Open Access Article
Open Access ArticleMitomycin C-loaded PTMC15–F127–PTMC15 hydrogel maintained better bleb function after filtering glaucoma surgery in monkeys with intraocular hypertension
Shu Tu,
Ziming Luo,
Runcai Yang ,
Dongpeng Hu,
Bikun Xian,
Feng Zhao and
Jian Ge*
,
Dongpeng Hu,
Bikun Xian,
Feng Zhao and
Jian Ge*
State Key Laboratory of Ophthalmology, Guangdong Provincial Key Laboratory of Ophthalmology and Visual Science, Zhongshan Ophthalmic Center, Sun Yat-sen University, 7 Jinsui Road, Tianhe District, Guangzhou, 510000, China. E-mail: gejian@mail.sysu.edu.cn; Tel: +86 20 66615460
First published on 3rd May 2023
Abstract
There is an unmet need for a safer and more effective approach for antimetabolite application to prevent bleb fibrosis after glaucoma filtering surgery. Here, we utilized our previously developed thermosensitive sustained-release agent, mitomycin C-loaded poly(trimethylene carbonate)15–F127–poly(trimethylene carbonate)15 (MMC-hydrogel), aiming to further evaluate the efficacy and safety of MMC-hydrogel in high intraocular pressure (IOP) primate eyes. Twelve primate eyes after high IOP induction were randomly divided into three groups, which respectively received phosphate-buffered saline (PBS)-hydrogel, MMC-hydrogel, and MMC treatment during trabeculectomy. IOP and bleb volume were measured using a Tonopen and anterior segment optical coherence tomography over 28 days. At the end of the experiment, all experimental primate eyes were enucleated. Histopathology and immunohistochemistry were performed to reveal myofibroblast cells and collagen deposition of filtering blebs. The MMC-hydrogel group had satisfactory IOP control (9.25 ± 4.80 mmHg) and maintained well-functioning blebs for a longer time. Fibrosis and scarring were significantly alleviated in this MMC-hydrogel group. There was no obvious toxicity to ocular surfaces or intraocular structures. Taken together, these data suggest that PTMC15–F127–PTMC15-loaded MMC-hydrogel plays a role in functional maintenance and scarring inhibition, showing high efficacy in reducing post-filtering surgery bleb fibrosis. This MMC-hydrogel may offer a new solution for filtering bleb management after glaucoma surgery.
Introduction
Filtration surgery is one of the main treatment modalities for glaucoma, a leading cause of irreversible blindness worldwide. Intraocular aqueous humor is drained out of the globe by filtration surgery to lower intraocular pressure (IOP). The aqueous humor is effluxed to a conjunctival-walled subconjunctival space, known as a filtrating bleb. However, postoperative scarring remains a critical issue, leading to bleb failure and hence uncontrolled IOP.1 Therefore, antimetabolites, such as mitomycin C (MMC) and 5-fluorouracil, have been used for many years to prevent bleb fibrosis and maintain long-term functional blebs.2 Although several studies showed similar efficacy of other drugs or small molecules, including siRNA,3 MMC and 5-FU are still the preferred antimetabolites in the clinic. Specifically, MMC inhibits fibroblasts and controls wound healing through DNA cross-linking alkylation. Adjuvant MMC has significantly improved the outcomes of trabeculectomy by preventing bleb scarring.4,5Currently, MMC is mainly applied by placing soaked sponges in the subconjunctival space during trabeculectomy surgery, and is then washed out with saline after a defined period.6 Alternatively, MMC may be applied by intraoperative subconjunctival injection.7 The dose and application time of MMC are variable. Typically, it ranges from 0.1 mg ml−1 to 0.5 mg ml−1, with the time varying between 1 to 5 minutes.8 It appears to be a trend that higher rates of hypotony are observed with higher strengths of MMC. Several studies also suggested that MMC application may be tailored according to patient factors, including race, age, prior intraocular surgery, and topical medication use.9 These variations contribute to potential risks of either bleb failure because of insufficient antimetabolic effect, or bleb leakage from over-suppression of wound healing. Balancing the pros and cons becomes one of the main concerns of the decision-making process during the surgery.
Therefore, sustained drug delivery could effectively address this unmet need. Sustained-released preparation allows delivery of MMC at a programmed rate for a prolonged period. Our group had previously reported a study on loading MMC into 5% (w/v) poly(trimethylene carbonate)15–F127–poly(trimethylene carbonate)15 (PTMC15–F127–PTMC15) hydrogel. This hydrogel demonstrated controlled, sustained release of MMC and reliable anti-scarring effect both in vitro and in vivo.10 Pluronic™ F127 is a triblock copolymer, of which aqueous solutions undergo sol–gel transition at 25 °C.11 This thermosensitive material can form a gel in the organism before solidifying within the desired tissue.12 PTMC15–F127–PTMC15 was synthesized with F127 and polycarbonate, improving the stability and providing a system for the controlled long-term continuous release of active substances.13 In our previous study, in vitro release experiment showed sustained release for up to 16 days, and no MMC burst-induced cell apoptosis was observed.10
However, our study was performed on normal rabbit eyes. It has been reported that immunoinflammatory markers are significantly increased in the conjunctival epithelium of glaucoma patients compared with normal eyes,14 which indicates that a more aggressive scarring process might happen under the disease. Thus, we include a chronic high IOP primate model in the present study. Moreover, a previous study showed that rabbit and monkey eyes had qualitative and quantitative differences in response to irritating surfactant solutions,14 which may be due to the anatomic difference of having a third eyelid and a less effective scarring mechanism in rabbits. On the contrary, primate eyes have highly similar anatomy and physiology to human eyes. Therefore, we aim to further evaluate the efficacy of MMC-loaded hydrogel in maintaining bleb function in pre-existing high IOP eyes.
Result
MMC-loaded hydrogel successfully maintained post-operation IOP as well as traditional MMC adjuvant treatment
Fig. 1A shows a schematic diagram depicting the preparation of the MMC-loaded hydrogel. Trabeculectomy is a filtering surgery where an ostium is created into the anterior chamber from underneath a partial-thickness scleral flap, allowing the aqueous humor to flow out of the eye and subsequently lowering IOP. The aqueous humor flowing into the subconjunctival space leads to an elevation of the conjunctiva, referred to as a filtering bleb.15 It is well-known that a diffuse, moderate-elevation and pale bleb is favorable and more likely to keep functioning in the long term.16,17 Although MMC has some side effects on ocular tissues, it is still the best method for assisting glaucoma surgery at present. The present study aims to compare the bleb development after traditional antimetabolite MMC adjuvant treatment during surgery and an MMC-loaded hydrogel injection. Twelve monkeys were involved in the present study after successfully inducing high IOP. They were randomly divided into three groups. A standard trabeculectomy was performed on all 12 monkey models' eyes. Briefly, a conjunctival incision was made, followed by a partial-thickness scleral flap. Subsequently, a sclerectomy and a peripheral iridectomy were performed after a paracentesis of the anterior chamber. The scleral incision was closed with 2 stitches using intermittent suture of 10-0 nylon thread, and the conjunctival incision was closed in a watertight fashion. Thus, the aqueous humor in the anterior chamber will efflux through a filter pathway to the subconjunctival space. Trabeculectomies were performed (Fig. 1B). In contrast, in the traditional MMC group, an MMC sponge was placed beneath the local conjunctiva and scleral flap for pre-treatment after making a partial-thickness scleral flap. For the other two groups, instead of applying MMC before sclerectomy, either PBS-hydrogel or MMC-hydrogel was injected subconjunctivally after closing the scleral incision (Fig. 1B). Slit-lamp photography was conducted thereafter for assessment.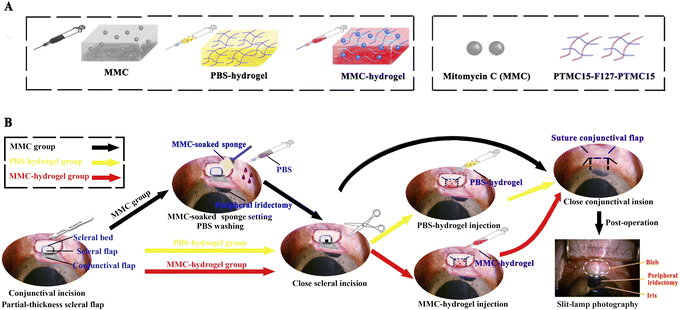 | ||
| Fig. 1 Standard trabeculectomy procedure with MMC adjuvant treatment versus MMC- or PBS-hydrogel injection. (A) Components of MMC-loaded hydrogel. MMC was dissolved in sterile PBS and prepared with PTMC15–F127–PTMC15 in a mass fraction of 5% under sterile operation. The MMC-loaded temperature-responsive PTMC15–F127–PTMC15 hydrogel was stored at 4 °C, and automatically formed a gel quickly at 37 °C. The detailed preparation method can be found in our previous publication.10 (B) Surgical procedures for the three groups. Slit-lamp photography showed the filtering bleb. | ||
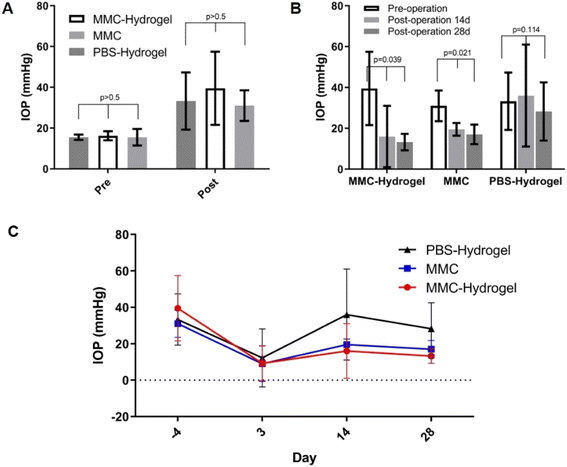
As shown in Fig. 2A, all 12 eyes in the three groups exhibited high IOP before the surgery. There was no significant difference in baseline IOP or post-modeling IOP among the groups. Three days after surgery, the IOP decreased into a normal range of 12.25 ± 7.97, 9.25 ± 4.80, and 9.00 ± 4.88 mmHg in the PBS-hydrogel, MMC-hydrogel, and MMC groups, respectively (Fig. 2B). This indicated that the surgeries were successful, and there was no significant variation in surgery outcomes among the groups (p = 0.916). At 2 and 4 weeks after surgery, the IOP increased slightly in the MMC-hydrogel and MMC groups, which were still significantly decreased compared to the pre-operation IOP (p = 0.039 and 0.021, respectively). However, in the PBS-hydrogel group, the IOP gradually went back to the pre-operation level, which showed no statistically significant difference to the pre-operation IOP (p = 0.114). Hence, MMC-hydrogel and traditional MMC pre-treatment both help maintain the blebs' filtering function and lower the IOP in 1 month. The trend of intraocular pressure changes after glaucoma surgery was displayed in Fig. 2C.
MMC-loaded hydrogel reduced scarring and maintained functional filtering blebs for a longer time
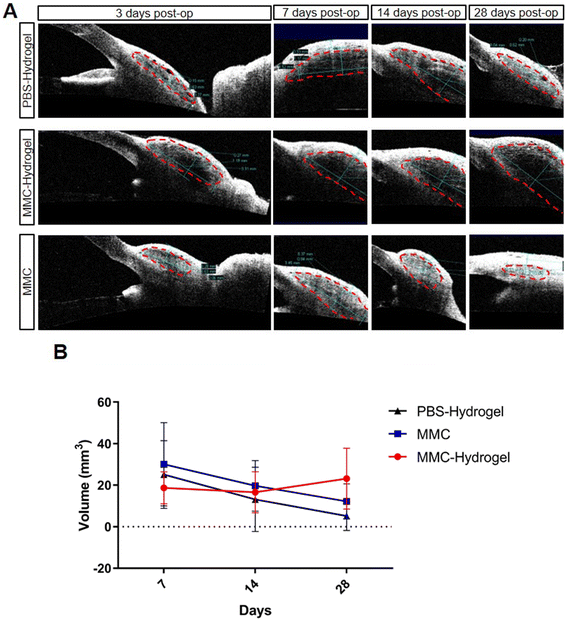
Scarring during the wound-healing process is the main reason for bleb failure. Many different grading systems and methods have been developed to evaluate bleb development and functional maintenance in the past few decades. Most of those grading systems are based on bleb morphology under slit-lamp evaluation and/or OCT scanning. Therefore, to evaluate the effect of the MMC-loaded hydrogel on preventing scarring and maintaining bleb function, we performed slit-lamp photography and AS-OCT measurements. Fig. 3A shows the bleb morphologies from slit-lamp photography at different time points after surgery. In both the MMC-hydrogel and MMC groups, blebs were well-preserved for up to 4 weeks, compared to the PBS-hydrogel group, which had almost no visible elevation on day 28. However, in the MMC group, the blebs were slightly smaller on day 28 compared to day 14, while in the MMC-hydrogel group, the blebs showed moderate bleb elevation during the whole follow-up period. On the other hand, vascularity is also one of the major risk factors leading to bleb failure in the first year after surgery.18 In the short term after surgery, both the PBS-hydrogel and MMC groups showed mild to moderate vascularity, which may be due to post-operational inflammation. Blebs in the MMC-hydrogel group demonstrated decreasing vascularity during the follow-up. We also applied the Indiana Bleb Appearance Grading Scale (IBAGS) to scale the blebs19 (Fig. 3B). The grading system scales bleb height (H, 0–3), extent (E, 0–3), and vascularity (V, 0–4) to quantitatively demonstrate the bleb morphology. Blebs were generally higher in the two hydrogel groups, probably because of the mechanical support. However, at the latest time point, the PBS-hydrogel blebs collapsed significantly owing to scarring, while the MMC-hydrogel blebs were maintained well. The MMC-hydrogel group still showed better horizontal bleb extension than the other two groups. The two groups with MMC demonstrated significantly less vascularity than the PBS-hydrogel group. Moreover, at the later time point, the MMC group showed a rebound of vascularization, while the MMC-hydrogel group was still avascular.Utilizing AS-OCT, we were able to assess the bleb volume. One week after surgery, blebs in the MMC-hydrogel group showed a smaller volume than in the other two groups (Fig. 4A and B). However, the MMC-hydrogel group showed relatively stable bleb volume in the following measurements. On the contrary, both the PBS-hydrogel and MMC groups demonstrated a reduction of the bleb volume. Meanwhile, the volume decreased much more dramatically in the PBS-hydrogel group compared to the two groups involving MMC. This result indicated that the bleb maintenance was mainly because of the antimetabolic effect from MMC, instead of the mechanical occupying effect from the injected hydrogel.
MMC-loaded hydrogel suppressed fibroblast proliferation and collagen deposition
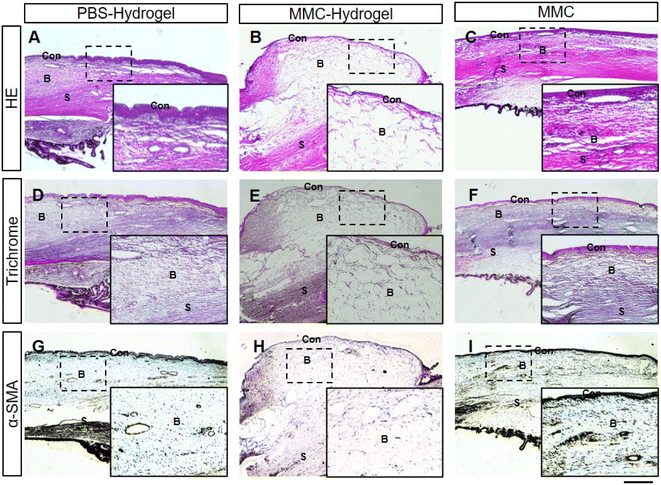
One month after the surgery, all eyes were enucleated. Histological examinations were performed, followed by HE and immunochemistry staining. HE staining showed consistent results with the AS-OCT in vivo evaluation. In the MMC-loaded hydrogel group, thin-walled blebs with large cavities were observed. Within the cavities, numerous microcystic structures were noticed, which suggested satisfactory filtering functions (Fig. 5B). However, blebs were flat and compact with thick walls in the PBS-hydrogel and MMC groups (Fig. 5A and C).Masson's trichrome staining was used to reveal the deposited collagen (blue). As shown in Fig. 5E, scant collagen deposition and loose connective tissue were seen in the subconjunctival space of the MMC-loaded hydrogel bleb. On the contrary, dense collagen deposition was noticed in the thick wall of blebs in the other two groups (Fig. 5D and F).
Furthermore, α-smooth muscle actin (α-SMA), a marker of fibroblast and myofibroblast (brown), was used to show the scarring of blebs. In immunohistochemistry, α-SMA were labeled by diaminobenzidine (DAB), which is brown. Compared to the other two groups, there were far fewer brown fibroblasts and myofibroblasts in MMC-hydrogel bleb (Fig. 5G–I).
MMC-loaded hydrogel was safe when applied for a longer duration
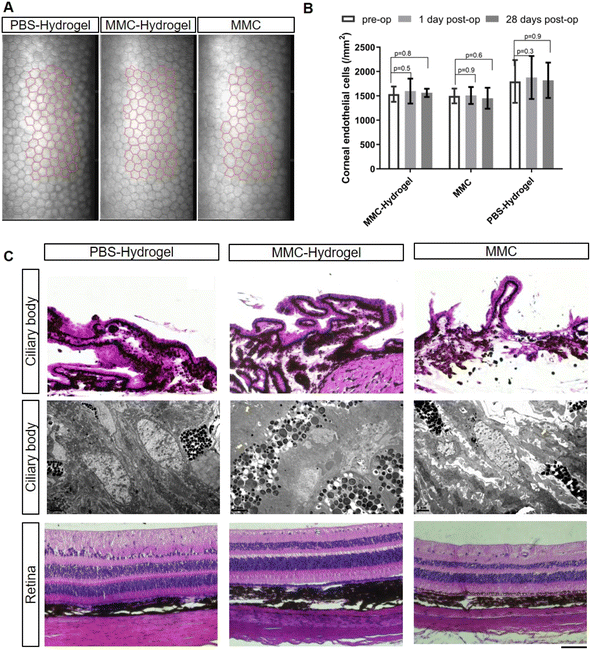
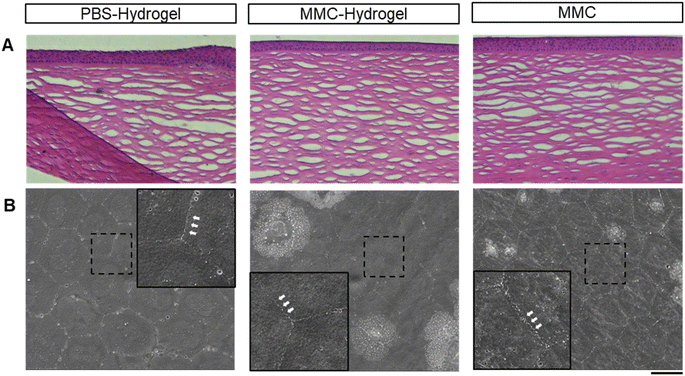
Next, the safety of MMC-loaded hydrogel application was addressed. In the traditional procedure, MMC pre-treatment was performed by applying an MMC-soaked sponge onto the scleral surgical site. This step is usually done prior to creating the incision in the anterior chamber, to avoid the side effects caused by MMC leaking into the anterior chamber. Then, the sponge is usually removed after 30 seconds to 5 minutes, since the longer the antimetabolite is applied, the higher the risk of wound-healing defects, which may lead to bleb leakage.In the present study, the MMC-loaded hydrogel was injected into the subconjunctival space after establishing a filtrating pathway. This might raise the concern of MMC diffusion into the anterior chamber and toxic to the inner eye structure. Therefore, the corneal endothelium, one of the most fragile structures within the globe, was examined to assess the safety of the MMC-loaded hydrogel. As shown in Fig. 6A and B, there were no significant morphological or quantitative defects in all three groups after surgeries. Meanwhile, except for the retinal nerve fibral layer thinning caused by the high IOP modeling, there were no significant histological abnormalities in the ciliary bodies and retinas in all three groups (Fig. 6C, upper and lower panels). We also utilized electron microscopy to further examine the ciliary body. The ultrastructure of the ciliary body showed no significant difference among the groups. Junctional complexes/interdigitations were well-preserved (as indicated by yellow arrows), and there were no signs of swelling, edema, degeneration, and necrosis (Fig. 6C, middle panel).
To address the concerns regarding toxic effects or wound-healing defects caused by prolonged drug exposure in the MMC-hydrogel group, we monitored bleb leakage by slit-lamp microscopy. No leak or hypotony was observed in any follow-up examinations. Moreover, the structure of the cornea did not show any architectural interference in histology (Fig. 7A). The corneal endothelial cells preserved their typically hexagonal morphologies with uniform size and interdigitate cell border (Fig. 7B).
Discussion
The present study focused on the efficacy and safety of MMC-loaded PTMC15–F127–PTMC15 hydrogel in maintaining filtering bleb function and preventing bleb scarring after glaucoma filtration surgery in high IOP monkey models. We demonstrated that MMC-loaded hydrogel had equivalent efficacy for postoperative IOP maintenance to the traditional MMC adjuvant treatment. Further, MMC-loaded hydrogel showed a better long-term effect at preserving bleb function and suppressing fibroblast proliferation and collagen deposition. Meanwhile, no obvious side effects were found in the present study. Thus, this thermosensitive, sustained-releasing antimetabolite is a promising solution for greater efficacy and safety for glaucoma filtration surgery.PTMC15–F127–PTMC15 hydrogel is a well-studied thermosensitive hydrogel that serves as one of the ocular drug delivery systems.20 Antimetabolites, including MMC, are critical drugs used in glaucoma filtration surgery to sustain well-functional filtering blebs and reduce postoperative bleb scarring. It had been reported that the frequency of encapsulated blebs without antimetabolites could be up to 28% in trabeculectomies,21 while with MMC adjuvant treatment, the frequency was low. However, MMC adjuvant treatment may not be able to provide sufficient long-term anti-scarring effect in pediatric glaucoma, and is associated with complications including bleb leakage, hypotony, and infective endophthalmitis.22 To address these issues, we developed an MMC-loaded PTMC15–F127–PTMC15 hydrogel. Our previous study demonstrated controlled, sustained release of MMC and reliable inhibition of fibroblast proliferation in vitro and in vivo (normal IOP rabbits).10
However, rabbits have different eye anatomy to humans, including much thinner and extendable sclera and smaller vitreous cavities. These may alter the reaction and adaptation to IOP changes. Meanwhile, it has been reported that the wound-healing response in the surgical sites of rabbits is very different from humans, and IOP could be low even when the bleb has collapsed.23 These findings raised our concerns. Moreover, it is well-known that inflammation is significantly more severe in glaucoma patients than in normal eyes.14 Moreover, higher pre-operative levels of inflammatory factors in the aqueous humor are associated with worse outcomes for glaucoma surgery.24 Thus, to further evaluate the efficacy and safety of MMC-loaded hydrogel more reliably, we adopted primate high IOP models in the present study. We had previously reported that the high IOP primate models closely mimic the anatomic and pathophysiological features of human glaucoma.25 Fortunately, in the present study, MMC-hydrogel could still reliably preserve filtering bleb function and lower the IOP.
Filtration bleb morphology has been mentioned in the literature. The aqueous humor flowing into the subconjunctival space leads to an elevation of the conjunctiva, referred to as a filtering bleb.15 It is well-known that a diffuse, moderate-elevation and pale bleb is favorable and more likely to keep functioning in the long term.16,17 The pale filtering bleb mentioned here refers to the absence of blood vessels. Non-functional filtering blebs after glaucoma filtration surgery often manifest as localized, flat, and vascularized. Non-functional filtering blebs after glaucoma surgery are mainly associated with the proliferation of fibroblasts in the surgical area and the obstruction of aqueous humor outflow due to the scarring of the filtration passage. In order to improve the success rate of surgery, some anti-scarring drugs have been used in experimental and clinical studies of glaucoma filtering surgery. Mitomycin C (MMC) has become the most common anti-scarring drug for assisted glaucoma filtration surgery. However, due to the nonspecific inhibition of the cell cycle, while suppressing the proliferation of fibroblasts in the surgical area, MMC also produces toxic and side effects on other normal cells in the surrounding tissues (such as conjunctival cells, goblet cells, and limbal stem cells), such as high sensitivity, filtration bleb leakage, and scleral lysis.26,27 In order to solve these problems and achieve efficient and safe improvement of the success rate of glaucoma filtering surgery, the experiment was conducted.
In the experiment, the MMC group served as a traditional positive control group. The eyes, including the sclera and conjunctiva, are exposed to high concentrations of MMC at one time. After that, the eye tissue will not be influenced by MMC, the proliferation of fibrous cells returns back to normal, and the function of filtering blebs will be reduced. In that case, the overlaying conjunctiva is likely to have a vascularized appearance or a rebound of vascularization. In the MMC-hydrogel group, MMC is embedded in the gel to achieve stable and continuous release of MMC after surgery. The low concentration of MMC continues to inhibit fibroblast proliferation. Because of the continuous release of MMC, the dispersion (width) and protrusion (height) of the filtering blebs in this group are bigger than those in the MMC group, and the state of no or little vascularization is prolonged, indicating good filtering function. The MMC-hydrogel group did not show lower IOP than the traditional MMC group, which indicated that no hypotony (over-low IOP) was induced by prolonged MMC exposure. There was a slight difference in the intraocular pressure measurements before modeling. While a significant difference in intraocular pressure measurements could be observed after modeling, the difference in IOP was small 3 days after surgery. However, over the postoperative period, the difference in IOP became significant. The PBS-hydrogel group, as the negative control group of this experiment, was supposed to gain no extra benefit from PBS and was expected to have naturally increasing IOP after surgery. The use of MMC is the best method for assisting glaucoma surgery at present. Therefore, the positive control group had relatively good IOP after surgery, especially in the early postoperative period. Despite the equivalent level of IOPs in the two MMC groups, bleb height was significantly better than that in the MMC group. Conjunctiva in primates is far more pigmented and vascularized than in humans; the avascular blebs indicated sufficient anti-scarring effect provided by MMC-loaded hydrogel. The later histological evaluation also demonstrated significantly less fibroblast/myofibroblast proliferation and collagen deposition in the MMC-hydrogel blebs.
Because previous studies had reported that MMC has good intraocular penetration and fast diffusion,28,29 the prolonged exposure in the present study may raise the concern of intraocular toxicity. Thus, we assessed several intraocular tissues, including the corneal endothelium, ciliary body, and retina. No obvious gross structural disruption was observed. A study reported that a high concentration of MMC would lead to significant ultrastructural changes in the ciliary body.30 Fortunately, junctional complexes and interdigitations were preserved well in both the cornea and ciliary body in our study. This is precisely what this study wants to confirm: the sustained low concentration release of MMC can interfere with postoperative fibroblast proliferation, thereby maintaining a good filter bleb morphology. It has also been confirmed that it will not have any side effects on the surrounding eye tissues. This MMC-hydrogel offers a new solution for filtering bleb management after glaucoma surgery.
Considering the long-term need for IOP control in glaucoma patients, one of the limitations of our study was the duration of follow-up. We only observed IOP and bleb maintenance for 28 days, which is the period that has the highest risk of postoperative bleb encapsulation.31 In future studies, longer observation periods are needed.
Conclusion
In conclusion, the thermosensitive sustained-release agent of PTMC15–F127–PTMC15 can be loaded with MMC. MMC-loaded PTMC15–F127–PTMC15 hydrogel led to improved functional maintenance and scarring inhibition, showing high efficacy in reducing postoperative filtering bleb fibrosis. This MMC-hydrogel offers a new solution for filtering bleb management after glaucoma surgery.Experimental section
Preparation and administration of MMC-loaded hydrogel
The following is a brief description of the preparation of this sustained-release formulation. The specific preparation method can be referenced in our previous publication.10 Powder of PTMC15–F127–PTMC15 was prepared and provided by Prof. Daping Quan's research group (Sun Yat-Sen University, School of Materials Science and Engineering).10,32 PTMC15–F127–PTMC15 was synthesized via the ring-opening polymerization of trimethylene carbonate (TMC) in tetrahydrofuran (THF) using F127 as the initiator and tetramethylethylenediamine (TMEDA) as the catalyst. The typical experimental procedure is described as follows: TMC (0.3788 g, 3.75 × 10−3 mol) was quickly added to stirred TMEDA (0.02 ml, 2.5 × 10−4 mol) and F127 (1.575 g, 1.25 × 10−4 mol) in THF (3 ml) in a glove box under an argon atmosphere. The reaction vessel was sealed and placed in a thermostated oil bath set at 55 °C. After 48 h of polymerization, the reaction was terminated using two drops of acetic acid. The rough product was isolated via precipitation in cold diethyl ether and dried in vacuo at room temperature to a constant weight. MMC (Roche Diagnostics GmbH, Molecular weight, ∼334.33 Da) was dissolved in sterile phosphate-buffered saline (PBS, pH 7.4) at a concentration of 1 mg ml−1 and prepared with PTMC15–F127–PTMC15 in a mass fraction of 5% under sterile operation. Then, 180 mg of F127 or 50 mg of PTMC15–F127–PTMC15 were dissolved in an aqueous solution of MMC to obtain 1 g sol. The MMC-loaded temperature-responsive PTMC15–F127–PTMC15 hydrogel was stored at 4 °C, and automatically formed a gel quickly at 37 °C. The in vitro release experiment showed that the drug could be released from PTMC15–F127–PTMC15 hydrogel for up to 16 days with only 57% of drug released in the first day. The dose of MMC used in this study was based upon the relative toxicity of MMC, and our previous experiment showed that MMC-loaded hydrogel of 0.1 mg ml−1 was sufficient to inhibit bleb fibrosis in the rabbit model.Animal and anesthesia
The experiment was conducted according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the guidelines developed by the Animal Care Committee at Zhongshan Center (Permit Number: 2020-140). Twelve adult rhesus monkeys (male, aged from 4 to 6 years old, weight between 4 and 8 kg), obtained from Zhongshan Ophthalmic Center, were used for studying the formation of glaucoma filtration blebs. The monkeys' life and health were monitored. The experimental procedures were performed under deep general anesthesia via an intramuscular injection of ketamine hydrochloride (5 mg kg−1, Ketalar 50®, GuTian Pharmaceuticals Ltd, Fujian, China) plus chlorpromazine hydrochloride (2.5 mg kg−1, chlorpromazine 50®, JiaoZuo Pharmaceuticals Ltd, Tianjin, China).Establishment of monkey chronic intraocular hypertension model
Twelve monkeys were successfully induced into monkey chronic high IOP model (picking one eye of each animal at random) by VISULAS Trion (VISULAS Trion; Carl Zeiss) using the slit-lamp delivery system and a laser gonioscope, as described previously.23,24 IOP, slit-lamp microscopy and indirect ophthalmoscopy were performed prior to laser photocoagulation to exclude any existing ocular disease. The laser treatment was carried out according to our previous publication.25 Color fundus photograph was done using a retinal camera (TRC-50DX RETINAL CAMERA; Topcon, Tokyo, Japan) with a Nikon 200 D digital camera. Cross-sectional imaging of the RNFL was scanned with the circular scan (3.4 mm diameter) procedure using the STRATUS OCT Instrument (Model 3000, Carl Zeiss Meditec, Germany). Successful chronic intraocular hypertension animal model establishment was confirmed by narrowed neuroretinal rims, enlarged optic cups, and damaged RNFL thicknesses.Surgical procedure for trabeculectomy
Trabeculectomy was performed on all monkeys (the 12 eyes from the 12 high IOP models) by an experienced surgeon. Monkeys were deeply anesthetized and the pupils were contracted with 1% pilocarpine before surgery. A cornea limbus-based conjunctival incision was designed 8–10 mm from the cornea limbus, and then a partial-thickness 3 × 4 mm rectangular scleral flap was made. A 2 × 2 mm sclerectomy and a peripheral iridectomy were performed after a paracentesis of anterior chamber. The scleral flap and Tenon's capsule were interrupted sutured and the conjunctival incision was closed in a watertight fashion. Thus, an opening leading to the anterior chamber and a filter pathway was formed. Efflux of aqueous humor into the subconjunctival space was produced. After surgery, the fornices were instilled with TobraDex ointment.Experimental animal grouping
Twelve monkeys were used in this experiment. Twelve eyes with high IOP from 12 modeling monkeys were equally divided into three groups, which were defined as PBS-hydrogel, MMC-hydrogel, and MMC groups (Fig. 1C). The PBS-hydrogel group received a subconjunctival injection with 0.1 ml of 5% w/v PTMC15–F127–PTMC15 after the conjunctival incision was closed; the MMC-hydrogel group received 0.1 ml of 5% w/v MMC-loaded PTMC15–F127–PTMC15 (0.1 mg ml−1) in the same way (Graphical Abstract); the MMC group received a 0.25 mg ml−1 MMC-soaked sponge covering (for 3 min) before the sclerectomy was done. The dose and administration of MMC and MMC-loaded hydrogel were decided according to clinical practice and our previous study.10Ocular examination
Filter bleb, ocular inflammation, anterior chamber depth, corneal edema, hemorrhage and bleb conditions were examined after the surgery (days 1, 3, 5, 7, 10, 14, 28) using slit-lamp microscopy. The morphological structure of the filtering blebs was observed on anesthetized animals using a slit-lamp microscope (Hagg-Streit BX900; Switzerland) with a Nikon 200D digital camera after the trabeculectomy (day 7, 14, 28). Blebs were graded by a blinded ophthalmologist, using The Indiana Bleb Appearance Grading Scale.Measurement of IOP
IOP was measured pre- and post-operation (day 1, 3, 5, 7, 10, 14, and 28) using a Tonopen® applanation tonometer (Reichert) according to the manufacturer's recommended procedures. The tonometer was calibrated before each measurement. The average value was automatically calculated after ten consecutive IOP readings were obtained by the tonometer. An experienced operator finished all IOP measurements.Anterior segment optical coherence tomography
Cross-sectional images of the filtering blebs were scanned with the line scan procedure using anterior segment optical coherence tomography (AS-OCT; Carl Zeiss Meditec AG) after the surgery (days 7, 14, and 28). The procedure with four lines scan model of cornea was applied to complete the AS-OCT measurement of the filtering bleb. The top of the filter bleb was located at the center of the scanning line. The bleb height, area, volume, and the thickness of bleb rampart were calculated.Corneal endothelium evaluation
The morphology and number of corneal endothelia were measured (SP-3000P; TOPCON) and compared pre- and post-operation. The operational approach was similar to the anterior segment photography measurement.Histology and immunochemistry
All eyes were enucleated after the animals were deeply anesthetized by intravenous injection of pentobarbital (50 mg kg−1) at the end of the experiment (day 28 after trabeculectomy). The filter blebs were well-preserved. The enucleated eyes were dissected clean of orbital tissue, rinsed with PBS and then placed in 10% formalin solution for 24 hours. The globes were then dehydrated and embedded in paraffin. Serial sections of 5 μm thickness were obtained. Hematoxylin and eosin (H&E) staining, Masson's trichrome staining, and α-smooth muscle actin (α-SMA) immunohistochemistry were performed as previously reported.10 These images were collected using a Zeiss Axioplan 2 imaging system (Axioplan 2 imaging; Zeiss, Thornwood, NY, USA).Electron microscopes
The cornea samples from the four groups were prepared with size of 1 mm3 and fixed in 2.5% glutaraldehyde for at least 2 hours. Subsequently, samples were prepared by ethanol gradient dehydration, critical-point drying, and gold coating. The corneal endothelial morphologies were assessed by scanning electron microscope (model XL-30E SEM; Philips, Eindhoven, The Netherlands).The ciliary body samples from the four groups were prepared with size of 1 mm3 and fixed in 2.5% glutaraldehyde overnight. Then samples were fixed in 1% osmium tetroxide and embedded in Epon epoxy resin, followed by uranyl acetate and lead citrate staining. The ultrastructure of the ciliary body was examined by transmission electron microscope (model CM10; Philips).
Statistical analysis
All data were expressed as means ± SD. Differences were considered statistically significant when p < 0.05. Data were analyzed by ANOVA and paired t-test, with a P-value of <0.05 considered statistically significant. Statistical analyses were performed using GraphPad Prism 6 (version 6.01).Author contributions
Conceptualization was by J. G. Methodology was by S. T. Investigation was conducted by R. Y., Z. L, B. X., F. Z. and D. H. Writing of the original draft was done by Z. L, S. T., R. Y. and D. H. Review and editing of the manuscript were done by J. G. Funding acquisition was done by J. G. and S. T., and supervision was done by J. G. and S. T.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Science and Technology Planning Projects of Guangdong Province (grant number: 2014B030301040, 2022A1515010914); National Natural Science Foundation of China (grant number: 81904000, 82271083); the Fundamental Research Funds for the Central Universities (grant number: 19ykpy156); and the Open Research Fund of the State Key Laboratory of Ophthalmology (grant number: 30306020240020314). We acknowledge Prof. Quan Daping for providing PTMC15–F127–PTMC15.References
- P. T. Khaw, L. Chang, T. T. Wong, A. Mead, J. T. Daniels and M. F. Cordeiro, Modulation of wound healing after glaucoma surgery, Curr. Opin. Ophthalmol., 2001, 12(2), 143–148 CrossRef CAS PubMed.
- G. Hollo, Wound Healing and Glaucoma Surgery: Modulating the Scarring Process with Conventional Antimetabolites and New Molecules, Dev. Ophthalmol., 2017, 59, 80–89 Search PubMed.
- H. Ye, Y. Qian, M. Lin, Y. Duan, X. Sun, Y. Zhuo and J. Ge, Cationic nano-copolymers mediated IKKbeta targeting siRNA to modulate wound healing in a monkey model of glaucoma filtration surgery, Mol. Vision, 2010, 16, 2502–2510 CAS.
- V. P. Costa, P. E. Comegno, J. P. Vasconcelos, R. F. Malta and N. K. Jose, Low-dose mitomycin C trabeculectomy in patients with advanced glaucoma, J. Glaucoma, 1996, 5(3), 193–199 CrossRef CAS PubMed.
- A. Reibaldi, M. G. Uva and A. Longo, Nine-year follow-up of trabeculectomy with or without low-dosage mitomycin-c in primary open-angle glaucoma, Br. J. Ophthalmol., 2008, 92(12), 1666–1670 CrossRef CAS PubMed.
- S. Yazdani, S. Rezai, M. Pakravan, M. Afrouzifar and E. Ghahari, Mitomycin-C Application Before versus After Scleral Flap Dissection in Trabeculectomy; a Randomized Clinical Trial, J. Ophthalmic Vision Res., 2015, 10(4), 391–399 CrossRef PubMed.
- M. Pakravan, H. Esfandiari, S. Yazdani, A. Douzandeh, N. Amouhashemi, M. Yaseri and P. Pakravan, Mitomycin C-augmented trabeculectomy: subtenon injection versus soaked sponges: a randomised clinical trial, Br. J. Ophthalmol., 2017, 101(9), 1275–1280 CrossRef PubMed.
- S. J. Lee, A. Paranhos and M. B. Shields, Does titration of mitomycin C as an adjunct to trabeculectomy significantly influence the intraocular pressure outcome?, Clin. Ophthalmol., 2009, 3, 81–87 CAS.
- K. Bell, B. de Padua Soares Bezerra, M. Mofokeng, G. Montesano, M. E. Nongpiur, M. V. Marti and M. Lawlor, Learning from the past: Mitomycin C use in trabeculectomy and its application in bleb-forming minimally invasive glaucoma surgery, Surv. Ophthalmol., 2021, 66(1), 109–123 CrossRef PubMed.
- L. Xi, T. Wang, F. Zhao, Q. Zheng, X. Li, J. Luo, J. Liu, D. Quan and J. Ge, Evaluation of an injectable thermosensitive hydrogel as drug delivery implant for ocular glaucoma surgery, PLoS One, 2014, 9(6), e100632 CrossRef PubMed.
- G. Dumortier, J. L. Grossiord, F. Agnely and J. C. Chaumeil, A review of poloxamer 407 pharmaceutical and pharmacological characteristics, Pharm. Res., 2006, 23(12), 2709–2728 CrossRef CAS PubMed.
- X. Y. Xiong, Q. H. Li, Y. P. Li, L. Guo, Z. L. Li and Y. C. Gong, Pluronic P85/poly(lactic acid) vesicles as novel carrier for oral insulin delivery, Colloids Surf., B, 2013, 111, 282–288 CrossRef CAS PubMed.
- S. Tang, J. J. Zhao, S. Y. Xu, J. F. Li, Y. Teng, D. P. Quan and X. D. Guo, Bone induction through controlled release of novel BMP-2-related peptide from PTMC11-F127-PTMC11 hydrogels, Biomed. Mater., 2012, 7, 1–9 Search PubMed.
- C. Baudouin, P. Hamard, H. Liang, C. Creuzot-Garcher, L. Bensoussan and F. Brignole, Conjunctival epithelial cell expression of interleukins and inflammatory markers in glaucoma patients treated over the long term, Ophthalmology, 2004, 111(12), 2186–2192 CrossRef PubMed.
- K. Jinza, S. Saika, K. Kin and Y. Ohnishi, Relationship between formation of a filtering bleb and an intrascleral aqueous drainage route after trabeculectomy: Evaluation using ultrasound biomicroscopy, Ophthalmic Res., 2000, 32(5), 240–243 CrossRef CAS PubMed.
- A. P. Wells, J. G. Crowston, J. Marks, J. F. Kirwan, G. Smith, J. C. Clarke, R. Shah, J. Vieira, C. Bunce, I. Murdoch and P. T. Khaw, A pilot study of a system for grading of drainage blebs after glaucoma surgery, J. Glaucoma, 2004, 13(6), 454–460 CrossRef CAS PubMed.
- G. Picht and F. Grehn, Classification of filtering blebs in trabeculectomy: biomicroscopy and functionality, Curr. Opin. Ophthalmol., 1998, 9(2), 2–8 CrossRef CAS PubMed.
- J. R. Marks, J. C. K. Clarke, T. Peto, D. Minassian and P. T. Khaw, Postoperative increased bleb vascularity persists for over one year and has implications for intraocular pressure control, Invest. Ophthalmol. Visual Sci., 2004, 45, U377 Search PubMed.
- L. B. Cantor, A. Mantravadi, D. WuDunn, K. Swamynathan and A. Cortes, Morphologic classification of filtering blebs after glaucoma filtration surgery: the Indiana Bleb Appearance Grading Scale, J. Glaucoma, 2003, 12(3), 266–271 CrossRef PubMed.
- Q. Wang, Z. Zuo, C. K. C. Cheung and S. S. Y. Leung, Updates on thermosensitive hydrogel for nasal, ocular and cutaneous delivery, Int. J. Pharm., 2019, 559, 86–101 CrossRef CAS PubMed.
- R. M. Feldman, R. L. Gross, G. L. Spaeth, W. C. Steinmann, R. Varma, L. J. Katz, R. P. Wilson, M. R. Moster and D. Spiegel, Risk-Factors for the Development of Tenons Capsule Cysts after Trabeculectomy, Ophthalmology, 1989, 96(3), 336–341 CrossRef CAS PubMed.
- A. Al-Hazmi, J. Zwaan, A. Awad, S. Al-Mesfer, P. B. Mullaney and D. T. Wheeler, Effectiveness and complications of mitomycin C use during pediatric glaucoma surgery, Ophthalmology, 1998, 105(10), 1915–1920 CrossRef CAS PubMed.
- M. H. Miller, I. Grierson, W. I. Unger and R. A. Hitchings, Wound healing in an animal model of glaucoma fistulizing surgery in the rabbit, Ophthalmic Surg., 1989, 20(5), 350–357 CAS.
- B. Cvenkel, A. N. Kopitar and A. Ihan, Inflammatory molecules in aqueous humour and on ocular surface and glaucoma surgery outcome, Mediators Inflammation, 2010, 2010, 939602 Search PubMed.
- S. Tu, K. Li, X. H. Ding, D. P. Hu, K. J. Li and J. Ge, Relationship between intraocular pressure and retinal nerve fibre thickness loss in a monkey model of chronic ocular hypertension, Eye, 2019, 33(12), 1833–1841 CrossRef PubMed.
- A. M. Palanca-Capistrano, et al., Long-term outcomes of intraoperative 5-fluorouracil versus intraoperative mitomycin C in primary trabeculectomy surgery, Ophthalmology, 2009, 116(2), 185–190 CrossRef PubMed.
- N. Anand, S. Arora and M. Clowes, Mitomycin C augmented glaucoma surgery: evolution of filtering bleb avascularity, transconjunctival oozing, and leaks, Br. J. Ophthalmol., 2006, 90(2), 175–180 CrossRef CAS PubMed.
- P. Chetoni, S. Burgalassi, D. Monti, M. Najarro and E. Boldrini, Liposome-encapsulated mitomycin C for the reduction of corneal healing rate and ocular toxicity, J. Drug Delivery Sci. Technol., 2007, 17(1), 43–48 CrossRef CAS.
- M. Georgopoulos, C. Vass, I. El Menyawi, S. Radda, W. Graninger and R. Menapace, In vitro diffusion of mitomycin-C into human sclera after episcleral application: Impact of diffusion time, Exp. Eye Res., 2000, 71(5), 453–457 CrossRef CAS PubMed.
- A. Cetinkaya, A. Akman, G. Take, B. Bilezikci and Y. A. Akova, Ciliary Body Toxicity of Subconjunctival Suramin Compared with Mitomycin-C in the Rabbit Eye: Determining the Toxic Concentration, Ophthalmic Res., 2009, 41(2), 91–97 CrossRef CAS PubMed.
- A. Azuara-Blanco and L. J. Katz, Dysfunctional filtering blebs, Surv. Ophthalmol., 1998, 43(2), 93–126 CrossRef CAS PubMed.
- M. Yan, W. Tao, Q. Na, Y. Huang and D. Quan, Controllable ring-opening polymerization of trimethylene carbonate catalyzed by aliphatic tertiary amines in the presence of benzyl alcohol or F127, Polym. Int., 2012, 10(61), 1525–1531 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |