DOI:
10.1039/D3RA02131A
(Paper)
RSC Adv., 2023,
13, 13586-13591
LZY3016, a novel geldanamycin derivative, inhibits tumor growth in an MDA-MB-231 xenograft model†
Received
31st March 2023
, Accepted 26th April 2023
First published on 3rd May 2023
Abstract
A novel geldanamycin derivative LZY3016 was synthesized as an antitumor agent. Compound LZY3016 exhibited potent anti-proliferation activity toward MDA-MB-231 (IC50 = 0.06 μM), which was more effective than positive drug 17-AAG. In vivo hepatotoxicity assay displayed that serum AST/ALT levels in LZY3016-treated mice were both significantly less than those in the geldanamycin (GA) group. LZY3016 showed potent antitumor activity in an MDA-MB-231 xenograft mouse model, suggesting LZY3016 is an up-and-coming antitumor candidate. The theoretical binding mode between LZY3016 and Hsp90 was obtained by molecular dynamics simulation.
1. Introduction
Heat shock protein 90 (Hsp90) is a very rich protein in cells, which plays essential regulatory roles by binding to several client proteins. Geldanamycin (GA) was identified as the first natural product inhibitor of Hsp90, which binds to the ATP-binding site of the Hsp90 N-terminal domain, leading to degradation of its client proteins.1,2 Because of its core role in carcinogenic signal transduction, Hsp90 is an attractive target for cancer treatment.3
Although GA has excellent antitumor potentiality, it has not been applied in the clinic due to its poor water solubility, and hepatotoxicity.4 Subsequently, more stable and soluble GA derivatives were synthesized and promoted to clinical trials,5,6 such as tanespimycin (17-AAG) and alvespimycin (17-DMAG) (Fig. 1). 17-AAG showed some prospect for the treatment of breast cancer in the phase II clinical trial, however, the clinical trial has been terminated, which indicates that the development of a new generation of GA derivatives with low or even no hepatotoxicity is imminent.
 |
| | Fig. 1 Known ansamycin Hsp90 inhibitors. | |
In this study, a novel GA derivative 17-propargylamine-17-demethoxygeldanamycin (LZY3016) was synthesized, because to modify the 17-position of GA with amino substituents could decrease the hepatotoxicity, such as 17-AAG.7 (Fig. 2). In this paper, the anti-proliferation activity of LZY3016 toward five cancer cells and its in vivo hepatotoxicity were determined. In addition, the binding target and antitumor activity in an MDA-MB-231 xenograft model of LZY3016 were also studied.
 |
| | Fig. 2 The tumor growth curve of the MDA-MB-231 xenograft in nude mice (A). Photo of dissected tumor tissues (B). | |
2. Materials and methods
2.1. General
All melting points were measured on the micro-melting point instrument without correction. 1H NMR spectra and 13C NMR spectra were both obtained by a 600 MHz Bruker Avance spectrometer. API 4000 Mass Spectrometer was used to record the ESI-MS spectra. Thin-layer chromatography (TLC) was carried out on GF254 glass pre-coated silica gel plates. Geldanamycin was purchased from Shanghai Yingrui Chemical Technology Co., Ltd. The AST/ALT Assay Kits were purchased from Shanghai Rongsheng Biological Pharmaceutical Co., Ltd.
2.2. Ethical statement
The experimental animal ethics committee of Shandong Provincial Hospital Affiliated to Shandong First Medical University approved all animal experiments and ensured they are carried out in accordance with the “Guidelines for the Care and Use of Experimental Animals”. All efforts are aimed at reducing the number of animals used and the suffering in the experiment.
2.3. Synthesis of LZY3016
Geldanamycin (28 mg, 0.05 mmol) was suspended in 5 mL dichloromethane and then propargylamine (130 μL, 2 mmol) was added, which was stirred at room temperature overnight. After the reaction finished, the mixture was washed with 1.5 N hydrochloric acid solution (2 × 20 mL), followed by brine (3 × 15 mL). The dichloromethane layer was then dehydrated by anhydrous magnesium sulfate, filtered, and evaporated to afford the crude product, which was purified by flash column chromatography on silica gel (petroleum ether/ethyl acetate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain LZY3016 (28 mg, 95.9%) as a purple solid. M.p. 126–127 °C; 1H NMR (600 MHz, chloroform-d) δ 9.09 (s, 1H), 7.32 (s, 1H), 6.95 (d, J = 11.7 Hz, 1H), 6.59 (t, J = 11.4 Hz, 1H), 6.35 (t, J = 6.0 Hz, 1H), 5.92–5.84 (m, 2H), 5.19 (s, 1H), 4.98 (s, 2H), 4.31 (dt, J = 6.2, 3.3 Hz, 3H), 4.07 (s, 1H), 3.57 (t, J = 7.1 Hz, 1H), 3.47–3.43 (m, 1H), 3.37 (s, 3H), 3.27 (s, 3H), 2.78–2.69 (m, 2H), 2.41 (d, J = 2.5 Hz, 1H), 2.38 (dd, J = 14.3, 10.9 Hz, 1H), 2.04–2.00 (m, 3H), 1.86–1.74 (m, 6H), 1.00 (t, J = 7.6 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 183.6, 181.6, 168.4, 156.1, 143.9, 140.7, 135.9, 134.9, 133.6, 132.9, 127.0, 126.5, 110.3, 109.1, 81.6, 81.3, 81.1, 78.2, 74.0, 72.6, 57.1, 56.7, 35.4, 34.9, 34.4, 32.3, 28.6, 22.9, 12.8, 12.6, 12.4; ESI-MS: m/z 606.5 [M + Na]+ (calcd 606.7).
1) to obtain LZY3016 (28 mg, 95.9%) as a purple solid. M.p. 126–127 °C; 1H NMR (600 MHz, chloroform-d) δ 9.09 (s, 1H), 7.32 (s, 1H), 6.95 (d, J = 11.7 Hz, 1H), 6.59 (t, J = 11.4 Hz, 1H), 6.35 (t, J = 6.0 Hz, 1H), 5.92–5.84 (m, 2H), 5.19 (s, 1H), 4.98 (s, 2H), 4.31 (dt, J = 6.2, 3.3 Hz, 3H), 4.07 (s, 1H), 3.57 (t, J = 7.1 Hz, 1H), 3.47–3.43 (m, 1H), 3.37 (s, 3H), 3.27 (s, 3H), 2.78–2.69 (m, 2H), 2.41 (d, J = 2.5 Hz, 1H), 2.38 (dd, J = 14.3, 10.9 Hz, 1H), 2.04–2.00 (m, 3H), 1.86–1.74 (m, 6H), 1.00 (t, J = 7.6 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 183.6, 181.6, 168.4, 156.1, 143.9, 140.7, 135.9, 134.9, 133.6, 132.9, 127.0, 126.5, 110.3, 109.1, 81.6, 81.3, 81.1, 78.2, 74.0, 72.6, 57.1, 56.7, 35.4, 34.9, 34.4, 32.3, 28.6, 22.9, 12.8, 12.6, 12.4; ESI-MS: m/z 606.5 [M + Na]+ (calcd 606.7).
2.4. In vitro anti-proliferation activity
The cytotoxicities of the compounds against cells were determined by MTT method.8 The cell inhibitory rate was calculated according to the following equation:
The cytotoxicities of the compounds are showed by IC50 value, which is defined as the concentration of the test compounds required to reduce cell viability by 50%.
2.5. In vivo hepatotoxicity test
The hepatotoxicity test in mice for the compounds were done according to our previously published study.8–12 The levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) of GA-treated and LZY3016-treated groups were measured using the AST and ALT Assay Kits.
2.6. In vitro Hsp90 inhibitory activity
The fluorescence polarization assay (FPA) was done as our previously published papers.8–12 Briefly, recombinant Hsp90α protein (50 nM), GA-FITC (5 nM), and the tested compounds were added to the reaction mixes (100 μL) in a black 96-well plates, which were recorded on POLARstar® Omega microplate reader (λex = 485 nm, λem = 520 nm) after incubation for 5 h. The IC50 is the concentration of the compound replaced 50% bound GA-FITC.
2.7. In vivo antitumor activity
Shortly, 5 × 106 MDA-MB-231 cells were implanted in the armpit subcutaneous layer of right foreleg of female athymic nude mice (6–7 weeks old). Eighteen mice were divided randomly into LZY3016-treated group, 17-AAG-treated group and vehicle group (six mice in each group) after the average tumor volume reached to 100 mm3. A dose of 10 mg kg−1 actual body-weight was given intravenously every 3 days. After fifteen days of drug treatment, all the mice were killed and then the tumors were excised and weighted. The tumor growth inhibition (TGI), RTV and relative increment ratio (T/C) were calculated with the following three equations:13| |
 | (1) |
| |
 | (2) |
| |
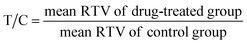 | (3) |
2.8. Molecular docking
We used Autodock Vina 1.1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 to study the binding pattern between LZY3016 and Hsp90. The Hsp90-GA complex (PDB code: 1YET) was retrieved from RCSB Protein Data Bank.15,16 AutoDock Tools 1.5.6 package17,18 was used to prepare PDBQT formats of Hsp90 and LZY3016. The Hsp90 pocket was set as center_x, y, z (40.695, −46.782, 65.693) and size_x, y, z (15, 15, 15). The best pose was chosen for subsequent MD simulation.
14 to study the binding pattern between LZY3016 and Hsp90. The Hsp90-GA complex (PDB code: 1YET) was retrieved from RCSB Protein Data Bank.15,16 AutoDock Tools 1.5.6 package17,18 was used to prepare PDBQT formats of Hsp90 and LZY3016. The Hsp90 pocket was set as center_x, y, z (40.695, −46.782, 65.693) and size_x, y, z (15, 15, 15). The best pose was chosen for subsequent MD simulation.
2.9. Molecular dynamics simulation
Amber 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19–21 was used to optimize the best docking pose. Initially 500 steps of steepest descent minimization followed by 500 steps of conjugate gradient minimization were done. The complex was equilibrated by 100 ps heating and 1000 ps density equilibration with weak restraints. Finally, 40 ns MD simulation was performed using the PMEMD module.
19–21 was used to optimize the best docking pose. Initially 500 steps of steepest descent minimization followed by 500 steps of conjugate gradient minimization were done. The complex was equilibrated by 100 ps heating and 1000 ps density equilibration with weak restraints. Finally, 40 ns MD simulation was performed using the PMEMD module.
2.10. MM/GBSA calculation and energy decomposition calculation
The equilibrated part of trajectories was used to estimate the binding free energy (ΔGbind) using the MM/GBSA method.22 To obtain more information with respect to the residues surrounding the binding pocket, the per-residue binding free energy was decomposed into van der Waals (ΔEvdw), solvation (ΔEsol), electrostatic (ΔEele) and total contribution (ΔEtotal).
3. Results and discussion
3.1. Preparation of LZY3016
The compound LZY3016 was synthesized by reacting the lead compound GA with the propargylamine as reported in the literature (Scheme 1).8–11,23,24 The structure of LZY3016 was characterized by 1H-NMR, 13C-NMR and MS spectral data.
 |
| | Scheme 1 Synthesis route of LZY3016. Reagents and conditions: (i) propargylamine, dichloromethane, room temperature, overnight. | |
3.2. In vitro anti-proliferative activity
The antitumor activities of LZY3016 on MDA-MB-231, HepG2, HeLa, A431 and SW480 cancer cell lines were screened by MTT method with 17-AAG and GA as positive controls. In vitro cytotoxicities evaluation showed that compound LZY3016 was approximate 8-fold less toxic than the positive control GA toward normal human hepatic cell HL7702 (Table 1). In the cancer cell lines, LZY3016 displayed potent cytotoxicity against MDA-MB-231 cells with an IC50 value of 0.06 μM, which was 3.5-fold, 3.1-fold, 12-fold and 3.9-fold more active than that toward HeLa, A431, SW480 and HepG2, respectively, suggesting that LZY3016 had selective anti-MDA-MB-231 proliferation activity against other four cancer cell lines (Table 1).
Table 1 In vitro cytotoxicities of LZY3016, 17-AAG and GA
| Compd |
IC50 (nM) |
| HL7702 |
HeLa |
MDA-MB-231 |
A431 |
SW480 |
HepG2 |
| LZY3016 |
1247 ± 263 |
218 ± 27 |
60 ± 19 |
186 ± 47 |
725 ± 86 |
236 ± 37 |
| 17-AAG |
262 ± 35 |
159 ± 21 |
338 ± 52 |
67 ± 5 |
657 ± 79 |
86 ± 12 |
| GA |
146 ± 15 |
651 ± 78 |
65 ± 16 |
48 ± 9 |
308 ± 37 |
41 ± 6 |
3.3. Hepatotoxicity evaluations
In vivo hepatotoxicity test displayed that GA had serious hepatotoxicity due to serum AST/ALT levels of GA-treated mice were significantly increased compared to vehicle group (P < 0.001) (Table 2). LZY3016-treated group had significantly lower serum AST level than 17-AAG group (P < 0.05), and no significant differences of serum ALT level were found between LZY3016-treated group and 17-AAG-treated group (P > 0.05). Interestingly, LZY3016-treated group had significantly lower serum AST/ALT levels than GA group (P < 0.001), and no significant differences were found between LZY3016-treated mice and vehicle control mice (P > 0.05).
Table 2 In vivo hepatotoxicity evaluation of LZY3016, GA and vehicle-treated mice
| Compd |
AST (U L−1) |
ALT (U L−1) |
| LZY3016 |
183.3 ± 29.4 |
75.5 ± 12.8 |
| 17-AAG |
248.9 ± 21.6 |
80.2 ± 14.3 |
| GA |
358.6 ± 45.2 |
298.1 ± 31.7 |
| Vehicle |
199.5 ± 18.3 |
65.6 ± 7.2 |
3.4. Hsp90 inhibitory activity
To identify the drug target of LZY3016, using 17-AAG and GA as the positive controls, the Hsp90 binding assay was performed using FPA method to evaluate the inhibitory activity between compound LZY3016 and recombinant Hsp90. It is indicated that Hsp90 inhibitory activity of compound LZY3016 (IC50 = 0.63 μM) was more active than 17-AAG (IC50 = 0.81 μM) (Table 3).
Table 3 The IC50 values of LZY3016, 17-AAG and GA at inhibiting Hsp90α
| Compd |
IC50 (μM) |
| LZY3016 |
0.63 ± 0.14 |
| 17-AAG |
0.81 ± 0.19 |
| GA |
0.21 ± 0.08 |
3.5. In vivo antitumor activity
Inspired with the potent in vitro antiproliferative activity of LZY3016 toward MDA-MB-231 cells with no hepatotoxicity, we continue to investigate the antitumor activity in vivo in an MDA-MB-231 xenograft model. The antitumor effects were unveiled by tumor weight and volume, which were reflected by calculating the TGI and T/C, respectively. After fifteen days of continuous treatment, compound LZY3016-treated group exhibited higher TGI (60.5%) and lower T/C (38.3%) values than those of 17-AAG-group (TGI = 53.9%, T/C = 43.5%), indicating the excellent in vivo antitumor activities of LZY3016 (Table 4). The tumor growth curve and the final tumor tissue size visualized in Fig. 2 explicitly showed the excellent antitumor potency of LZY3016.
Table 4 The inhibitory effect of LZY3016 on MDA-MB-231 xenografts in nude mice
| Compd |
TGI (%) |
T/C (%) |
| LZY3016 |
60.5 |
38.3 |
| 17-AAG |
53.9 |
43.5 |
3.6. Molecular modeling
MD simulation of Hsp90-LZY3016 complex was done using Amber 12 package. The free protein and Hsp90-LZY3016 complex were equilibrated after 40 ns MD simulation, and the plot of RMSDs (in ångstrom) of the two systems were shown in Fig. 3A.
 |
| | Fig. 3 The root-mean-square deviations (RMSDs) (in ångstrom) of all the atoms of free Hsp90 and Hsp90-LZY3016 complex during the 40 ns simulation (A). RMSF of residues of the whole protein in free Hsp90 and Hsp90-LZY3016 complex during the 40 ns simulation (B). | |
The flexibility of the residues was revealed by calculating the root mean square fluctuations (RMSF) of the whole protein residues in the free protein and Hsp90-LZY3016 complex. The RMSF values of Hsp90 residues in the presence and absence of LZY3016 were clearly exhibited in Fig. 3B. It was showed that most of the residues in the Hsp90 binding site showed minor flexibility with the RMSF less than 2 Å, revealing that these residues seemed to be more rigid due to their binding to LZY3016.
The energy decomposition demonstrated that the residue Lys-112 has a moderate electrostatic contribution, with the ΔEele of < −4.5 kcal mol−1 (Fig. 4A), because the amino acid Lys-112 was oriented to the benzoquinone group of the LZY3016, forming the cation-π interaction (Fig. 4B–D).25 In addition, the residue Asn-106 has an excellent van der Waals contribution with the ΔEvdw value less than −3.5 kcal mol−1 (Fig. 4A) due to the close-range between Asn-106 and LZY3016 (Fig. 4B–D). Except for the amino acid Asn-106, most of energy decomposition interactions were manifested as van der Waals interactions (Fig. 4A), mainly through hydrophobic interactions binding to LZY3016, such as Ala-55, Ile-96, Met-98, Leu-107 and Phe-138. Besides, the total binding free energy of Hsp90-LZY3016 complex was calculated based on the MMGBSA method, and the estimated ΔGbind of LZY3016 was −43.1 kcal mol−1, suggesting that LZY3016 can strongly bind to Hsp90.
 |
| | Fig. 4 Decomposition of the binding energy on a per-residue basis in the Hsp90-LZY3016 complex (A). The predicted binding mode of LZY3016 in Hsp90 binding pocket obtained from MD simulation (B–D). | |
4. Conclusions
In summary, a novel geldanamycin derivative LZY3016 was synthesized, which demonstrated potent anti-proliferation activity against MDA-MB-231 cell line, with an IC50 value of 0.06 μM. Hepatotoxicity evaluation test showed that the AST and ALT levels of LZY3016-treated group were both lower than those in GA-treated group. In addition, compared to 17-AAG, LZY3016 displayed more potent antitumor activity in the MDA-MB-231 xenograft model. The molecular modeling study revealed the theoretical binding model between LZY3016 and Hsp90 at molecular level. All in all, LZY3016 has emerged as a potential hit for further design and development of novel Hsp90 inhibitors.
Conflicts of interest
We declare that we have no conflict of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81502921), and Shandong Provincial Natural Science Foundation (ZR2020MH399).
Notes and references
- C. DeBoer, P. A. Meulman, R. J. Wnuk and D. H. Peterson, J. Antibiot., 1970, 23, 442–447 CrossRef CAS PubMed.
- C. E. Stebbins, A. A. Russo, C. Schneider, N. Rosen, F. U. Hartl and N. P. Pavletich, Cell, 1997, 89, 239–250 CrossRef CAS PubMed.
- C. Moser, S. A. Lang and O. Stoeltzing, Anticancer Res., 2009, 29, 2031–2042 CAS.
- J. G. Supko, R. L. Hickman, M. R. Grever and L. Malspeis, Cancer Chemother. Pharmacol., 1995, 36, 305–315 CrossRef CAS PubMed.
- E. A. Ronnen, G. V. Kondagunta, N. Ishill, S. M. Sweeney, J. K. DeLuca and L. Schwartz, et al., Invest. New Drugs, 2006, 24, 543–546 CrossRef CAS PubMed.
- E. R. Glaze, A. L. Lambert, A. C. Smith, J. G. Page, W. D. Johnson and D. L. McCormick, et al., Cancer Chemother. Pharmacol., 2005, 56, 637–647 CrossRef CAS PubMed.
- H. Behrsing, K. Amin, C. Ip, L. Jimenez and C. Tyson, Toxicol. In Vitro, 2005, 19, 1079–1088 CrossRef CAS PubMed.
- Z. Li, L. Jia, J. Wang, X. Wu, G. Shi and C. Lu, et al., Chem. Biol. Drug Des., 2015, 85, 181–188 CrossRef CAS PubMed.
- Z. Li, L. Jia, J. Wang, X. Wu, H. Hao and Y. Wu, et al., Eur. J. Med. Chem., 2014, 87, 346–363 CrossRef CAS PubMed.
- Z. Li, L. Jia, J. Wang, X. Wu, H. Hao and H. Xu, et al., Eur. J. Med. Chem., 2014, 85, 359–370 CrossRef CAS PubMed.
- Z. Li, L. Jia, H. Xu, C. Lu and Y. Shen, Med. Chem., 2015, 11, 482–488 CrossRef CAS PubMed.
- Z. Li, L. Jia, H. Tang, Y. Shen and C. Shen, RSC Adv., 2019, 9, 42509–42515 RSC.
- Y. Zhang, H. Fang, J. Feng, Y. Jia, X. Wang and W. Xu, J. Med. Chem., 2011, 54, 5532–5539 CrossRef CAS PubMed.
- O. Trott and A. J. Olson, J. Comput. Chem., 2010, 31, 455–461 CAS.
- H. M. Berman, J. Westbrook, Z. Feng, G. Gilliland, T. N. Bhat and H. Weissig, et al., Nucleic Acids Res., 2000, 28, 235–242 CrossRef CAS PubMed.
- S. K. Burley, C. Bhikadiya, C. Bi, S. Bittrich, H. Chao and L. Chen, et al., Nucleic Acids Res., 2023, 51, D488–D508 CrossRef PubMed.
- M. F. Sanner, J. Mol. Graphics Modell., 1999, 17, 57–61 CAS.
- G. M. Morris, R. Huey, W. Lindstrom, M. F. Sanner, R. K. Belew and D. S. Goodsell, et al., J. Comput. Chem., 2009, 30, 2785–2791 CrossRef CAS PubMed.
- L. C. Pierce, R. Salomon-Ferrer, C. A. F. de Oliveira, J. A. McCammon and R. C. Walker, J. Chem. Theory Comput., 2012, 8, 2997–3002 CrossRef CAS PubMed.
- A. W. Götz, M. J. Williamson, D. Xu, D. Poole, S. Le Grand and R. C. Walker, J. Chem. Theory Comput., 2012, 8, 1542–1555 CrossRef PubMed.
- R. Salomon-Ferrer, A. W. Götz, D. Poole, S. Le Grand and R. C. Walker, J. Chem. Theory Comput., 2013, 9, 3878–3888 CrossRef CAS PubMed.
- A. A. Elfiky, H. A. Mahran, I. M. Ibrahim, M. N. Ibrahim and W. M. Elshemey, RSC Adv., 2022, 12, 2741–2750 RSC.
- Y. Kasuya, Z. Lu, P. Kopeckova and J. Kopecek, Bioorg. Med. Chem. Lett., 2001, 11, 2089–2091 CrossRef CAS PubMed.
- F. Wuest, V. Bouvet, B. Mai and P. LaPointe, Org. Biomol. Chem., 2012, 10, 6724–6731 RSC.
- J. C. Ma and D. A. Dougherty, Chem. Rev., 1997, 97, 1303–1324 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2023 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Lejiao Jiab,
Hui Tanga,
Yuemao Shenc and
Chengwu Shen
a,
Lejiao Jiab,
Hui Tanga,
Yuemao Shenc and
Chengwu Shen *a
*a

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain LZY3016 (28 mg, 95.9%) as a purple solid. M.p. 126–127 °C; 1H NMR (600 MHz, chloroform-d) δ 9.09 (s, 1H), 7.32 (s, 1H), 6.95 (d, J = 11.7 Hz, 1H), 6.59 (t, J = 11.4 Hz, 1H), 6.35 (t, J = 6.0 Hz, 1H), 5.92–5.84 (m, 2H), 5.19 (s, 1H), 4.98 (s, 2H), 4.31 (dt, J = 6.2, 3.3 Hz, 3H), 4.07 (s, 1H), 3.57 (t, J = 7.1 Hz, 1H), 3.47–3.43 (m, 1H), 3.37 (s, 3H), 3.27 (s, 3H), 2.78–2.69 (m, 2H), 2.41 (d, J = 2.5 Hz, 1H), 2.38 (dd, J = 14.3, 10.9 Hz, 1H), 2.04–2.00 (m, 3H), 1.86–1.74 (m, 6H), 1.00 (t, J = 7.6 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 183.6, 181.6, 168.4, 156.1, 143.9, 140.7, 135.9, 134.9, 133.6, 132.9, 127.0, 126.5, 110.3, 109.1, 81.6, 81.3, 81.1, 78.2, 74.0, 72.6, 57.1, 56.7, 35.4, 34.9, 34.4, 32.3, 28.6, 22.9, 12.8, 12.6, 12.4; ESI-MS: m/z 606.5 [M + Na]+ (calcd 606.7).
1) to obtain LZY3016 (28 mg, 95.9%) as a purple solid. M.p. 126–127 °C; 1H NMR (600 MHz, chloroform-d) δ 9.09 (s, 1H), 7.32 (s, 1H), 6.95 (d, J = 11.7 Hz, 1H), 6.59 (t, J = 11.4 Hz, 1H), 6.35 (t, J = 6.0 Hz, 1H), 5.92–5.84 (m, 2H), 5.19 (s, 1H), 4.98 (s, 2H), 4.31 (dt, J = 6.2, 3.3 Hz, 3H), 4.07 (s, 1H), 3.57 (t, J = 7.1 Hz, 1H), 3.47–3.43 (m, 1H), 3.37 (s, 3H), 3.27 (s, 3H), 2.78–2.69 (m, 2H), 2.41 (d, J = 2.5 Hz, 1H), 2.38 (dd, J = 14.3, 10.9 Hz, 1H), 2.04–2.00 (m, 3H), 1.86–1.74 (m, 6H), 1.00 (t, J = 7.6 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 183.6, 181.6, 168.4, 156.1, 143.9, 140.7, 135.9, 134.9, 133.6, 132.9, 127.0, 126.5, 110.3, 109.1, 81.6, 81.3, 81.1, 78.2, 74.0, 72.6, 57.1, 56.7, 35.4, 34.9, 34.4, 32.3, 28.6, 22.9, 12.8, 12.6, 12.4; ESI-MS: m/z 606.5 [M + Na]+ (calcd 606.7).


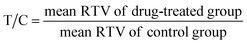
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 to study the binding pattern between LZY3016 and Hsp90. The Hsp90-GA complex (PDB code: 1YET) was retrieved from RCSB Protein Data Bank.15,16 AutoDock Tools 1.5.6 package17,18 was used to prepare PDBQT formats of Hsp90 and LZY3016. The Hsp90 pocket was set as center_x, y, z (40.695, −46.782, 65.693) and size_x, y, z (15, 15, 15). The best pose was chosen for subsequent MD simulation.
14 to study the binding pattern between LZY3016 and Hsp90. The Hsp90-GA complex (PDB code: 1YET) was retrieved from RCSB Protein Data Bank.15,16 AutoDock Tools 1.5.6 package17,18 was used to prepare PDBQT formats of Hsp90 and LZY3016. The Hsp90 pocket was set as center_x, y, z (40.695, −46.782, 65.693) and size_x, y, z (15, 15, 15). The best pose was chosen for subsequent MD simulation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19–21 was used to optimize the best docking pose. Initially 500 steps of steepest descent minimization followed by 500 steps of conjugate gradient minimization were done. The complex was equilibrated by 100 ps heating and 1000 ps density equilibration with weak restraints. Finally, 40 ns MD simulation was performed using the PMEMD module.
19–21 was used to optimize the best docking pose. Initially 500 steps of steepest descent minimization followed by 500 steps of conjugate gradient minimization were done. The complex was equilibrated by 100 ps heating and 1000 ps density equilibration with weak restraints. Finally, 40 ns MD simulation was performed using the PMEMD module.





