. The oxidation pretreatment of CL involved adding 5 g of untreated CL to 250 ml of 2% NaOH solution and boiling for 30 min. The fiber was then filtered and transferred to 18% NaOH solution (bath ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) and stirred at room temperature for 2 h. After filtration and washing, the activated CL was obtained by drying at 45 °C for 12 h. The activated CL was placed into a 12 g L−1 NaIO4 solution (bath ratio of 1
30) and stirred at room temperature for 2 h. After filtration and washing, the activated CL was obtained by drying at 45 °C for 12 h. The activated CL was placed into a 12 g L−1 NaIO4 solution (bath ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30), and the reaction was stirred continuously at 50 °C for 2 h in the absence of light. After the oxidation reaction, 3 ml of glycerol was added to remove the unreacted NaIO4. Finally, the fibers were filtered, washed, and dried at 45 °C to form the treated CL-O. The precursor of Fe3O4/CL-D was synthesized by using the deep eutectic solvent (DES) dissolution method: 55.84 g of choline chloride and 55.04 g of oxalic acid dihydrate were stirred in a beaker at 90 °C until the reagent turned into a fully clarified liquid. Then, 1.0 g of CL was added into the solution until dissolved, it was filtered with ethanol and deionized water three times, and dried at 45 °C for 12 h. Subsequently, the dried fibers were mixed with deionized water at a ratio of 0.05% and ultrasonicated for 20 min, filtered with deionized water, and dried at 45 °C for 12 h to obtain CL-D.
30), and the reaction was stirred continuously at 50 °C for 2 h in the absence of light. After the oxidation reaction, 3 ml of glycerol was added to remove the unreacted NaIO4. Finally, the fibers were filtered, washed, and dried at 45 °C to form the treated CL-O. The precursor of Fe3O4/CL-D was synthesized by using the deep eutectic solvent (DES) dissolution method: 55.84 g of choline chloride and 55.04 g of oxalic acid dihydrate were stirred in a beaker at 90 °C until the reagent turned into a fully clarified liquid. Then, 1.0 g of CL was added into the solution until dissolved, it was filtered with ethanol and deionized water three times, and dried at 45 °C for 12 h. Subsequently, the dried fibers were mixed with deionized water at a ratio of 0.05% and ultrasonicated for 20 min, filtered with deionized water, and dried at 45 °C for 12 h to obtain CL-D.
2.2 Adsorbent characterization
The true content of Fe on the adsorbents was measured using inductively coupled plasma spectrometry (ICP-MS: Agilent 7800, USA). To analyze the crystal phases of all adsorbents, a Smartlab 9 kW advance powder X-ray Cu Kα radiation diffractometer (Japan) was used to record the X-ray diffraction (XRD) patterns of the samples, with the voltage at 40 kV, current at 40 mA, and the range of scan angle (2θ) set as 5–90°. The morphology and microstructure of the adsorbents were studied using field emission scanning electron microscopy (SEM) on a ZEISS Sigma 300 (Germany). The Fourier transform infrared spectra of all adsorbents were recorded by a Thermo Scientific Nicolet iS50 FT-IR spectrometer (USA) in the range of 4000–450 cm−1. The X-ray photoelectron spectroscopy (XPS) measurements of all adsorbents were carried out on a Thermo Scientific K-Alpha spectrometer (UK) equipped with a monochromatic Al K radiation source (1486.6 eV), and the values of binding energies were calibrated using the criterion reference of the C 1s signal at 284.8 eV. The thermogravimetric (TG) analysis of all adsorbents was carried out under a nitrogen atmosphere at a heating rate of 10 °C min−1 using a TA TGA5500 (USA).2.3 Adsorption experiments
A series of adsorption experiments was conducted using the as-prepared adsorbents. In each experiment, 0.2 g of adsorbent was added to a 50 ml solution containing 1.0 g L−1 Cu2+ and stirred for 120 min using a magnetic agitator with varying speeds ranging from 200 to 600 rpm. The concentrations of remaining metal ions in the filtrate were determined using an atomic adsorption spectrophotometer (TAS-986, Beijing Purkinje General Instrument Co., Ltd). The adsorption capacity of the adsorbent was calculated as the difference between the initial and remaining concentrations of Cu2+. The equilibrium adsorption capacity (eqn (1)) and the percentage removal of heavy metal ions (eqn (2)) were calculated using the following formulas:44
 | (1) |
 | (2) |
3 Results and discussion
3.1 Characterization of adsorbents
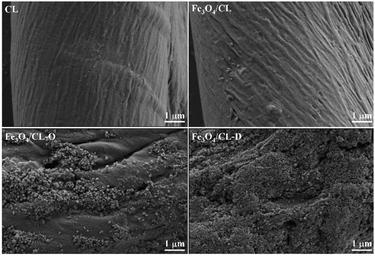
The actual Fe loadings recorded by ICP were 0, 0.19, 8.1, and 15.2 wt% for the CL, Fe3O4/CL, Fe3O4/CL-O, and Fe3O4/CL-D adsorbents, respectively. The results suggested that compared with untreated CL, the treated CL achieved higher Fe loadings, and the DES modified CL presented the maximum Fe loading. The XRD patterns of the adsorbents are shown in Fig. 2. The cotton linter (CL) exhibited obvious diffraction peaks at 14.9°, 16.5°, 22.7°, and 34.4°, which indicated that the natural cotton fibers used in the experiment had the typical cellulose type I crystal structure.45 The characteristic peaks of Fe3O4/CL were mostly coincident with CL, which was due to the low loading of Fe3O4 on untreated CL and the limitations of XRD. Interestingly, only the characteristic peaks of cellulose could be observed for Fe3O4/CL-O, which may have been related to the high dispersion of Fe3O4 on the surface of CL-O, and this phenomenon was confirmed by SEM (Fig. 3). In addition to the presence of the obvious cellulose type I crystal characteristic peaks, diffraction peaks at 30.1°, 35.5°, 43.1°, 53.5°, 57.0°, and 62.7° were observed for Fe3O4/CL-D, which matched well with the pure magnetite reflections of Fe3O4 (PDF: 19-0629).46 Moreover, the diffraction peak intensity of Fe3O4/CL-O and Fe3O4/CL-D decreased when modified by NaIO4 oxidation or DES dissolution. At the same time, the macroscopic length and strength of the prepared Fe3O4/CL-O and Fe3O4/CL-D fibers became shorter and weaker, which indicated that the chemical modification destroyed the internal structure of the cotton fibers. In other words, the crystallinity of Fe3O4/CL-O and Fe3O4/CL-D decreased and the fiber microstructure became looser, which we speculated was conducive to the diffusion of heavy metals into the fibers and favorable for adsorption.Fig. 3 shows the SEM images of CL before and after modification. It can be observed that chemical modification changed the surface morphology of CL. Among them, the surface of CL appeared relatively smooth, while a few particles appeared on the surface of Fe3O4/CL. We speculated that these particles were Fe3O4 loaded on the surface of the fibers, and this phenomenon was more obvious on Fe3O4/CL-O and Fe3O4/CL-D. It is worth mentioning that this phenomenon was consistent with the increase of Fe loading in the ICP results. Besides, there were many grooves and folds on the surface of Fe3O4/CL-O, which was due to the oxidation and hydrolysis reaction between NaIO4 and cellulose macromolecular chains on the surface of the cotton fibers, leading to the possible breakage of some cellulose molecular chains and the roughness of the fiber surface.47 However, the surface of Fe3O4/CL-D was covered with so much Fe3O4 that it was difficult to distinguish, and the Fe3O4 underwent a certain extent of aggregation. It can be seen from the SEM images that the surface morphology of CL changed during chemical modification, and the surface changes of CL were most obvious due to the oxidation by NaIO4 and the dissolution by the DES.
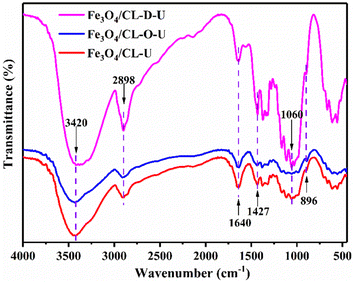
The crystalline structure of the adsorbents was analyzed using FTIR spectroscopy measurement, and the results are shown in Fig. 4. In the FTIR spectra, all samples presented six or more peaks corresponding to the typical cellulose crystal structure, which agreed with the XRD results. The peaks at 3420 cm−1, 2900 cm−1, 1640 cm−1, 1427 cm−1, 1060 cm−1, and 896 cm−1 corresponded to O–H stretch vibrations, C–H stretch sp3 vibrations, adsorbed water H2O bending vibrations, C–H bending vibrations, C–O symmetric stretch vibrations, and glucoside bond vibrations, respectively.48,49 These peaks are the characteristic adsorption peaks of cellulose, indicating that the main composition structure of cotton fiber did not change significantly after chemical modification. However, clear blue shifts for Fe3O4/CL-O and Fe3O4/CL-D were observed after chemical modification, such as the C–H stretch sp3 vibration peak shift from 2921 cm−1 to 2895 cm−1, and the glucoside bond shift from 899 cm−1 to 896 cm−1, which indicated that the crystalline structure of CL slightly changed after NaIO4 oxidation, DES dissolution, and Fe3O4 loading.50,51
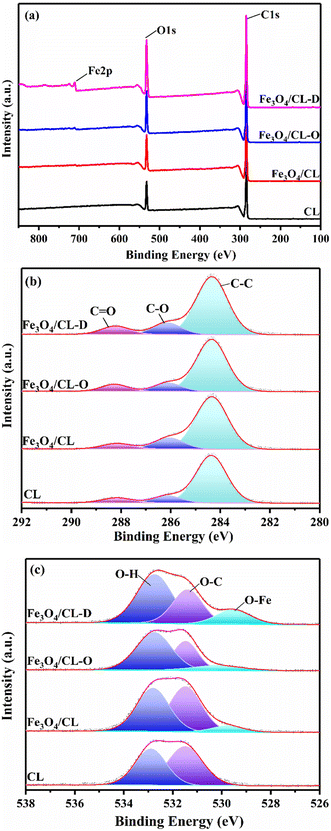
The chemical valence states of each element in all adsorbents were further studied using X-ray photoelectron spectroscopy (XPS). In the broad spectrum (Fig. 5a), it was found that all the samples showed characteristic peaks belonging to C 1s and O 1s at 284.8 eV and 532.0 eV, indicating that there were carbon and oxygen elements in all the adsorbents and the content of carbon was higher than that of oxygen. Regarding the Fe 2p characteristic peak with binding energy near 711.0 eV, it was observed for Fe3O4/CL, Fe3O4/CL-O, and Fe3O4/CL-D, and it was most obvious for Fe3O4/CL-D, implying that the content of Fe element was the highest in Fe3O4/CL-D, and this result was consistent with the ICP result.52 Subsequently, the high-resolution electronic spectra of C 1s were deconvolved (Fig. 5b), and the results showed that the carbon element in all samples had three kinds of chemical bonds, namely, C–C, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and the corresponding binding energy was around 284.5 eV, 286.2 eV, and 288.2 eV.53,54 It should be noted that the presence of C
O, and the corresponding binding energy was around 284.5 eV, 286.2 eV, and 288.2 eV.53,54 It should be noted that the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in all the samples was caused by the interference of CO2 in the air during the measurement, which was confirmed by the absence of C
O in all the samples was caused by the interference of CO2 in the air during the measurement, which was confirmed by the absence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration peaks in the FTIR characterization. For the deconvolved O element (Fig. 5c), it was found that except for CL, the other adsorbents exhibited three valence forms of O, namely Fe–O at 529.7 eV, O–C at 531.5 eV, and O–H at 532.8 eV, respectively.55,56 This result further indicated that except for CL, the other adsorbents were loaded with Fe to varying degrees and the Fe was present in the form of Fe–O bonds. Combined with XRD analysis, it was speculated that Fe was loaded on the cotton fibers in the form of Fe3O4, which provided the material basis for improving adsorption of metal ions (Cu2+). Moreover, the surface atomic content of the samples was analyzed (Table 1), and it was found that with the chemical modification of CL, the proportion of C atoms gradually decreased, and correspondingly the proportion of Fe and O atoms gradually increased. The increased O atom content could greatly improve the coordination capacity of the samples, which would help to improve the adsorption capacity of the adsorbent for pollutants. Besides, it should be noted that the content of Fe atoms was significantly lower than that of the ICP results due to XPS being a surface-based technique.
O vibration peaks in the FTIR characterization. For the deconvolved O element (Fig. 5c), it was found that except for CL, the other adsorbents exhibited three valence forms of O, namely Fe–O at 529.7 eV, O–C at 531.5 eV, and O–H at 532.8 eV, respectively.55,56 This result further indicated that except for CL, the other adsorbents were loaded with Fe to varying degrees and the Fe was present in the form of Fe–O bonds. Combined with XRD analysis, it was speculated that Fe was loaded on the cotton fibers in the form of Fe3O4, which provided the material basis for improving adsorption of metal ions (Cu2+). Moreover, the surface atomic content of the samples was analyzed (Table 1), and it was found that with the chemical modification of CL, the proportion of C atoms gradually decreased, and correspondingly the proportion of Fe and O atoms gradually increased. The increased O atom content could greatly improve the coordination capacity of the samples, which would help to improve the adsorption capacity of the adsorbent for pollutants. Besides, it should be noted that the content of Fe atoms was significantly lower than that of the ICP results due to XPS being a surface-based technique.
| Sample code | C atomic content | Fe atomic content | O atomic content |
|---|---|---|---|
| CL | 84.46% | — | 15.54% |
| Fe3O4/CL | 83.40% | 0.61% | 15.99% |
| Fe3O4/CL-O | 80.43% | 0.98% | 18.59% |
| Fe3O4/CL-D | 75.76% | 2.09% | 22.15% |
The TG analysis of the samples is illustrated in Fig. 6. The TG curves show that two weight-loss processes occur in all the samples. The first weight loss stage occurred in the temperature range of 30–200 °C, which could be attributed to residual water evaporation.57 The weight loss ratio of all samples at this stage was about 7%. When the temperature was higher than 200 °C, the main weight loss process occurred at 200–400 °C for all adsorbents, which resulted from the decomposition of the cotton fibers.58 It should be noted that the weight loss amount for all samples was different; CL without any treatment underwent the highest weight loss (up to ∼69%, water-loss part below 200 °C is not included in the calculation), followed by Fe3O4/CL at ∼67% and Fe3O4/CL-O at ∼59%, and the lowest weight loss rate of ∼52% for Fe3O4/CL-D. Therefore, it was speculated that the reason for the lower weight loss amounts of the other samples compared with CL was the successful loading of Fe3O4. ICP results showed that the content of Fe in Fe3O4 CL-D was the highest, followed by Fe3O4/CL-O and Fe3O4/CL, which further confirmed this phenomenon. The TG results suggested that the CL samples treated by NaIO4 oxidation and DES dissolution were more likely to be loaded with Fe3O4, which had a positive influence on the adsorption of Cu2+.
3.2 Evaluation of adsorbents
 | ||
| Fig. 7 Effect of temperature (a), stirring rate (b), and initial pH value (c) on the removal of Cu2+; the adsorption capacity of all samples (d). | ||
The adsorption capacity of all samples is presented in Fig. 7d. It was observed that CL showed a low adsorption activity of 11.0 mg g−1 before chemical modification and Fe3O4 loading. After the chemical modification and Fe3O4 loading treatment, the adsorption capacity of Fe3O4/CL, Fe3O4/CL-O, and Fe3O4/CL-D gradually increased and reached a maximum of 93.1 mg g−1 for Fe3O4/CL-D. These results indicated that Fe3O4 loading, NaIO4 oxidation, and DES dissolution pretreatment positively contributed to further improving the adsorption property. We speculated that the increased adsorption capacity was due to the loading of Fe3O4, which increased the proportion of O atoms and improved the complexation ability of Cu ions. Moreover, NaIO4 oxidation and DES dissolution caused significant damage to the structure of the cotton fibers, resulting in decreased fiber crystallinity and increased amorphous regions containing free hydroxyl groups, which was favorable for surface adsorption.
Furthermore, we compared the adsorption capacity of Fe3O4/CL-D with other biomaterials reported in previous studies and summarized the results in Table 2. One can find that the adsorption capacity of Fe3O4/CL-D for Cu2+ was higher than that of the reported related adsorbents. We believe that our Fe3O4/CL-D adsorbents have several advantages over other biomaterials. Firstly, our adsorbents have a high specific surface area due to the porous nature of cellulose, which provides more adsorption sites for heavy metal ions. Secondly, the use of Fe3O4 nanoparticles enhances the magnetic separation ability of the adsorbents, making them more efficient and cost-effective. Finally, the synthesis of the Fe3O4/CL-D adsorbents involves a simple and environmentally friendly method, which makes them suitable for large-scale production.
| Adsorbent | Cu2+ adsorption capacity (mg g−1) | Ref. |
|---|---|---|
| Fe3O4–cellulose–chitosan | 44.7 ± 5 | 63 |
| Magnetic chitosan/cellulose microspheres | 88.21 | 64 |
| p(HEMA-co-TACYC) hydrogels | 17.24 | 65 |
| Opuntia activated biochar (OFI) | 49.36 | 66 |
| SA-PAM/GO | 68.76 | 67 |
| Magnetic pine cone gel beads (MPCB) | 69.80 | 60 |
| Fe3O4/CL-D | 93.1 | This work |
Langmuir model:
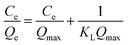 | (3) |
On the other hand, the Freundlich isotherm model assumes multilayer adsorption on a heterogeneous surface, and there are many interactions between adsorbent and adsorbate. Multilayer adsorption can be understood as the phenomenon whereby adsorbed molecules can re-adsorb with other molecules, meaning that indirect adsorption exists. The Freundlich isotherm model can be expressed by eqn (4):
Freundlich model:
 | (4) |
Fig. 8a shows the fitting results of the Langmuir and Freundlich adsorption isotherm models to the experimental data, and the correlation coefficients of each model were calculated from the slope and intercept, as shown in Table 3. The models and parameter results indicated that, compared to the Langmuir isotherm model, the Freundlich isotherm model provided a better fit to the experimental data. This suggests that the adsorption of Cu2+ onto Fe3O4/CL-D is a multilayer process. We hypothesized that Cu2+ occupying the adsorption sites of Fe3O4 also undergoes hydrogen bonding or intermolecular adsorption with cotton fibers, facilitating continued indirect adsorption and leading to the diffusion of Cu ions into the interior of the adsorbent and the occurrence of multilayer adsorption.
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| Qm,cal (mg g−1) | KL (L mg−1) | R2 | KF | n | R2 |
| 35.47 | 0.0118 | 0.9228 | 5.5259 | 4.2735 | 0.9540 |
The adsorption kinetics of a solute on an adsorbent is a fundamental parameter that determines the rate at which the reaction pollutant is adsorbed and the time required for the adsorbent to reach equilibrium. By controlling the residence time on the adsorbent at the solid–liquid interface, the efficiency of wastewater treatment can be improved. In this study, we investigated the adsorption kinetics of the adsorbent using both quasi-first-order and quasi-second-order kinetic models. The expressions for these models are given by eqn (5) and (6), respectively:60
Quasi-first-order kinetic model:
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qt − k1t Qt − k1t
| (5) |
Quasi-second-order kinetic model:
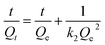 | (6) |
Fig. 8b illustrates the adsorption kinetics curve of Cu2+ (experimental data), which shows that the adsorption equilibrium was reached after approximately 2 h. The adsorption process of Cu2+ was divided into three stages. The first stage was characterized by a rapid rise in the curve within the first 30 min of adsorption, indicating a high adsorption rate during this period, and adsorption mainly occurred on the surface of the adsorbent. In the second stage, the curve rose at a slower rate, and the adsorption capacity gradually stabilized between 30 and 120 min. The third stage, which occurred between 120 and 420 min, showed a stable adsorption capacity, indicating that the adsorption had reached equilibrium.61 The initial rapid adsorption was attributed to the presence of numerous active adsorption sites or active groups on the surface of Fe3O4–CL-D that could combine with Cu2+. As adsorption proceeded, the adsorption sites became progressively occupied, and competitive adsorption increased, resulting in a slower adsorption rate.
The fitted parameters of the adsorption kinetics curve of Cu2+ are listed in Table 4. Compared with the quasi-first-order kinetic model, the quasi-second-order kinetic model fits the experimental data better, with a fitting coefficient (R2) close to 1.0, indicating that the quasi-second-order kinetic model can more reasonably describe the adsorption process of Cu2+ on Fe3O4–CL-D. The quasi-second-order kinetic model represents a second-order proportionality between the reaction rate and the square of the reactant concentration, reflecting the composite effect of adsorption and multiple adsorption mechanisms.
| Pesudo-first-order | Pesudo-second-order | ||||
|---|---|---|---|---|---|
| Qe (mg g−1) | k1 (min−1) | R2 | Qe (mg g−1) | k2 (min−1) | R2 |
| 39.9599 | 0.08435 | 0.9023 | 42.5894 | 0.00302 | 0.9997 |
According to the presented information, Fe3O4–CL-D demonstrates a multilayer adsorption phenomenon for Cu2+ and conforms to both the Freundlich isothermal model and quasi-second-order adsorption mechanism. Specifically, Cu2+ adsorbed onto the surface of Fe3O4–CL-D to form a multilayer adsorption structure. This adsorption phenomenon can be accurately described by the Freundlich isothermal model, which indicates a power-law relationship between the adsorption capacity and the concentration of the adsorbate. Furthermore, this adsorption phenomenon follows the quasi-second-order adsorption mechanism, where the adsorption rate is proportional to the square of the concentration of the adsorbate. It is important to note that these findings have significant implications for understanding the adsorption behavior of Cu2+ on Fe3O4–CL-D and for developing new approaches for effective adsorption removal of heavy metals from contaminated water.
3.3 Characterization of adsorbents after adsorption
To further elucidate the morphological and structural changes of the adsorbent after Cu2+ adsorption, Fourier-transform infrared (FTIR) spectroscopy and scanning electron microscopy (SEM) characterizations were performed on the corresponding samples, denoted by the suffix “U”. Following the adsorption of Cu2+, the material's functional groups and surface morphology underwent significant changes. The FTIR spectrum analysis revealed a weakening of peak intensities at 1427 cm−1, 1060 cm−1, and 896 cm−1, corresponding to C–H, C–O, and glucoside bond vibrations after Cu2+ adsorption (Fig. 10).49 This observation suggested that these functional groups played a significant role in the adsorption process. The overall FTIR peaks of all materials did not show any significant reduction, indicating that cellulose remained the primary constituent of the adsorbent.Furthermore, the SEM images of the adsorbent before and after Cu2+ adsorption revealed changes in surface morphology (Fig. 11). Before adsorption, the surface of Fe3O4/CL was smooth and relatively uniform, whereas, after adsorption, the surface became porous and irregular with the formation of clusters. This suggested that the adsorption process led to the aggregation of adsorbent particles, which enhanced the adsorption capacity. The formation of these agglomerates may be attributed to the electrostatic attraction between the negatively charged surface of Fe3O4/CL-U and the positively charged Cu2+. SEM analysis also revealed that the Fe3O4/CL-D-U sample retained its morphology and did not undergo significant structural changes after Cu2+ adsorption, indicating its stability and potential for reuse. These results provide further insights into the adsorption mechanism and structural properties of the Fe3O4/CL-D adsorbent, which can guide the design and optimization of similar adsorbents for efficient wastewater treatment applications.
3.4 Discussion of adsorption mechanism
A set of adsorbents comprising Fe3O4 on modified CL were synthesized using the coprecipitation method. Characterization using ICP, SEM, TG, and XRD revealed that Fe3O4 loading was maximal on Fe3O4/CL-D following DES dissolution, and crystallinity was correspondingly reduced to the greatest extent. Moreover, FTIR and XPS analyses demonstrated that the crystalline structure of CL underwent slight changes after chemical modification, with an increase in the proportion of O atoms that was favorable for Cu2+ adsorption. These findings contribute to our understanding of the adsorption behavior of Cu2+ on Fe3O4/CL-D and have important implications for the development of novel approaches for the effective removal of heavy metals from contaminated water sources.The results presented in Fig. 7d demonstrate that the adsorption capacity of Cu2+ was significantly greater over Fe3O4/CL than CL. This enhanced performance can be attributed to the following factors: as indicated by the experimental results in Part 3.2.1 (Fig. 7c), the initial adsorption solution was weakly acidic, resulting in a large number of surface hydroxyl groups on the Fe3O4 molecule. These hydroxyl groups can interact with Cu2+ in the solution via ion exchange, leading to the efficient removal of Cu2+. Furthermore, the pristine CL molecule possesses a multitude of hydroxyl groups, hydrogen bonds, and multilevel cavities, which facilitate chelation reactions with Cu2+. However, the high crystallinity of CL hinders the entry of Cu2+ into the crystallization zone for coordination reactions, resulting in a very low adsorption rate for the unmodified CL. Therefore, oxidation by NaIO4 and dissolution in DES were employed to decrease the crystallinity of cellulose and increase the accessibility of hydroxyl groups, resulting in an improved adsorption capacity of CL.
Through fitting the experimental data of the CL-based adsorbent using adsorption isotherm and kinetics models, we found that the adsorption of Cu2+ on Fe3O4/CL-D follows the Freundlich isotherm model and quasi-second-order adsorption model. Namely, the adsorption process of Cu2+ on Fe3O4/CL-D is multilayer and involves multiple adsorption mechanisms. Therefore, the adsorption mechanism of Cu2+ by Fe3O4/CL-D in aqueous solution was proposed as shown in Fig. 12.62 Initially, Fe3O4 molecules were loaded onto the surface of CL, and the abundant O atoms in the molecular structure of Fe3O4 formed hydrogen bonds with the hydroxyl groups in CL molecules, thus stabilizing Fe3O4 on CL. Subsequently, the adsorption of Cu2+ on Fe3O4 was primarily driven by an ion exchange reaction, while the chelation reaction was mainly observed on the surface and interface of CL.
4 Conclusions
In this study, we successfully synthesized a magnetic Fe3O4/CL adsorption material using low-value cotton linter as the raw material. The chemical modification pretreatment of CL facilitated the loading of a higher amount of Fe3O4, particularly with the use of DES treatment, which effectively improved the adsorption capacity of Cu2+. ICP analysis indicated that the Fe loading on Fe3O4/CL-D reached 15.2 wt% and existed in the form of Fe3O4, which was confirmed by XRD. Moreover, the XRD results suggested that the crystallinity of cotton linter treated with DES was significantly decreased, contributing to the increased accessibility of Cu2+ to hydroxyl groups and thus improving the adsorption capacity. As a result, the adsorption capacity of Fe3O4/CL-D reached 93.1 mg g−1, which was much higher than that of untreated CL (11.0 mg g−1). The adsorption process of Cu2+ on Fe3O4/CL-D from aqueous solution was an ion exchange and chelation reaction, and the adsorption behavior mainly involved Freundlich multimolecular layer adsorption and quasi-second-order multiple adsorption mechanisms. Our findings offer a potential strategy for the efficient utilization of low-cost cotton linter in the future, with broad application prospects in the adsorption of heavy metals and other pollutants. However, the current study also has limitations. For instance, the study only focused on the adsorption of Cu2+, and the effectiveness of Fe3O4/CL-D for the adsorption of other pollutants was not investigated. In future research, the application of Fe3O4/CL-D in the removal of other heavy metals and pollutants will be explored. Additionally, the study only examined the feasibility of using cotton linter as a raw material for the synthesis of Fe3O4/CL-D. The scalability and economic feasibility of the production process should also be considered for practical applications.Author contributions
S. Y.: conceptualization, formal analysis, resources; M. X.: formal analysis, investigation, writing – original draft; H. S.: methodology, formal analysis, writing – original draft; Y. N.: formal analysis, investigation, writing – review and editing; R. W.: resources, formal analysis; R. Y.: methodology, writing – review and editing; L. W.: methodology, validation; F. L.: project administration, funding acquisition, writing – review and editing; X. D.: visualization, funding acquisition, writing – review and editing; J. W.: conceptualization, formal analysis, writing – review and editing, supervision. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from Young Talents Program of Anhui Academy of Agricultural Sciences, the Key Science and Technology Project of Anhui 334 Province (202003B06020009), Anhui Provincial Finance Agricultural Scientific and Technological Achievements Transformation Project (2022ZH011), and the Research Team Project of Anhui Academy of Agricultural Sciences.References
- J. Zhang, Z. Liu, B. Tian, J. Li, J. Luo, X. Wang, S. Ai and X. Wang, Assessment of soil heavy metal pollution in provinces of China based on different soil types: From normalization to soil quality criteria and ecological risk assessment, J. Hazard. Mater., 2023, 441, 129891 CrossRef CAS PubMed.
- R. S. Ahmed, M. E. Abuarab, M. M. Ibrahim, M. Baioumy and A. Mokhtar, Assessment of environmental and toxicity impacts and potential health hazards of heavy metals pollution of agricultural drainage adjacent to industrial zones in Egypt, Chemosphere, 2023, 137872 CrossRef CAS PubMed.
- H. Binner, T. Sullivan, M. A. K. Jansen and M. E. McNamara, Metals in urban soils of Europe: A systematic review, Sci. Total Environ., 2022, 158734 CAS.
- J. d. S. O. Filho and M. G. Pereira, Is Environmental Contamination a Concern in Global Technosols? A Bibliometric Analysis, Water, Air, Soil Pollut., 2023, 234(3), 142 CrossRef.
- Z. Wang, P. Luo, X. Zha, C. Xu, S. Kang, M. Zhou, D. Nover and Y. Wang, Overview assessment of risk evaluation and treatment technologies for heavy metal pollution of water and soil, J. Cleaner Prod., 2022, 134043 CrossRef CAS.
- M. Wang, A. B. G. Janssen, J. Bazin, M. Strokal, L. Ma and C. Kroeze, Accounting for interactions between Sustainable Development Goals is essential for water pollution control in China, Nat. Commun., 2022, 13(1), 730 CrossRef CAS PubMed.
- Y. Yu, Y. Zhong, M. Wang and Z. Guo, Electrochemical behavior of aluminium anode in super-gravity field and its application in copper removal from wastewater by electrocoagulation, Chemosphere, 2021, 272, 129614 CrossRef CAS PubMed.
- Y. Yu, Y. Zhong, W. Sun, J. Xie, M. Wang and Z. Guo, A novel electrocoagulation process with centrifugal electrodes for wastewater treatment: Electrochemical behavior of anode and kinetics of heavy metal removal, Chemosphere, 2023, 310, 136862 CrossRef CAS PubMed.
- J. Gao, Y. Yuan, Q. Yu, B. Yan, Y. Qian, J. Wen, C. Ma, S. Jiang, X. Wang and N. Wang, Bio-inspired antibacterial cellulose paper-poly(amidoxime) composite hydrogel for highly efficient uranium(vi) capture from seawater, Chem. Commun., 2020, 56(28), 3935–3938 RSC.
- V. Mohanapriya, R. Sakthivel, N. D. K. Pham, C. K. Cheng, H. S. Le and T. M. H. Dong, Nanotechnology - A ray of hope for heavy metals removal, Chemosphere, 2023, 311, 136989 CrossRef CAS PubMed.
- S. Rajendran, A. K. Priya, P. S. Kumar, T. K. A. Hoang, K. Sekar, K. Y. Chong, K. S. Khoo, H. S. Ng and P. L. Show, A critical and recent developments on adsorption technique for removal of heavy metals from wastewater-A review, Chemosphere, 2022, 303, 135146 CrossRef CAS PubMed.
- Z. Long, Q. Li, T. Wei, G. Zhang and Z. Ren, Historical development and prospects of photocatalysts for pollutant removal in water, J. Hazard. Mater., 2020, 395, 122599 CrossRef CAS PubMed.
- A. Guleria, R. Sharma, P. Shandilya, Photocatalytic and adsorptional removal of heavy metals from contaminated water using nanohybrids, Materials and Research Foundations, 2021, vol. 100, pp. 113–160 Search PubMed.
- A. E. Mouden, N. E. Messaoudi, A. E. Guerraf, A. Bouich, V. Mehmeti, A. Lacherai, A. Jada and F. Sher, Multifunctional cobalt oxide nanocomposites for efficient removal of heavy metals from aqueous solutions, Chemosphere, 2023, 137922 CrossRef PubMed.
- G. Lin, B. Zeng, J. Li, Z. Wang, S. Wang, T. Hu and L. Zhang, A systematic review of metal organic frameworks materials for heavy metal removal: synthesis, applications and mechanism, Chem. Eng. J., 2023, 460, 141710 CrossRef CAS.
- D. Li, X. Tian, Z. Wang, Z. Guan, X. Li, H. Qiao, H. Ke, L. Luo and Q. Wei, Multifunctional adsorbent based on metal-organic framework modified bacterial cellulose/chitosan composite aerogel for high efficient removal of heavy metal ion and organic pollutant, Chem. Eng. J., 2020, 383, 123127 CrossRef CAS.
- R. Liu, S. Wen, Y. Sun, B. Yan, J. Wang, L. Chen, S. Peng, C. Ma, X. Cao, C. Ma, G. Duan, S. Shi, Y. Yuan and N. Wang, A nanoclay enhanced Amidoxime-Functionalized Double-Network hydrogel for fast and massive uranium recovery from seawater, Chem. Eng. J., 2021, 422, 130060 CrossRef CAS.
- J. Yu, A. C. Wang, M. Zhang and Z. Lin, Water treatment via non-membrane inorganic nanoparticles/cellulose composites, Mater. Today, 2021, 50, 329–357 CrossRef CAS.
- A. K. Rana, S. Guleria, V. K. Gupta and V. K. Thakur, Cellulosic pine needles-based biorefinery for a circular bioeconomy, Bioresour. Technol., 2023, 367, 128255 CrossRef CAS PubMed.
- A. K. Rana, F. Scarpa and V. K. Thakur, Cellulose/polyaniline hybrid nanocomposites: Design, fabrication, and emerging multidimensional applications, Ind. Crops Prod., 2022, 187, 115356 CrossRef CAS.
- A. K. Badawi, M. Abd Elkodous and G. A. M. Ali, Recent advances in dye and metal ion removal using efficient adsorbents and novel nano-based materials: an overview, RSC Adv., 2021, 11(58), 36528–36553 RSC.
- A. Sczostak, Cotton Linters: An Alternative Cellulosic Raw Material, Macromol. Symp., 2010, 294(2), 151 CrossRef CAS.
- J. P. Morais, F. Rosa Mde, S. de Souza Filho Mde, L. D. Nascimento, D. M. do Nascimento and A. R. Cassales, Extraction and characterization of nanocellulose structures from raw cotton linter, Carbohydr. Polym., 2013, 91(1), 229–235 CrossRef CAS PubMed.
- X. Zhou, P. Wang, Y. Zhang, X. Zhang and Y. Jiang, From Waste Cotton Linter: A Renewable Environment-Friendly Biomass Based Carbon Fibers Preparation, ACS Sustainable Chem. Eng., 2016, 4(10), 5585–5593 CrossRef CAS.
- A. Tutuş, A. Özdemir and M. Çiçekler, Evaluation of Linter Cellulose as an Alternative Raw Material for Tissue Paper Production, Drvna Ind., 2017, 68(4), 291–298 CrossRef.
- Y. Sun, Q. Yue, B. Gao, Q. Li, L. Huang, F. Yao and X. Xu, Preparation of activated carbon derived from cotton linter fibers by fused NaOH activation and its application for oxytetracycline (OTC) adsorption, J. Colloid Interface Sci., 2012, 368(1), 521–527 CrossRef CAS PubMed.
- A. Etale, A. J. Onyianta, S. R. Turner and S. J. Eichhorn, Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment, Chem. Rev., 2023, 123(5), 2016–2048 CrossRef CAS PubMed.
- T. Li, C. Chen, A. H. Brozena, J. Y. Zhu, L. Xu, C. Driemeier, J. Dai, O. J. Rojas, A. Isogai, L. Wagberg and L. Hu, Developing fibrillated cellulose as a sustainable technological material, Nature, 2021, 590(7844), 47–56 CrossRef CAS PubMed.
- H. Wang, G. Gurau and R. D. Rogers, Ionic liquid processing of cellulose, Chem. Soc. Rev., 2012, 41(4), 1519–1537 RSC.
- O. M. Vanderfleet and E. D. Cranston, Production routes to tailor the performance of cellulose nanocrystals, Nat. Rev. Mater., 2020, 6(2), 124–144 CrossRef.
- Z. Ling, J. V. Edwards, Z. Guo, N. T. Prevost, S. Nam, Q. Wu, A. D. French and F. Xu, Structural variations of cotton cellulose nanocrystals from deep eutectic solvent treatment: micro and nano scale, Cellulose, 2018, 26(2), 861–876 CrossRef.
- Z. Ling, T. Wang, M. Makarem, M. Santiago Cintrón, H. N. Cheng, X. Kang, M. Bacher, A. Potthast, T. Rosenau, H. King, C. D. Delhom, S. Nam, J. Vincent Edwards, S. H. Kim, F. Xu and A. D. French, Effects of ball milling on the structure of cotton cellulose, Cellulose, 2019, 26(1), 305–328 CrossRef CAS.
- L. Zhou, F. Pan, Y. Liu, Z. Kang, S. Zeng and Y. Nie, Study on the regularity of cellulose degradation in ionic liquids, J. Mol. Liq., 2020, 308, 113153 CrossRef CAS.
- A. J. Sayyed, N. A. Deshmukh and D. V. Pinjari, A critical review of manufacturing processes used in regenerated cellulosic fibres: viscose, cellulose acetate, cuprammonium, LiCl/DMAc, ionic liquids, and NMMO based lyocell, Cellulose, 2019, 26(5), 2913–2940 CrossRef CAS.
- R. Sescousse, R. Gavillon and T. Budtova, Aerocellulose from cellulose–ionic liquid solutions: Preparation, properties and comparison with cellulose–NaOH and cellulose–NMMO routes, Carbohydr. Polym., 2011, 83(4), 1766–1774 CrossRef CAS.
- R. P. Swatloski, J. D. Holbrey and R. D. Rogers, Ionic liquids are not always green: hydrolysis of 1-butyl-3-methylimidazolium hexafluorophosphate, Green Chem., 2003, 5, 361–363 RSC.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, Dissolution of Cellose with Ionic Liqui, J. Am. Chem. Soc., 2002, 124(18), 4974–4975 CrossRef CAS PubMed.
- R. D. Rogers and K. R. Seddon, CHEMISTRY: Ionic Liquids--Solvents of the Future?, Science, 2003, 302(5646), 792–793 CrossRef PubMed.
- A. Mnasri, R. Khiari, H. Dhaouadi, S. Halila and E. Mauret, Acidic and alkaline deep eutectic solvents pre-treatment to produce high aspect ratio microfibrillated cellulose, Bioresour. Technol., 2023, 368, 128312 CrossRef CAS PubMed.
- J. Xie, J. Xu, Z. Zhang, B. Wang, S. Zhu, J. Li and K. Chen, New ternary deep eutectic solvents with cycle performance for efficient pretreated radiata pine forming to lignin containing cellulose nanofibrils, Chem. Eng. J., 2023, 451, 138591 CrossRef CAS.
- X. Wu, Y. Yuan, S. Hong, J. Xiao, X. Li and H. Lian, Controllable preparation of nano-cellulose via natural deep eutectic solvents prepared with lactate and choline chloride, Ind. Crops Prod., 2023, 194, 116259 CrossRef CAS.
- M. P. Rayaroth, D. Oh, C.-S. Lee and Y.-S. Chang, Simultaneous removal of heavy metals and dyes in water using a MgO-coated Fe3O4 nanocomposite: Role of micro-mixing effect induced by bubble generation, Chemosphere, 2022, 294, 133788 CrossRef CAS PubMed.
- E. S. Behbahani, K. Dashtian and M. Ghaedi, Fe3O4-FeMoS4: Promise magnetite LDH-based adsorbent for simultaneous removal of Pb (II), Cd (II), and Cu (II) heavy metal ions, J. Hazard. Mater., 2021, 100 Search PubMed.
- M. E. Mahmoud, S. M. El-Bahy and S. M. T. Elweshahy, Decorated Mn-ferrite nanoparticle@Zn-Al layered double hydroxide@Cellulose@ activated biochar nanocomposite for efficient remediation of methylene blue and mercury (II), Bioresour. Technol., 2021, 342, 126029 CrossRef CAS PubMed.
- X. Deng, S. Ye, L. Wan, J. Wu, H. Sun, Y. Ni and F. Liu, Study on Dissolution and Modification of Cotton Fiber in Different Growth Stages, Materials, 2022, 15(7), 2685 CrossRef CAS PubMed.
- H. L. Fan, S. F. Zhou, W. Z. Jiao, G. S. Qi and Y. Z. Liu, Removal of heavy metal ions by magnetic chitosan nanoparticles prepared continuously via high-gravity reactive precipitation method, Carbohydr. Polym., 2017, 174, 1192–1200 CrossRef CAS PubMed.
- X. Yue, F. Jiang, D. Zhang, H. Lin and Y. Chen, Preparation of adsorbent based on cotton fiber for removal of dyes, Fibers Polym., 2017, 18(11), 2102–2110 CrossRef CAS.
- Y. Yue, G. Han and Q. Wu, Transitional Properties of Cotton Fibers from Cellulose I to Cellulose II Structure, BioResources, 2013, 8(4), 6460–6471 CrossRef.
- T. Chen, J. Hong, C. Peng, G. Chen, C. Yuan, Y. Xu, B. Zeng and L. Dai, Superhydrophobic and flame retardant cotton modified with DOPO and fluorine-silicon-containing crosslinked polymer, Carbohydr. Polym., 2019, 208, 14–21 CrossRef CAS PubMed.
- N. Abidi, L. Cabrales and C. H. Haigler, Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy, Carbohydr. Polym., 2014, 100, 9–16 CrossRef CAS PubMed.
- M. Martinez-Sanz, F. Pettolino, B. Flanagan, M. J. Gidley and E. P. Gilbert, Structure of cellulose microfibrils in mature cotton fibres, Carbohydr. Polym., 2017, 175, 450–463 CrossRef CAS PubMed.
- T. Yamashita and P. Hayes, Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials, Appl. Surf. Sci., 2008, 254(8), 2441–2449 CrossRef CAS.
- X. Chen, X. Wang and D. Fang, A review on C1s XPS-spectra for some kinds of carbon materials, Fullerenes, Nanotubes, Carbon Nanostruct., 2020, 28(12), 1048–1058 CrossRef CAS.
- M. Ayiania, M. Smith, A. J. R. Hensley, L. Scudiero, J.-S. McEwen and M. Garcia-Perez, Deconvoluting the XPS spectra for nitrogen-doped chars: An analysis from first principles, Carbon, 2020, 162, 528–544 CrossRef CAS.
- Q. Li, H. Wang, Z. Chen, X. He, Y. Liu, M. Qiu and X. Wang, Adsorption-reduction strategy of U(VI) on NZVI-supported zeolite composites via batch, visual and XPS techniques, J. Mol. Liq., 2021, 339, 116719 CrossRef CAS.
- P. S. Bagus, C. J. Nelin, C. R. Brundle, N. Lahiri, E. S. Ilton and K. M. Rosso, Analysis of the Fe 2p XPS for hematite alpha Fe2O3: Consequences of covalent bonding and orbital splittings on multiplet splittings, J. Chem. Phys., 2020, 152(1), 014704 CrossRef CAS PubMed.
- G. Zhu, L. Yang, Y. Gao, J. Xu, H. Chen, Y. Zhu, Y. Wang, C. Liao, C. Lu and C. Zhu, Characterization and pelletization of cotton stalk hydrochar from HTC and combustion kinetics of hydrochar pellets by TGA, Fuel, 2019, 244, 479–491 CrossRef CAS.
- M. Zhong, S. Chen, T. Wang, J. Liu, M. Mei and J. Li, Co-pyrolysis of polyester and cotton via thermogravimetric analysis and adsorption mechanism of Cr (VI) removal by carbon in aqueous solution, J. Mol. Liq., 2022, 354, 118902 CrossRef CAS.
- M. S. Almughamisi, Z. A. Khan, W. Alshitari and K. Z. Elwakeel, Recovery of Chromium(VI) Oxyanions from Aqueous Solution Using Cu(OH)2 and CuO Embedded Chitosan Adsorbents, J. Polym. Environ., 2019, 28(1), 47–60 CrossRef.
- M. Touihri, F. Guesmi, C. Hannachi, B. Hamrouni, L. Sellaoui, M. Badawi, J. Poch and N. Fiol, Single and simultaneous adsorption of Cr(VI) and Cu (II) on a novel Fe3O4/pine cones gel beads nanocomposite: Experiments, characterization and isotherms modeling, Chem. Eng. J., 2021, 416, 129101 CrossRef CAS.
- X. Yue, J. Huang, F. Jiang, H. Lin and Y. Chen, Synthesis and characterization of cellulose-based adsorbent for removal of anionic and cationic dyes, J. Eng. Fibers Fabr., 2019, 14 DOI:10.1177/1558925019828194.
- S. Ye, H. Sun, J. Wu, L. Wan, Y. Ni, R. Wang, Z. Xiang and X. Deng, Supercritical CO2 Assisted TiO2 Preparation to Improve the UV Resistance Properties of Cotton Fiber, Polymers, 2022, 14, 5513 CrossRef CAS PubMed.
- Z. Liu, H. Wang, C. Liu, Y. Jiang, G. Yu, X. Mu and X. Wang, Magnetic cellulose-chitosan hydrogels prepared from ionic liquids as reusable adsorbent for removal of heavy metal ions, Chem. Commun., 2012, 48(59), 7350–7352 RSC.
- X. Luo, J. Zeng, S. Liu and L. Zhang, An effective and recyclable adsorbent for the removal of heavy metal ions from aqueous system: Magnetic chitosan/cellulose microspheres, Bioresour. Technol., 2015, 194, 403–406 CrossRef CAS PubMed.
- H. Ozay, Z. Gungor, B. Yilmaz, P. Ilgin and O. Ozay, Dual use of colorimetric sensor and selective copper removal from aqueous media with novel p(HEMA-co-TACYC) hydrogels: Cyclen derivative as both monomer and crosslinker, J. Hazard. Mater., 2020, 389, 121848 CrossRef CAS PubMed.
- M. Choudhary, R. Kumar and S. Neogi, Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu(+2) and Ni(+2) from water, J. Hazard. Mater., 2020, 392, 122441 CrossRef CAS PubMed.
- H. Jiang, Y. Yang, Z. Lin, B. Zhao, J. Wang, J. Xie and A. Zhang, Preparation of a novel bio-adsorbent of sodium alginate grafted polyacrylamide/graphene oxide hydrogel for the adsorption of heavy metal ion, Sci. Total Environ., 2020, 744, 140653 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |