Bi3+/Sm3+ co-doped LiTaO3 photochromic perovskites: an ultrafast erasable optical information storage medium†
Ruiting
Zhang
a,
Yahong
Jin
 *a,
Yanmei
Li
*b,
Haoyi
Wu
*a,
Yanmei
Li
*b,
Haoyi
Wu
 a and
Yihua
Hu
a and
Yihua
Hu
 *a
*a
aSchool of Physics and Optoelectronic Engineering, Guangdong University of Technology, WaiHuan Xi Road, No. 100, Guangzhou, Guangdong 510006, China. E-mail: yhjin@gdut.edu.cn; huyh@gdut.edu.cn
bSchool of Electronics and Electrical Engineering, Zhaoqing University, Yingbin Avenue, Duanzhou District, Zhaoqing, Guangdong 526061, China. E-mail: 2010020017@zqu.edu.cn
First published on 11th July 2023
Abstract
Inorganic photochromic materials hold great scientific and technological promise in the field of optical information storage due to their numerous advantages, including an extended color change duration, exceptional fatigue resistance, and robust thermal stability. However, their practical application has been hindered by relatively slow coloring and bleaching rates. In this study, we present a P-type photochromic perovskite material, LiTaO3:Bi3+/Sm3+, with an ultra-fast optical response. LiTaO3:Bi3+/Sm3+ exhibits a rapid coloring time of approximately 25 seconds and an ultra-fast bleaching time of approximately 1 second, achieved through alternating 254 nm and 365 nm light irradiation. Notably, the photobleaching rate of LiTaO3:Bi3+/Sm3+ is currently the fastest among all reported inorganic photochromic materials. The prepared LiTaO3:Bi3+/Sm3+ material exhibits a maximum color change contrast of 26.49% and excellent fatigue resistance. The codopant Bi3+ plays a critical role in creating traps within the host lattice, which greatly enhances photochromism contrast and enables efficient long afterglow luminescence. These dual properties make the material suitable for dual-mode optical information storage in both bright and dark fields. Finally, the excellent optical properties of LiTaO3:Bi3+/Sm3+ are demonstrated in its application for erasable optical information storage vividly.
1. Introduction
The storage of information is the basis for the transmission of information in the dimension of time, and it is also the basis for the further synthesis, processing, accumulation and regeneration of information, which has great significance in the development of human history. In this highly informationized society, the amount of information is exploding with the development of people's lives and society, which has been a huge test and put a huge demand on information storage technology. Optical storage technology has attracted a lot of research attention due to its advantages such as sustainability, high storage density, a long storage life, non-contact reading and writing and erasing.1–7 Under specific light irradiation conditions, photochromic materials can undergo specific chemical reactions that result in a change in the material's color or absorption peak of light. The initial state can be restored by heating or exposure to another wavelength of light.8,9 Defining the two states before and after photochromic coloration enables the storage of information data. Inorganic photochromic materials, which exhibit reversible color changes under specific light source irradiation, possess good fatigue resistance, thermal stability, and strong mechanical properties10–12 and are among the most promising media in the field of optical information storage applications. In recent years, many inorganic photochromic materials with excellent performance, such as Ba(Zr0.16Mg0.28Ta0.56)O3: Pr3+, Ba3MgSi2O8: Pr3+, PbWO4:Yb3+, Er3+, La4GeO8:Eu2+, Er3+, Na0.5Bi2.5Nb2O9:Er3+,Pr3+ and TiO2:Yb3+,Er3+, have been reported.13–18 These materials combine reversible fluorescence modulation, long-afterglow luminescence, up-conversion luminescence and other luminescence characteristics based on reversible photochromism, providing a rich combination of information storage technology and media selection.Photochromic materials can be divided into T-type and P-type, where the T-type needs to be heated back to its original state, while the P-type can only be restored to its original state only by irradiation with a different wavelength of light.19 Recently, inorganic photochromic materials bleached by thermal stimulation have been widely reported.14,20–23 Compared with photo-stimulation to induce bleaching, thermal stimulation has many limitations in practical applications. In practical information storage applications, materials often need to be processed into ceramics, films or other devices,14,23–26 and in these states, thermal stimulation bleaching may cause irreversible effects on the physical properties of the devices and the surrounding environment. P-type photochromic materials can be bleached by irradiation with light of a specific wavelength, which causes little damage to the physical properties of the materials and makes the data read-out more convenient. However, the photobleaching process of P-type photochromic materials, which has been reported so far, takes a long time (>20 s), and some of them cannot even completely restore their initial state by photo-stimulation,27–31 which greatly hinders their practical application potential. Therefore, to promote the practical application of memory storage media, new P-type photochromic materials with a fast photo-bleaching speed are necessary.
Some luminescent materials can absorb and store energy after being excited by a high-energy light source and then release the stored energy in the form of light after the removal of the excitation source to achieve long persistent luminescence for several seconds to several hours. This property is called persistent luminescence (PersL) or long afterglow.32,33 PersL materials have attracted much attention in the fields of night vision surveillance,34,35 anti-counterfeiting identification,36,37 and information storage38,39 due to their unique delayed luminescence characteristics. We envisage that if we can combine photochromic and delayed luminescence properties in a single material, it will be able to write and read data in both light and dark fields, significantly improving the applicability of information storage media. However, the photochromic process and the delayed luminescence process are two opposite reactions, where photochromism requires storing as many electrons as possible and delayed luminescence requires releasing the trapped electrons. How to introduce suitable defects into materials is a key factor. Combining the above issues and requirements, it will be very interesting and meaningful to design and develop a P-type inorganic photochromic material combining the fast light stimulation response and excellent PersL properties.
In this work, we report a new P-type inorganic photochromic material LiTaO3:Bi3+/Sm3+. The material can achieve rapid coloring in ∼25 s and ultrafast bleaching in ∼1 s with good fatigue resistance under irradiation with 254 nm and 365 nm light, respectively. It also releases PersL luminescence and exhibits a bright orange-red afterglow after the excitation source is removed. The optical properties, reaction process, and luminescence mechanism of the material were studied and analyzed by diffuse reflectance spectroscopy (DRS), photoluminescence (PL), thermoluminescence (TL), PersL, and X-ray photoelectron spectroscopy (XPS) characterization. A flexible composite film was also prepared to demonstrate the proof-of-concept applications in the field of optical data storage.
2. Experimental section
2.1 Preparation
LiTaO3 and LiTaO3:0.5 mol% Sm3+/x mol% Bi3+ (x = 0, 0.25, 0.5, 0.75, 1, 1.25, 1.5) were prepared by a traditional high-temperature solid phase method. Li2CO3 (Macklin, AR, 99.99%), Ta2O5 (Aladdin, AR, 99.99%), Sm2O3 (Aladdin, AR, 99.99%), and Bi2O3 (Aladdin, AR, 99.99%) were selected as raw materials and weighed according to the stoichiometric formula. The weighed powders were thoroughly mixed and ground in an agate mortar for one hour. Subsequently, the resulting mixtures were transferred to a tubular furnace and calcined in air at 1250 °C for six hours to obtain the powder samples.2.2 Flexible composite film preparation
The PDMS (SYLGRD184) and curing agent were mixed and stirred in a beaker at a ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to obtain a silica gel precursor. LiTaO3:Bi3+/Sm3+ powder was fully mixed with the silica gel precursor at a ratio of 1
1 to obtain a silica gel precursor. LiTaO3:Bi3+/Sm3+ powder was fully mixed with the silica gel precursor at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 and allowed to stand for 20 min to eliminate bubbles generated by stirring. A copper plate with a size of 100 × 100 mm2 was placed on a heating table preheated to 50 °C. The mixture was then slowly poured onto the template until it completely covered the copper plate. Finally, the temperature of the heating table was slowly raised to 100 °C and held for 10 min to obtain a composite flexible film.
15 and allowed to stand for 20 min to eliminate bubbles generated by stirring. A copper plate with a size of 100 × 100 mm2 was placed on a heating table preheated to 50 °C. The mixture was then slowly poured onto the template until it completely covered the copper plate. Finally, the temperature of the heating table was slowly raised to 100 °C and held for 10 min to obtain a composite flexible film.
2.3 Sample characterization
The phase structure of the material was characterized using a D8 ADVANCE X-ray diffractometer (Bruker, Germany) with Cu Kα irradiation (λ = 1.5406 Å). The X-ray diffraction (XRD) patterns were analyzed using the Rietveld refinement method. An electron paramagnetic resonance (EPR) spectrometer (EMXplus-10/12, Bruker, Germany) was used to obtain EPR spectra at a frequency of 9.18 GHz at room temperature in air. Field-emission scanning electron microscopy (FE-SEM, Hitachi-SU8220 Instruments) was used to examine the surface micromorphology and energy-dispersive spectroscopy (EDS) elemental mapping images. DRS was conducted using a UV-visible spectrophotometer (UV-3600 Plus, Shimadzu, China). Thermal stimulation bleaching was achieved by placing the sample-loaded carrier on a pre-set temperature heating stage for heating. The light source used in the photobleaching experiment is a 365 nm laser with adjustable power. The power density of the 254 nm light source used for sample coloring is 5.73 mW cm−2. The PL spectra, photoluminescence excitation (PLE) spectra, PersL spectra, and fluorescence decay curves were obtained using a fluorescence spectrophotometer (FLS980, Edinburgh Instruments). TL curves were characterized using a TL meter (SL08-L and Risø TL/OSL-DA-20) with a heating rate of 3 °C s−1. XPS was conducted on an X-ray photoelectron spectrometer (Escalab 250Xi, Thermo Fisher, USA) to analyze elemental valence states.3. Results and discussion
3.1 Phase identification and structural analysis
XRD patterns of LiTaO3 and LiTaO3:0.5 mol% Sm3+/x mol% Bi3+ (x = 0, 0.25, 0.5, 0.75, 1, 1.25, 1.5) are shown in Fig. 1(a). All diffraction peaks can be well indexed to the standard card PDF#29-0836, indicating the successful incorporation of Sm3+ and Bi3+ into the LiTaO3 host lattice. The (0 1 2) plane shifts towards a lower angle with the increase of Bi3+ doping concentration. Due to the low melting point of lithium, it is easy to volatilize and lose during high-temperature sintering, resulting in a large number of lithium vacancies in the crystal. To maintain electrical neutrality, a small amount of tantalum is introduced to occupy some of the lithium vacancies
in the crystal. To maintain electrical neutrality, a small amount of tantalum is introduced to occupy some of the lithium vacancies  , resulting in the formation of anti-site tantalum (Ta4+Li).40,41 In addition, oxygen vacancies may also be generated in the crystal to maintain charge balance. Fig. S1† shows the EPR spectra of the LiTaO3 host lattice before and after irradiation with 254 nm light. There is a very weak isotropic electron signal from oxygen vacancies
, resulting in the formation of anti-site tantalum (Ta4+Li).40,41 In addition, oxygen vacancies may also be generated in the crystal to maintain charge balance. Fig. S1† shows the EPR spectra of the LiTaO3 host lattice before and after irradiation with 254 nm light. There is a very weak isotropic electron signal from oxygen vacancies  at 3510 G (g = 2.003), indicating that the density of oxygen vacancies is very low. Under 0.5 mol% Sm3+ doping, considering the ionic radius (Sm3+ CN = 6, r = 0.96 Å, Li+ CN = 6, r = 0.76 Å, Ta5+ CN = 6, r = 0.64 Å) and the element valence state, Sm3+ ions will preferentially occupy the
at 3510 G (g = 2.003), indicating that the density of oxygen vacancies is very low. Under 0.5 mol% Sm3+ doping, considering the ionic radius (Sm3+ CN = 6, r = 0.96 Å, Li+ CN = 6, r = 0.76 Å, Ta5+ CN = 6, r = 0.64 Å) and the element valence state, Sm3+ ions will preferentially occupy the  . At this time, the impact on the cell volume is not significant, and the peak position hardly shifts. After introducing Bi3+ (CN = 6, r = 1.03 Å,) ion doping, Sm3+ and Bi3+ compete to replace lithium vacancies and even replace anti-site defects. As the co-doping concentration increases, Bi3+ begins to replace the conventional Li+ and Ta5+ sites, leading to lattice expansion and the diffraction peaks in XRD shifting towards lower angles.42 The title compounds have an octahedral structure and belong to the trigonal crystal system with a space group of R3c. As shown in Fig. 1(b), the Li+ and Ta5+ ions in the LiTaO3 crystal are regularly arranged in the interstices of oxygen octahedra, forming [LiO6] and [TaO6] as the basic repeating units after occupying the interstices. In the oxygen octahedron, both Li+ and Ta5+ ions are surrounded by six O2− ions. In order to further analyze the structural changes of the doped samples and obtain detailed crystal information, Rietveld refinement was performed on all samples. The refinement results of LiTaO3 and LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ are shown in Fig. S2† and Fig. 1(c). The refined parameters were compared with the information of the LiTaO3 standard crystal and are recorded in Table 1, while the cell parameters of the other samples are recorded in Table S1.† The refined parameters Rwp, Rp, and χ2 are all within a reliable range, indicating that the crystal structure of the material has not been damaged after ion doping. Compared to the host, the volume of LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ slightly increases, which is consistent with the discussed lattice expansion caused by the co-doping of Sm3+/Bi3+ ions. Fig. 1(d) shows the SEM and elemental mapping images of LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ powder, while Fig. S3† shows the SEM images of LiTaO3 and LiTaO3:0.5 mol% Sm3+. From the image, it can be seen that the LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ sample has a larger particle size and irregular agglomeration phenomenon, which can be attributed to the addition of Bi2O3 acting as a flux. The elemental mapping image also reflects the uniform distribution of doped ions Sm3+ and Bi3+ in the material.
. At this time, the impact on the cell volume is not significant, and the peak position hardly shifts. After introducing Bi3+ (CN = 6, r = 1.03 Å,) ion doping, Sm3+ and Bi3+ compete to replace lithium vacancies and even replace anti-site defects. As the co-doping concentration increases, Bi3+ begins to replace the conventional Li+ and Ta5+ sites, leading to lattice expansion and the diffraction peaks in XRD shifting towards lower angles.42 The title compounds have an octahedral structure and belong to the trigonal crystal system with a space group of R3c. As shown in Fig. 1(b), the Li+ and Ta5+ ions in the LiTaO3 crystal are regularly arranged in the interstices of oxygen octahedra, forming [LiO6] and [TaO6] as the basic repeating units after occupying the interstices. In the oxygen octahedron, both Li+ and Ta5+ ions are surrounded by six O2− ions. In order to further analyze the structural changes of the doped samples and obtain detailed crystal information, Rietveld refinement was performed on all samples. The refinement results of LiTaO3 and LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ are shown in Fig. S2† and Fig. 1(c). The refined parameters were compared with the information of the LiTaO3 standard crystal and are recorded in Table 1, while the cell parameters of the other samples are recorded in Table S1.† The refined parameters Rwp, Rp, and χ2 are all within a reliable range, indicating that the crystal structure of the material has not been damaged after ion doping. Compared to the host, the volume of LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ slightly increases, which is consistent with the discussed lattice expansion caused by the co-doping of Sm3+/Bi3+ ions. Fig. 1(d) shows the SEM and elemental mapping images of LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ powder, while Fig. S3† shows the SEM images of LiTaO3 and LiTaO3:0.5 mol% Sm3+. From the image, it can be seen that the LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ sample has a larger particle size and irregular agglomeration phenomenon, which can be attributed to the addition of Bi2O3 acting as a flux. The elemental mapping image also reflects the uniform distribution of doped ions Sm3+ and Bi3+ in the material.
| Parameter | LiTaO3 | LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ |
|---|---|---|
| Space group | R3c | R3c |
| Z | 6 | 6 |
| a (Å) | 5.159 | 5.161 |
| b (Å) | 5.159 | 5.161 |
| c (Å) | 13.763 | 13.766 |
| V (Å3) | 317.279 | 317.505 |
| R wp (%) | 9.08 | 9.49 |
| R p (%) | 7.16 | 7.19 |
| χ 2 | 2.032 | 2.07 |
3.2 Photochromism properties
The samples doped with Sm3+/Bi3+ exhibit good photochromic behavior after 254 nm light irradiation. For further investigation, the DRS of all samples before and after 254 nm light irradiation were recorded, as shown in Fig. 2(a)–(c) and Fig. S4(a)–(e).† To quantify the degree of color change, ΔR was defined, which can be calculated using the formula ΔR = (R0 − Rt) × 100%, where R0 and Rt are the reflectivity at 437 nm before and after UV irradiation, respectively. The non-doped and Sm3+ singly doped samples only show minor color changes with ΔR = 5.45% and 8.23%, respectively. However, the co-doping of Bi3+ significantly enhances the coloration degree to 26.49%. The photo of the sample in the illustration intuitively reflects the color change after UV irradiation. With the co-doping of Bi3+, the surface of the powder can be seen changing from white to yellowish brown after UV irradiation. Fig. 2(d) shows the difference in reflectance of all samples before and after discoloration. It can be seen that the Bi3+ co-doped samples notably improve the photochromic performance by enhancing the coloration degree. As the concentration of Bi3+ doping increases, it first increases and then decreases, reaching the maximum at x = 1.The reflectivity of the photochromic samples shows a strong dependence on UV irradiation time. From the illustration in Fig. 3(a), it can be observed that with the increase of 254 nm light irradiation time, the reflectivity rapidly decreases and stabilizes after ∼25 s. The illustration in the lower right corner shows the variation of ΔR of the sample with irradiation time at 437 nm, indicating that ΔR has remained basically unchanged after 25 s. The photos of the sample also visually demonstrate this characteristic. We have organized and recorded the coloring rates of inorganic photochromic materials reported in recent years in Table S2.† By comparing the data in the table, it can be seen that the material has a good photochromic response speed. Interestingly, LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ can recover to its initial color under 365 nm light irradiation, indicating that it is a P-type photochromic material. To investigate the impact of light power on bleaching, the DRS of the fully colored samples were obtained after irradiation with 365 nm light with varied powers for 1 s. As shown in Fig. 3(b), high-power light is more effective for bleaching and the colored sample almost completely returns to its initial state as the power approaches 272.6 mW cm−2. Subsequently, 365 nm light (272.6 mW cm−2) was used as the bleaching light source to measure its bleaching rate. As shown in Fig. 3(c), the reflectivity of the sample almost returns to its initial state within ∼1 s and completely recovers to its original position after 2 s, demonstrating an ultrafast bleaching speed. The bleaching speed of ∼1 s is the fastest so far compared with the previously reported results summarized in Table S3,† which greatly improves the application potential in repeatable erasable information storage. Besides, LiTaO3:0.5 mol% Sm3+/1 mol% can also be bleached through thermal stimulation. DRS was conducted at different heating temperatures for 5 s to observe the effect of temperature on the bleaching effect. As depicted in Fig. 3(d), a higher temperature leads to faster recovery of the reflectance and the DRS almost recovers to its initial level at 170 °C. Fig. 3(e) shows the reflectivity at 170 °C for different heating times, showing that the colored sample can be completely bleached after 7 s of heating. Fatigue resistance is one of the important properties of inorganic photochromic materials. Therefore, the reflectivity of LiTaO3:0.5 mol% Sm3+/1 mol% at 437 nm was tested for 7 cycles by alternative 254 nm and 365 nm light irradiation/heating treatment at 170 °C. As shown in Fig. 3(f) and (g), LiTaO3:0.5 mol% Sm3+/1 mol% exhibits excellent fatigue resistance. Above all, good reversibility, fast coloration rate, ultrafast photobleaching speed, and excellent fatigue resistance have been achieved in LiTaO3:0.5 mol% Sm3+/1 mol%.
3.3 PersL properties in LiTaO3:Sm3+/Bi3+
After 30 s of irradiation with 254 nm light, strong orange red PersL was observed in LiTaO3:Sm3+/Bi3+ powder. Therefore, the afterglow spectrum of LiTaO3:0.5 mol% Sm3+/x mol% Bi3+ (x = 0, 0.25, 0.5, 0.75, 1, 1.25, 1.5) samples was obtained, as shown in Fig. 4(a). As a reference, the excitation and emission spectra of LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ were obtained and are recorded in Fig. S5.† Under monitoring at 648 nm, an excitation peak composed of two sub-peaks was observed in the range of 220–340 nm, which were attributed to the charge transfer band of the host and the 1S0 → 3P1 transition of Bi3+.31,41 The sharp peaks in the range of 340–510 nm were attributed to the transitions between 6H5/2 → 6H13/2, 6H5/2 → 4D3/2, 6H5/2 → 6P7/2, 6H5/2 → 4F7/2 and 6H5/2 → 4I13/2. Under excitation at 409 nm, the orange red emission peak of Sm3+ is displayed in the range of 550–750 nm. The peaks at 566 nm, 605 nm, 648 nm, and 709 nm are attributed to the energy-level transitions of 4G5/2 → 6H5/2, 4G5/2 → 6H7/2, 4G5/2 → 6H9/2, and 4G5/2 → 6H11/2, respectively.43 The afterglow spectra in Fig. 4(a) match well with the emission spectrum of Sm3+, indicating the origin of orange red afterglow from Sm3+ ions. With the co-doping of Bi3+ ions, the PersL intensity is significantly enhanced, reaching its maximum at x = 1. The afterglow decay curves of the co-doped samples were also recorded. As shown in Fig. 4(b), it can be seen that the afterglow intensity decreases rapidly within the first 2.5 min and then gradually stabilizes. The intensity of the afterglow decay curves exhibits the same change trend as that of the afterglow spectrum, approaching the maximum at x = 1. The afterglow photos of the LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ sample after being excited by 254 nm light and placed in a dark field for different times are recorded in Fig. S6.† Even after 120 min, a faint red luminescence can be still observed.3.4 Thermoluminescence
In general, defects in inorganic materials are the main cause of photochromism and PersL. TL is an efficient tool and usually used for the study of traps, including the trap depth and distribution.44,45Fig. 5(a) shows the TL spectra of the host and LiTaO3:0.5 mol% Sm3+/x mol% Bi3+ (x = 0, 0.25, 0.5, 0.75, 1, 1.25, 1.5) after 30 s of exposure to 254 nm light. It was observed that the TL peak intensities of LiTaO3 and LiTaO3:0.5 mol% Sm3+ samples were very weak in the range of 50–150 °C. After the introduction of Bi3+ ion doping, the trap density of the co-doped sample significantly increased in the temperature range of 60–140 °C. As the co-doping concentration of Bi3+ ions increases, the TL peak intensity first increases and then decreases, reaching its maximum at x = 1. Under the excitation of ultraviolet light, the traps capture electrons, and after the light source is removed, the traps spontaneously release electrons and produce afterglow luminescence. The emission spectrum of the LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ sample during TL measurements was recorded, as shown in Fig. S7.† The observed emission spectrum in the figure matches well with the Sm3+ luminescence, indicating that Sm3+ serves as a composite luminescent center. The higher trap density is more favorable for afterglow luminescence. The PersL spectrum in Fig. 4(a) also confirms this point. In addition, a high trap density is more favorable for capturing electrons excited by 254 nm light. After being captured, the electrons form a composite center, namely a color center. The increase in the number of color centers and their absorption of specific light leads to an increase in the degree of color change in the material. Combining Fig. 5(a) and 2(d), it can be concluded that the increase in trap density level after co-doping significantly enhances the photochromic effect. Besides, in previous reports by Lyu et al., Bi3+ can introduce an electron capture center of 0.8 ± 0.5 eV depth in the LiTaO3 host.31,46 For the TL peak of 0.5 mol% Sm3+/x mol% Bi3+ samples, the corresponding trap depth was calculated using Chen's formula: , where Tm is the temperature of the TL peak.47 The calculated trap depths of LiTaO3:0.5 mol% Sm3+/x mol% Bi3+ (x = 0.25, 0.5, 0.75, 1, 1.25, 1.5) were 0.772 eV, 0.741 eV, 0.761 eV, 0.753 eV, 0.761 eV, and 0.749 eV, respectively. In the study by Maldiney et al., traps within this depth range were beneficial for PersL.48 This suggests that the trap depth in the co-doped samples provides strong support for their PersL. The TL spectra of LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ were characterized after being irradiated with 254 nm light for different times to understand the process of carrier capture, as shown in Fig. 5(b). As the irradiation time is prolonged, the TL intensity of the sample gradually increases and reaches stability at 30 s. During the charging process, the TL curves shift to higher temperature, indicating that electrons first fill shallow traps and then deep traps. However, the storage of charge carriers in traps is not stable. The TL curves were tested at different delay times after being irradiated with 254 nm light for 30 s, as shown in Fig. S8(a).† After 240 min, the shallow trap almost disappeared, while the deep trap still had weaker peaks, which was caused by the spontaneous escape of the captured carriers in the trap. Fig. S8(b)† shows the DRS and the corresponding surface photos of LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ at different delay times after being irradiated with 254 nm light for 30 s. With the increase of delay time, the reflectance of the samples gradually recovered to the initial value, showing a consistent rule with the TL spectrum. However, photochromism involves trapping electrons as color centers, while PersL involves electrons being released by traps and returning to the luminescent center, which is an opposite process. As the afterglow luminescence continues, the charge carriers are gradually released, resulting in a gradual decrease in photochromic contrast. Light or heat stimulation can accelerate the release process of carriers in defects. Therefore, the TL curves of the irradiated samples were characterized at different times under 365 nm light or 170 °C thermal stimulation and are recorded in Fig. 5(c) and (d). Under the irradiation with 365 nm light, the TL peak almost disappears after about 1 s, indicating that the 365 nm light strongly promotes the escape of trapped carriers. Similarly, under stimulation at 170 °C, the charge carriers in the trap were rapidly released and it was completely emptied within 7 s.
, where Tm is the temperature of the TL peak.47 The calculated trap depths of LiTaO3:0.5 mol% Sm3+/x mol% Bi3+ (x = 0.25, 0.5, 0.75, 1, 1.25, 1.5) were 0.772 eV, 0.741 eV, 0.761 eV, 0.753 eV, 0.761 eV, and 0.749 eV, respectively. In the study by Maldiney et al., traps within this depth range were beneficial for PersL.48 This suggests that the trap depth in the co-doped samples provides strong support for their PersL. The TL spectra of LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ were characterized after being irradiated with 254 nm light for different times to understand the process of carrier capture, as shown in Fig. 5(b). As the irradiation time is prolonged, the TL intensity of the sample gradually increases and reaches stability at 30 s. During the charging process, the TL curves shift to higher temperature, indicating that electrons first fill shallow traps and then deep traps. However, the storage of charge carriers in traps is not stable. The TL curves were tested at different delay times after being irradiated with 254 nm light for 30 s, as shown in Fig. S8(a).† After 240 min, the shallow trap almost disappeared, while the deep trap still had weaker peaks, which was caused by the spontaneous escape of the captured carriers in the trap. Fig. S8(b)† shows the DRS and the corresponding surface photos of LiTaO3:0.5 mol% Sm3+/1 mol% Bi3+ at different delay times after being irradiated with 254 nm light for 30 s. With the increase of delay time, the reflectance of the samples gradually recovered to the initial value, showing a consistent rule with the TL spectrum. However, photochromism involves trapping electrons as color centers, while PersL involves electrons being released by traps and returning to the luminescent center, which is an opposite process. As the afterglow luminescence continues, the charge carriers are gradually released, resulting in a gradual decrease in photochromic contrast. Light or heat stimulation can accelerate the release process of carriers in defects. Therefore, the TL curves of the irradiated samples were characterized at different times under 365 nm light or 170 °C thermal stimulation and are recorded in Fig. 5(c) and (d). Under the irradiation with 365 nm light, the TL peak almost disappears after about 1 s, indicating that the 365 nm light strongly promotes the escape of trapped carriers. Similarly, under stimulation at 170 °C, the charge carriers in the trap were rapidly released and it was completely emptied within 7 s.
3.5 The mechanism of photochromism and PersL
In order to discuss the photochromic and afterglow processes of LiTaO3:Sm3+/Bi3+, a schematic diagram for the illustration of the mechanism is proposed. As shown in Fig. 6, the melting point of lithium is around 180 °C, so it is prone to volatilization and loss during high-temperature sintering, which will generate a large number of in LiTaO3. When Sm3+ and Bi3+ are doped into the LiTaO3 crystal, they will compete to replace
in LiTaO3. When Sm3+ and Bi3+ are doped into the LiTaO3 crystal, they will compete to replace  . When the doping concentration increases to a certain extent, they will start to replace conventional Li+ and Ta5+, and these unequal substitutions will cause charge imbalance within the crystal, resulting in the corresponding
. When the doping concentration increases to a certain extent, they will start to replace conventional Li+ and Ta5+, and these unequal substitutions will cause charge imbalance within the crystal, resulting in the corresponding  for charge compensation. From the TL curve, it can be concluded that the co-doping of Bi3+ ions introduces a trap with a level of approximately 0.75–0.77 eV in the crystal. According to the DRS of LiTaO3, the band gap (Eg) was calculated to be 4.78 eV using the formula. The calculation process is recorded in Fig. S9† and explained in the ESI accordingly†. Based on the traps introduced by Bi3+ (Bi-traps), they are located near the conduction band (CB), which can function as electron capture traps. Upon excitation with 254 nm light, the charge carriers in the valence band (VB) absorb energy and transit to the CB, leaving behind hole carriers in the VB. Some of the hole carriers will be captured by
for charge compensation. From the TL curve, it can be concluded that the co-doping of Bi3+ ions introduces a trap with a level of approximately 0.75–0.77 eV in the crystal. According to the DRS of LiTaO3, the band gap (Eg) was calculated to be 4.78 eV using the formula. The calculation process is recorded in Fig. S9† and explained in the ESI accordingly†. Based on the traps introduced by Bi3+ (Bi-traps), they are located near the conduction band (CB), which can function as electron capture traps. Upon excitation with 254 nm light, the charge carriers in the valence band (VB) absorb energy and transit to the CB, leaving behind hole carriers in the VB. Some of the hole carriers will be captured by  near the VB and recombine into V color centers (process I). Process I generates only a small number of V-centers, which have a weak impact on the color change of the crystal. The electrons that enter the CB will move towards lower energy states in a non-radiative transition. During this process, electrons will be captured by
near the VB and recombine into V color centers (process I). Process I generates only a small number of V-centers, which have a weak impact on the color change of the crystal. The electrons that enter the CB will move towards lower energy states in a non-radiative transition. During this process, electrons will be captured by  defects and Bi-traps in the crystal to form F color centers, which are processes II and III, respectively. The trap density in this part dominates the material, resulting in a significant number of F-centers being formed. The selective absorption of visible light by color centers leads to a change in the color of the crystal, known as photochromism. The process of filling electrons in the photochromic process of Bi-trap is given in Fig. 5(b). To investigate the changes in
defects and Bi-traps in the crystal to form F color centers, which are processes II and III, respectively. The trap density in this part dominates the material, resulting in a significant number of F-centers being formed. The selective absorption of visible light by color centers leads to a change in the color of the crystal, known as photochromism. The process of filling electrons in the photochromic process of Bi-trap is given in Fig. 5(b). To investigate the changes in 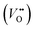 , the O1s signal of the sample before and after 254 nm light irradiation was characterized by XPS, as shown in Fig. S10.† The O1s signal is well fitted into three peaks, which correspond to lattice oxygen (530.1 eV),
, the O1s signal of the sample before and after 254 nm light irradiation was characterized by XPS, as shown in Fig. S10.† The O1s signal is well fitted into three peaks, which correspond to lattice oxygen (530.1 eV),  (531.7 eV), and absorbed oxygen (533.5 eV).49–52 Following irradiation, the proportion of the fitted area for
(531.7 eV), and absorbed oxygen (533.5 eV).49–52 Following irradiation, the proportion of the fitted area for  increased from 42.19% to 45.86%, indicating a rise in the
increased from 42.19% to 45.86%, indicating a rise in the  content within the crystal, which is conducive to photochromism. The emission of Sm3+ mainly comes from the transition between the energy of 4G5/2 → 6Hi/2 (i = 5, 7, 9, 11). According to the formula
content within the crystal, which is conducive to photochromism. The emission of Sm3+ mainly comes from the transition between the energy of 4G5/2 → 6Hi/2 (i = 5, 7, 9, 11). According to the formula 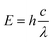 , the energy level bandwidth from 6H5/2 to 4G5/2 can be calculated to be approximately 2.2 eV, where h is the Planck constant, c is the speed of light, and λ is the wavelength of light. By comparison, it can be concluded that the above energy levels are located close to the VB in the bandgap, below the
, the energy level bandwidth from 6H5/2 to 4G5/2 can be calculated to be approximately 2.2 eV, where h is the Planck constant, c is the speed of light, and λ is the wavelength of light. By comparison, it can be concluded that the above energy levels are located close to the VB in the bandgap, below the  and Bi-trap positions. Under thermal agitation, the captured electrons will spontaneously escape from the trap. The escaped electrons will move to the 4G5/2 energy level of Sm3+ and generate luminescence through energy-level transitions (process IV), which is the mechanism of PersL generation. As process IV continues, the number of F-centers will decrease, causing the crystal color to slowly return to its initial state. In addition, 365 nm light irradiation/thermal stimulation can promote the release of trapped electrons in the F-centers (process V). The excited electrons will return to the CB and then move to the high energy levels of Sm3+ in the bandgap through non-radiative transitions. After relaxation to the 4G5/2 energy level, luminescence is generated through radiative transitions and eventually returns to the VB. The temperature range of trap distribution is wide, with the majority of traps concentrated in shallow levels, which facilitates the release of trapped electrons. Consequently, when stimulated by an appropriate external light source, the process of electron escape is accelerated, resulting in the rapid fading of the material. Simultaneously, the V-centers undergo decomposition and the holes within it return to the valence band (process VI). The schematic diagram in Fig. 6 visually displays the above discussion on photochromism and PersL.
and Bi-trap positions. Under thermal agitation, the captured electrons will spontaneously escape from the trap. The escaped electrons will move to the 4G5/2 energy level of Sm3+ and generate luminescence through energy-level transitions (process IV), which is the mechanism of PersL generation. As process IV continues, the number of F-centers will decrease, causing the crystal color to slowly return to its initial state. In addition, 365 nm light irradiation/thermal stimulation can promote the release of trapped electrons in the F-centers (process V). The excited electrons will return to the CB and then move to the high energy levels of Sm3+ in the bandgap through non-radiative transitions. After relaxation to the 4G5/2 energy level, luminescence is generated through radiative transitions and eventually returns to the VB. The temperature range of trap distribution is wide, with the majority of traps concentrated in shallow levels, which facilitates the release of trapped electrons. Consequently, when stimulated by an appropriate external light source, the process of electron escape is accelerated, resulting in the rapid fading of the material. Simultaneously, the V-centers undergo decomposition and the holes within it return to the valence band (process VI). The schematic diagram in Fig. 6 visually displays the above discussion on photochromism and PersL.
3.6 Rewritable optical information storage applications
Flexible composite films containing LiTaO3:Sm3+/Bi3+ phosphors were prepared to verify their potential for optical information storage. The preparation process for the thin films is illustrated in Fig. 7(a), with a detailed description provided in the Experimental section. Fig. 7(b) demonstrates the use of the flexible composite films for storing optical information. The process involves covering the hollow mask carrying the designed information on the film, followed by 30 s of 254 nm light irradiation. The mask is then removed to reveal the information generated by photochromism under a bright field. The pattern information emits bright orange-red light in a dark field owing to the PersL from LiTaO3:Sm3+/Bi3+. The recorded information in the thin film can be quickly erased by 365 nm illustration for 1 s. Videos were used to demonstrate the excellent light response speed of the LiTaO3:Sm3+/Bi3+ material in the process of writing and erasing of the designed letters, as presented in ESI Videos 1 and 2.† The recording and erasing of the actual text and pattern information is shown in Fig. 7(c). On a thin film with dimensions of 100 × 100 mm2, mask 1 was used to record the Chinese idiom “Emulate those better than oneself”. Due to the excellent PersL intensity and photochromic contrast of LiTaO3:Sm3+/Bi3+, the text on the composite film exhibits ideal resolution under both dark field and bright field conditions. With the help of masks 2–4, the writing and erasing of a series of patterns such as “lion dance paper cuttings”, “dog”, and “horse” were realized. The results demonstrate the application potential of the inorganic photochromic materials LiTaO3:Sm3+/Bi3+ as erasable optical information storage media.4. Conclusions
In summary, we prepared a novel P-type inorganic photochromic material by co-doping Bi3+/Sm3+ in LiTaO3 using a traditional high-temperature solid-state method. LiTaO3:Sm3+/Bi3+ achieved a coloring speed of ∼25 s and a bleaching speed of ∼1 s under the alternating action of 254 nm and 365 nm light, and the photobleaching speed is the fastest among the reported inorganic photochromic materials. After irradiation with 254 nm light, LiTaO3:Sm3+/Bi3+ demonstrated a maximum photochromic contrast of 26.49% and exhibited excellent fatigue resistance. In LiTaO3 crystals, Sm3+ acts as the luminescent center, while the co-doping of Bi3+ introduces new traps that give the material excellent delayed luminescence properties. The photochromism and afterglow luminescence were investigated by means of characterization methods such as DRS, TL, and XPS, and a mechanism diagram was constructed to provide a reasonable explanation for their processes. The combination of photochromic and afterglow properties makes it possible for materials to be applied to information storage and reading in both light and dark fields. A flexible film was prepared to validate this idea. It is hoped that this work will promote the development of inorganic photochromic materials for optical information storage by improving their photobleaching speed.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (No. 51972065 & 51802045) and the Guangzhou Basic and Applied Basic Research Project (202102020871). We also would like to thank the Analysis and Test Center of Guangdong University of Technology for the XRD, SEM and XPS analyses.References
- H. Wu, M. Wang, L. Huai, W. Wang, J. Zhang and Y. Wang, Optical storage and operation based on photostimulated luminescence, Nano Energy, 2021, 90, 106546 CrossRef CAS.
- X. Lin, K. Deng, H. Wu, B. Du, B. Viana, Y. Li and Y. Hua, Photon energy conversion and management in SrAl12O19: Mn2+, Gd3+ for rewritable optical information storage, Chem. Eng. J., 2021, 420, 129844 CrossRef CAS.
- Y. Xie, Y. Song, G. Sun, P. Hu, A. Bednarkiewicz and L. Sun, Lanthanide-doped heterostructured nanocomposites toward advanced optical anti-counterfeiting and information storage, Light: Sci. Appl., 2022, 11, 150 CrossRef CAS PubMed.
- Y. Wang, D. Chen, Y. Zhuang, W. Chen, H. Long, H. Chen and R. Xie, NaMgF3:Tb3+@NaMgF3 nanoparticles containing deep traps for optical information storage, Adv. Opt. Mater., 2021, 2100624 CrossRef CAS.
- T. Xu, S. Guo, W. Qi, S. Li, M. Xu and W. Wang, Organic transistor nonvolatile memory with three-level information storage and optical detection functions, ACS Appl. Mater. Interfaces, 2020, 12, 21952–21960 CrossRef CAS PubMed.
- D. Liu, L. Yuan, Y. Jin, H. Wu, Y. Lv, G. Xiong, G. Ju, L. Chen, S. Yang and Y. Hua, Tailoring multidimensional traps for rewritable multilevel optical data storage, ACS Appl. Mater. Interfaces, 2019, 11, 35023–35029 CrossRef CAS PubMed.
- H. Wang, Y. Lei, L. Wang, M. Sakakura, Y. Yu, C. Shayeganrad and P. Kazansky, 100-layer error-free 5D optical data storage by ultrafast laser nanostructuring in glass, Laser Photonics Rev., 2022, 16, 2100563 CrossRef CAS.
- R. Exelby and R. Grintetr, Phototropy (or Photochromism), Chem. Rev., 1965, 65, 247–260 CrossRef CAS.
- G. H. Brown, Photochromism, Tech. Chem., 1971, 853 Search PubMed.
- F. Yang, B. Jia, T. Wei, C. Zhao, Q. Zhou, Z. Li, M. Du, M. Wang, Y. Liu and C. Xie, Reversible regulation of upconversion luminescence in new photochromic ferroelectric materials: Bi4−xErxTi3O12 ceramics, Inorg. Chem. Front., 2019, 6, 2756 RSC.
- H. Sun, Y. Zhang, J. Liu, D. Peng, Q. Zhang and X. Hao, Reversible upconversion switching for Ho/Yb codoped (K,Na)NbO3 ceramics with excellent luminescence readout capability, J. Am. Ceram. Soc., 2018, 101, 5659–5674 CrossRef CAS.
- L. Yuan, Y. Jin, Y. Su, H. Wu, Y. Hu and S. Yang, Optically stimulated luminescence phosphors: Principles, applications, and prospects, Laser Photonics Rev., 2020, 2000123 CrossRef CAS.
- W. Tang, C. Zuo, C. Ma, Y. Wang, Y. Li, X. Yuan, E. Wang, Z. Wen and Y. Cao, Designing photochromic materials with high photochromic contrast and large luminescence modulation for hand-rewritable information displays and dual-mode optical storage, Chem. Eng. J., 2022, 435, 134670 CrossRef CAS.
- W. Tang, C. Zuo, C. Ma, C. Chang, F. Dang, H. Liu, Y. Li, X. Yuan, Z. Wen, L. Wu and Y. Cao, Rapid high-contrast reversible coloration of Ba3MgSi2O8:Pr3+ photochromic materials for rewritable light-printing, J. Mater. Chem. C, 2022, 10, 18375 RSC.
- X. Bai, Z. Yang, Y. Zhan, Z. Hu, Y. Ren, M. Li, Z. Xu, A. Ullah, I. Khan, J. Qiu, Z. Song, B. Liu and Y. Wang, Novel strategy for designing photochromic ceramic: Reversible upconversion luminescence modification and optical information storage application in the PbWO4:Yb3+, Er3+ photochromic ceramic, ACS Appl. Mater. Interfaces, 2022, 12, 21936–21943 CrossRef PubMed.
- P. Pei, R. Wei, B. Wang, J. Su, Z. Zhang and W. Liu, An advanced tunable multimodal luminescent La4GeO8: Eu2+, Er3+ phosphor for multicolor anticounterfeiting, Adv. Funct. Mater., 2021, 31, 2102479 CrossRef CAS.
- J. Gong, P. Du, W. Li, G. Yuan, X. Mao and L. Luo, The enhancement of photochromism and luminescence modulation properties of ferroelectric ceramics via chemical and physical strategies, Laser Photonics Rev., 2022, 16, 2200170 CrossRef CAS.
- A. A. Haider, Y. Cun, X. Bai, Y. Zi, J. Qiu, Z. Song, A. Huang and Z. Yang, Anti-counterfeiting applications by photochromism induced modulation of reversible upconversion luminescence in TiO2:Yb3+, Er3+ ceramic, J. Mater. Chem. C, 2022, 10, 6243–6251 RSC.
- A. Bianco, S. Perissinotto, M. Garbugli, G. Lanzani and C. Bertarelli, Control of optical properties through photochromism: a promising approach to photonics, Laser Photonics Rev., 2011, 5, 711–736 CrossRef CAS.
- Q. Du, J. Ueda, R. Zheng and S. Tanabe, Photochromism and long persistent luminescence in Pr3+-doped garnet transparent ceramic via UV or blue light up-conversion charging, Adv. Opt. Mater., 2023, 2202612 CrossRef CAS.
- Z. Yang, J. Hu, D. V. d. Heggen, M. Jiao, A. Feng, H. Vrielinck, P. F. Smet and D. Poelman, A versatile photochromic dosimeter enabling detection of X-ray, ultraviolet, and visible photons, Laser Photonics Rev., 2023, 2200809 CrossRef CAS.
- F. Yu, P. Wang, J. Lin, P. Zhou, Y. Ma, X. Wu, C. Lin, C. Zhao, M. Gao and Q. Zhang, (K0.5Na0.5)NbO3-based photochromic transparent ceramics for high-security dynamic anti-counterfeiting and optical storage applications, J. Lumin., 2022, 252, 119345 CrossRef CAS.
- Y. Mu, J. Yao, X. Wan, X. Mao and L. Luo, The integration of multi-luminescence in Sr2SnO4:xEr3+/Sm3+, J. Alloys Compd., 2022, 909, 164801 CrossRef CAS.
- X. Li, H. Lin, S. Lin, P. Li, P. Wang, J. Xu, Y. Cheng, Q. Zhang and Y. Wang, Rare-earth-ion doped Bi1.5ZnNb1.5O7 photochromics: A fast self-recoverable optical storage medium for dynamic anti-counterfeiting with high security, Laser Photonics Rev., 2023, 2200734 CrossRef CAS.
- Y. Zhou, A. Huang, S. Ji, H. Zhou, P. Jin and R. Li, Scalable preparation of photochromic composite foils with excellent reversibility for light printing, Chem. – Asian J., 2018, 13, 457–462 CrossRef CAS PubMed.
- Z. Hu, X. Huang, Z. Yang, J. Qiu, Z. Song, J. Zhang and G. Dong, Reversible 3D optical data storage and information encryption in photo-modulated transparent glass medium, Light: Sci. Appl., 2021, 10, 140 CrossRef CAS PubMed.
- Y. Wang, X. Yuan, Y. Cao and C. Ma, Multiple anti-counterfeiting strategy by integrating up-conversion, down-shifting luminescence, phosphorescence and photochromism into NaYTiO4 : Bi/Er phosphors, J. Mater. Sci. Technol., 2022, 130, 219–226 CrossRef.
- L. Sun, B. Wang, G. Xing, C. Liang, W. Ma and S. Yang, Bi-induced photochromism and photo-stimulated luminescence with fast photochromic response for multi-mode dynamic anti-counterfeiting and optical information storage, Chem. Eng. J., 2023, 455, 140752 CrossRef CAS.
- L. Shen, T. Wei, Y. Shi, X. Wang, L. Wu, J. Cui, T. Zhang, H. Yu and Z. Li, Light−heat and light−light dual-controlled upconversion luminescence and photochromic processes in Er-activated Bi7Ti4NbO21 for Optical Information Storage, ACS Appl. Elecrton. Mater., 2022, 4, 776–786 CrossRef CAS.
- Z. Yang, J. Du, L. I. D. J. Martin, A. Feng, E. Cosaert, B. Zhao, W. Liu, R. V. Deun, H. Vrielinck and D. Poelman, Designing photochromic materials with large luminescence modulation and strong photochromic efficiency for dual-mode rewritable optical storage, Adv. Opt. Mater., 2021, 9, 2100669 CrossRef CAS.
- T. Lyu, P. Dorenbos, C. Li and Z. Wei, Wide range X-ray to infrared photon detection and energy storage in LiTaO3:Bi3+,Dy3+ perovskite, Laser Photonics Rev., 2022, 16, 2200055 CrossRef CAS.
- L. Liang, J. Chen, K. Shao, X. Qin, Z. Pan and X. Liu, Controlling persistent luminescence in nanocrystalline phosphors, Nat. Mater., 2023, 22, 289 CrossRef CAS PubMed.
- S. Wu, Y. Li, W. Ding, L. Xu, Y. Ma and L. Zhang, Recent advances of persistent luminescence nanoparticles in bioapplications, Nano-Micro Lett., 2020, 12, 70 CrossRef CAS PubMed.
- J. Ueda, S. Miyano and S. Tanabe, Formation of deep electron traps by Yb3+ codoping leads to super-long persistent luminescence in Ce3+-doped yttrium aluminum gallium garnet phosphors, ACS Appl. Mater. Interfaces, 2018, 10, 20652–20660 CrossRef CAS PubMed.
- M. Li, Y. Jin, L. Yuan, B. Wang, H. Wu, Y. Hu and F. Wang, Near-infrared long afterglow in Fe3+-activated Mg2SnO4 for self-sustainable night vision, ACS Appl. Mater. Interfaces, 2023, 15, 13186–13194 CrossRef CAS PubMed.
- S. Tian, P. Feng, S. Ding, Y. Wang and Y. Wang, A color-tunable persistent luminescence material LiTaO3:Pr3+ for dynamic anti-counterfeiting, J. Alloys Compd., 2022, 899, 163325 CrossRef CAS.
- C. Wang, Y. Jin, J. Zhang, X. Li, H. Wu, R. Zhang, Q. Yao and Y. Hu, Linear charging-discharging of an ultralong UVA persistent phosphor for advanced optical data storage and wide-wavelength-range detector, Chem. Eng. J., 2023, 453, 139558 CrossRef CAS.
- Y. Zhuang, D. Chen, W. Chen, W. Zhang, X. Su, R. Deng, Z. An, H. Chen and R. Xie, X-ray-charged bright persistent luminescence in NaYF4:Ln3+@NaYF4 nanoparticles for multidimensional optical information storage, Light: Sci. Appl., 2021, 10, 132 CrossRef CAS PubMed.
- Z. Zhou, X. Wang, X. Yi, H. Ming, Z. Ma and M. Peng, Rechargeable and sunlight-activated Sr3Y2Ge3O12:Bi3+ UV–Visible-NIR persistent luminescence material for night-vision signage and optical information storage, Chem. Eng. J., 2021, 421, 127820 CrossRef CAS.
- L. Li, Y. Li and X. Zhao, Hybrid density functional theory insight into the stability and microscopic properties of Bi-doped LiNbO3: Lone electron pair effect, Phys. Rev. B, 2017, 96, 115118 CrossRef.
- R. Hu, Y. Zhao, Y. Zhang, X. Wang, G. Li and M. Deng, Opto-mechano-thermo-sensitive allochroic luminescence based on coupled dual activators in tantalate towards multidimensional stimulus sensing, Inorg. Chem. Front., 2023, 10, 1225 RSC.
- R. Hu, Y. Zhang, Y. Zhao, X. Wang, G. Li and M. Deng, Synergistic defect engineering and microstructure tuning in lithium tantalate for high-contrast mechanoluminescence of Bi3+: toward application for optical information display, Mater. Chem. Front., 2021, 5, 6891–6903 RSC.
- X. Geng, Y. Xie, S. Chen, J. Luo, S. Li, T. Wang, S. Zhao, H. Wang, B. Deng, R. Yu and W. Zhou, Enhanced local symmetry achieved zero-thermal-quenching luminescence characteristic in the Ca2InSbO6:Sm3+ phosphors for W-LEDs, Chem. Eng. J., 2021, 410, 128396 CrossRef CAS.
- Y. Jin, Y. Hu, Y. Fu, L. Chen, G. Ju and Z. Mu, Reversible colorless-cyan photochromism in Eu2+-doped Sr3YNa(PO4)3F powders, J. Mater. Chem. C, 2015, 3, 9435–9443 RSC.
- J. Du, K. Li, R. V. Deun, D. Poelman and H. Lin, Near-infrared persistent luminescence and trap reshuffling in Mn4+ doped Alkali-earth metal tungstates, Adv. Opt. Mater.Adv. Opt. Mater., 2021, 10, 2101714 CrossRef.
- T. Lyu and P. Dorenbos, Vacuum-referred binding energies of bismuth and lanthanide levels in LiTaO3 perovskite: Toward designing energy storage phosphor for anti-counterfeiting, X-ray imaging, and mechanoluminescence, Laser Photonics Rev., 2022, 16, 2200304 CrossRef CAS.
- R. Chen, On the calculation of activation energies and frequency factors from glow curves, J. Appl. Phys., 1969, 40, 570 CrossRef CAS.
- T. Maldiney, A. Lecointre, B. Viana, A. Bessiere, M. Bessodes;, D. Gourier, C. Richard and D. Scherman, Controlling electron trap depth to enhance optical properties of persistent luminescence nanoparticles for in vivo imaging, J. Am. Chem. Soc., 2011, 133, 11810–11815 CrossRef CAS PubMed.
- H. Yuan, J. Li, W. Yang, Z. Zhuang, Y. Zhao, L. He, L. Xu, X. Liao, R. Zhu and L. Mai, Oxygen vacancy-determined highly efficient oxygen reduction in NiCo2O4/hollow carbon spheres, ACS Appl. Mater. Interfaces, 2018, 10, 16410–16417 CrossRef CAS PubMed.
- Y. Song, H. Zhao, Y. Zi, J. Qiu, Z. Song, X. Bai, J. Liao and Z. Yang, X-ray-irradiation-induced discoloration and persistent radioluminescence for reversible dual-mode imaging and detection applications, ACS Energy Lett., 2023, 8, 2232–2240 CrossRef CAS.
- X. Lu, D. Wu, R. Li, Q. Li, S. Ye, Y. Xiang and G. Li, Hierarchical NiCo2O4 nanosheets@hollow microrod arrays for high-performance asymmetric supercapacitors, J. Mater. Chem. A, 2014, 2, 4706 RSC.
- G. Ou, Y. Xu, B. Wen, R. Lin, B. Ge, Y. Tang, Y. Liang, C. Yang, K. Huang, D. Zu, R. Yu, W. Chen, J. Li, H. Wu, L. Liu and Y. Li, Tuning defects in oxides at room temperature by lithium reduction, Nat. Commun., 2018, 9, 1302 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3qi00956d |
| This journal is © the Partner Organisations 2023 |







