A multifunctional molecular ferroelectric with a high Curie temperature and electrical–thermal double-switch coexistence: (C8H14NO)[FeCl4]†
Fang-Xin
Wang
,
Yun-Zhi
Tang
 *,
Yu-Hui
Tan
*,
Yu-Hui
Tan
 *,
Yi-Ran
Zhao
,
Jia-Ying
Wang
,
Yu-Kong
Li
,
Hao
Zhang
,
Ting-Ting
Ying
and
Ming-Yang
Wan
*,
Yi-Ran
Zhao
,
Jia-Ying
Wang
,
Yu-Kong
Li
,
Hao
Zhang
,
Ting-Ting
Ying
and
Ming-Yang
Wan
Faculty of Materials Metallurgy and Chemistry, Jiangxi University of Science and Technology, Ganzhou 341000, Jiangxi Province, P.R. China. E-mail: tangyunzhi75@163.com; tyxcn@163.com
First published on 13th July 2023
Abstract
Quasi-spherical molecules are used to construct high-performance molecular ferroelectrics, but accurately producing polar crystals with low symmetry remains a challenge. Herein, we report a novel high-temperature multiaxial molecular ferroelectric (C8H14NO)[FeCl4] (1) (C8H14NO = 1-methyl-3-quininocyclone). Dielectric and differential scanning calorimetry (DSC) measurements showed that compound 1 had a high Curie temperature of 341.8 K. It crystallizes in the Cmc21 space group at room temperature, showing switchable SHG response, moderate spontaneous polarization (Ps = 0.24 μC cm−2), and clear ferroelectric domains. In addition, DSC and dielectric cycling tests indicate that compound 1 can be used as an excellent electric double-switch material. Notably, the distinct symmetry breaking, that is, 4/mmmFmm2, leads to a biaxial ferroelectric with four equivalent directions of polarization. This discovery provides an effective approach for constructing multiaxial high-temperature molecular ferroelectrics.
Introduction
A ferroelectric material is a kind of crystalline material that has spontaneous polarization and reverses or reorients under an applied electric field.1,2 It has important applications in data storage, sensors, photoelectric equipment and so on. Inorganic ferroelectric ceramics have been dominant in research for a long time because of their outstanding piezoelectric properties.3,4 However, traditional ferroelectric ceramics have little mechanical flexibility and require high machining temperatures, and the increasing demand for flexible and thin-film integrated devices poses challenges to traditional ferroelectric ceramics.5,6 Molecular ferroelectrics have made remarkable progress in recent years, becoming a promising complement to traditional inorganic ferroelectrics due to their advantages of structural tunability, low impedance, environmental friendliness, mechanical flexibility and ease of preparation.7–9 Among them, organic–inorganic hybrid metal halide ferroelectrics have the advantages of both organic and inorganic parts. They have not only rich structural diversity, but also rich physical properties.10–16 At present, they receive wide attention in the exploration of multi-ferroelectrics, semiconductors, photovoltaics and other ferroelectric based multi-functional materials.17,18 The preparation of organic–inorganic hybrid metal halides using organic amines and metal halides is usually an effective strategy to obtain temperature-triggered phase change materials, the mechanism of which is usually the ordered and disordered motion of flexible organic cations.19–21 However, the phase transition temperatures of most molecular ferroelectrics reported are very low, mostly around or even below room temperature, such as those of (C6H5CH2CH2NH3)2[CdI4] (Tc = 301 K),22 (CH3)3NHBr (Tc = 286 K),23 (DMA)4[PbI6] (Tc = 252 K),24 and [(4-methoxyanilinium)(18-crown-6)][ReO4] (Tc = 153 K),25 and a small number of high temperature molecular ferroelectrics are affected by uniaxial properties, such as (3-pyrrolinium)[MnCl3] (Tc = 376 K),26 which seriously hinders their application potential. Thanks to the multiaxial nature their ferroelectric and piezoelectric properties can be optimized,27 and it is important to explore molecular ferroelectrics with high Curie temperature and multiaxial properties.Hybrid metal halides with spherical organic molecules, such as quinine, 1,4-diazocyclic [2.2.2] octane (dabco), and (Me4N)+, have low rotational energy barriers and can induce structural phase transitions. However, due to the high symmetry, these spherical molecules usually crystallize in the center symmetric space group. Xiong et al. proposed the “quasi-spherical theory”,28 that is, through modifying spherical molecules the symmetry of molecules can be reduced, in order to promote crystallization in the polar space group that is not highly symmetrical, which is beneficial for the ferroelectric behavior. Recently, quasi-spherical molecules have been used to construct high performance molecular ferroelectrics.29–31 For example, the molecular modification of (Me4N)+ cations, by substituting a methyl group with chloromethyl, iodomethyl, and hydroxyl groups, can successfully reduce the symmetry of the molecule from point group Td to C3v. When these modified organic cations were assembled with anions, molecular ferroelectrics (Me3NCH2Cl)[CdCl3],32 (Me3NCH2I)[PbI3],33 and (Me3NOH)[KFe(CN)6]34 were obtained successfully. Another example is the modification of dabco. A methyl group is added to an N atom to break the symmetry structure of dabco, thus reducing the molecular symmetry from D3h to C3v. (Mdabco)[RbI3]33 was obtained by assembling it with metal halides.
Based on the quasi-spherical theory, we used low symmetry 1-methyl-3-quininocyclone cations as polar components to prepare a new multifunctional organic–inorganic hybrid ferroelectric (C8H14NO)[FeCl4] (Scheme 1). It shows an obvious switchable nonlinear optical response, excellent ferroelectricity (Tc = 345 K and moderate spontaneous polarization Ps = 0.24 μC cm−2) and clear ferroelectric domains. Specifically, 1 is a multiaxial ferroelectric (Aizu notation: 4/mmmFmm2). Therefore, the discovery of compound 1 enriches the high-temperature multiaxial organic–inorganic hybrid ferroelectric library, opening up the way for multifunctional ferroelectric applications.
 | ||
| Scheme 1 (a) Synthesis flow chart of the organic cation [C8H14NO]+. (b) Schematic diagram of the synthesis of compound 1. | ||
Results and discussion
Differential scanning calorimetry (DSC) and dielectric properties
In order to determine the existence of reversible structural phase transitions, the thermal anomalies of compound 1 during heating/cooling were tested by DSC in the temperature range of 318–358 K (Fig. 1a). The endothermic and exothermic peaks of compound 1 were 341.8 K and 328.7 K, respectively. In addition, the wide reversible peak and the thermal hysteresis of 13 K in the DSC curve are consistent with the characteristics of first-order phase transition.35 We further calculated that the entropy changes (ΔS) of compound 1 during heating and cooling are about 37.92 J mol−1 K−1 and 37.39 J mol−1 K−1, respectively. According to the Boltzmann equation ΔS = nR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N (where N represents the possible orientation), the estimated N values of 95.679 and 89.747 are significantly greater than 1, and the ΔS and N obtained in both phases are roughly the same, suggesting that compound 1 undergoes a solid-state reversible phase transition. These high Curie temperatures, giant entropy changes and N values indicate that compound 1 has serious molecular disordered motion and structural deformation during the phase transition. In addition, we conducted DSC cycling tests on compound 1 using a scanning rate of 10 K min−1 (Fig. 1c). The test results indicate that even after 6 cycles of endothermic and exothermic cycles, the phase transition can still maintain its initial endothermic and exothermic effects. This indicates that compound 1 can be used as an excellent thermal switch material. The TG-DTA measurements (Fig. S2†) of compound 1 showed that the decomposition began at 573 K, which is much higher than the structural phase transition temperature (Tc = 341.8 K), indicating that compound 1 has good thermal stability.
N (where N represents the possible orientation), the estimated N values of 95.679 and 89.747 are significantly greater than 1, and the ΔS and N obtained in both phases are roughly the same, suggesting that compound 1 undergoes a solid-state reversible phase transition. These high Curie temperatures, giant entropy changes and N values indicate that compound 1 has serious molecular disordered motion and structural deformation during the phase transition. In addition, we conducted DSC cycling tests on compound 1 using a scanning rate of 10 K min−1 (Fig. 1c). The test results indicate that even after 6 cycles of endothermic and exothermic cycles, the phase transition can still maintain its initial endothermic and exothermic effects. This indicates that compound 1 can be used as an excellent thermal switch material. The TG-DTA measurements (Fig. S2†) of compound 1 showed that the decomposition began at 573 K, which is much higher than the structural phase transition temperature (Tc = 341.8 K), indicating that compound 1 has good thermal stability.
The phase transition behavior of crystals is usually accompanied by dielectric anomalies. The dielectric behavior can be expressed by measuring the temperature dependence of the real part of the complex dielectric constant ε’ (where ε = ε’ − iε′′ is the complex electric permittivity). In general, once a structural phase transition occurs, it results in an abnormal transition from a low dielectric state to a high dielectric state.36 In order to further confirm the structural phase transition of compound 1, the dielectric properties of compound 1 were measured by pressing it into a sheet. As expected, the dielectric anomaly of compound 1 shows a perfect step shape near the phase transition temperature during heating and cooling, with the real part of the dielectric constant increasing sharply at 336 K when heating and decreasing sharply at 331 K when cooling (Fig. 1b). These results are consistent with those of DSC measurements. As shown in Fig. 1d, the dielectric switch of compound 1 showed no significant dielectric loss after four consecutive cycles, demonstrating significant reversible dielectric switching and fatigue resistance. In the dielectric cycling test, the low and high dielectric constant states are similar to “off” and “on”, respectively.37 This indicates that compound 1 can be used as an excellent electric switch material.
Crystal structure analysis
In order to reveal the ferroelectric transformation mechanism of compound 1, the single crystal structure of compound 1 was measured at 300 K (RTP) (RTP = room temperature phase). Under RTP, compound 1 crystallizes in the non-centrosymmetric space group Cmc21 (orthorhombic system, point group mm2 with four symmetric elements (E, C2, and 2σv)) with cell parameters a = 9.1323(5) Å, b = 10.3481(6) Å, c = 14.8559(8) Å, α = β = γ = 90°, V = 1403.91(14) Å3, Z = 4 (Table S1†). As shown in Fig. 2a, the asymmetric unit of compound 1 at RTP consists of a protonated (C8H14NO)+ cation and a twisted [FeCl4]− anionic tetrahedron, consistent with charge conservation, and double disorder of organic cations can be seen. The bond lengths and angles are shown in Tables S2 and S3.† The distance range of the Fe–Cl bond is 2.1746(18) Å–2.1954(10) Å, and the angle range of Fe–Cl–Fe is 107.68(7)°–113.20(10)°. From the packing structure along the b-axis, as shown in Fig. 2c, the disordered (C8H14NO)+ organic cations and [FeCl4]− inorganic anion tetrahedra are arranged alternately, forming a zero-dimensional framework that provides more freedom for the movement of organic cations, resulting in ferroelectric phase transitions. It is worth noting that in the molecule, organic cations are distributed in the upper left or upper right direction. This arrangement results in a net polarization along the c-axis. (Fig. 2b).38Because the crystal quality of compound 1 is affected by high temperature, the diffraction data of a single crystal at high temperature are poor, and the complete crystal structure of compound 1 cannot be obtained. Fortunately, the reversible structural phase transition of compound 1 can be confirmed by thermostatic PXRD. At 298 K, according to the single crystal structure, the PXRD patterns were in good agreement with the simulated results (Fig. S3†), indicating a high purity of compound 1. As shown in Fig. 3a, the diffraction peak of compound 1 does not change significantly when heated from 298 K to 338 K, indicating that the structure remains relatively stable over a wide temperature range below Tc. With the increase of temperature, the number of diffraction peaks at 348–358 K decreases obviously, indicating that the crystal structure changes from low symmetry to high symmetry. When the temperature was cooled down to 298 K, these reduced peaks reappeared and returned to the original state, indicating that compound 1 had undergone a reversible structural phase transition, which was consistent with the results of DSC tests. At the same time, Le Bail refinement was performed on PXRD data at HTP (HTP = high temperature phase) to further understand the structural information at high temperature (Fig. 3b). The PXRD data after Le Bail refinement at 358 K provide cell parameters of the high temperature phase: a = 10.1902 Å, b = 10.1902 Å, c = 3.3976 Å, α = γ = β = 90°, V = 352.8 Å3. Based on the related physical properties, it is speculated that the high temperature phase of compound 1 may adopt the 4/mmm point group, of which the most likely space group is P4/mmm. More specifically, this symmetry breaking occurs in the Aizu notation 4/mmmFmm2.
 | ||
| Fig. 3 Variable-temperature PXRD patterns of 1 (a). Structural refinement results of PXRD data for compound 1 at 358 K (HTP) (b). | ||
SHG response and ferroelectric properties
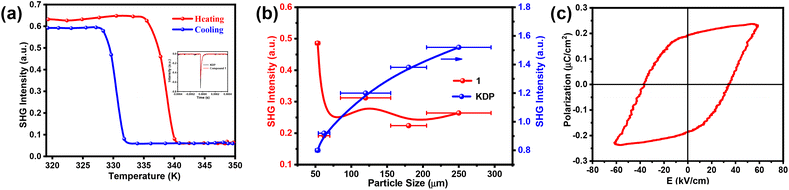
The second harmonic generation (SHG) method is the most direct method to detect the symmetry of crystals. The SHG signal is very sensitive to the transition from a non-centrosymmetric structure to a centrosymmetric structure, and the structural phase transition can be verified. Variable temperature SHG signal measurement is an effective method to detect the symmetry breaking of a crystal structure.39,40 Compound 1 crystallizes in the polar non-centrosymmetric space group Cmc21. Therefore, the variation of SHG signal intensity with temperature was measured. As shown in Fig. 4a, below Tc, a clear SHG signal can be observed, and its strength is stable around 0.60 (a.u.). When the temperature rises to about 336 K (HTP), the SHG signal strength drops sharply to close to 0 (a.u.). The strong SHG intensities below Tc and zero SHG intensities above Tc indicate non-centrosymmetric and centrosymmetric phases, respectively, corresponding to the phase transition from the non-centrosymmetric space group Cmc21 in the low temperature phase to the central space group P4/mmm in the high temperature phase. At the same time, the trend of temperature-dependent SHG signal intensity confirms the first-order phase transition in compound 1. Meanwhile, SHG intensity dependence on particle size was analyzed according to the Kurtz–Perry method; however, compound 1 did not display phase matching (Fig. 4b). This nonphase-matching behavior is well consistent with that reported by Kurtz and Perry and may be attributed to the value of L/![[r with combining circumflex]](https://www.rsc.org/images/entities/i_char_0072_0302.gif) (where L represents the powder-layer thickness and
(where L represents the powder-layer thickness and ![[r with combining circumflex]](https://www.rsc.org/images/entities/i_char_0072_0302.gif) represents the average particle size).41–43
represents the average particle size).41–43
The symmetry breaking diagram of compound 1 is shown in Fig. 5; compound 1 clearly undergoes a paraelectric-to-ferroelectric phase transition belonging to the 4/mmmFmm2 type according to the Aizu rule. The paraelectric point group 4/mmm and the ferroelectric point group mm2 have 16 and 4 symmetry elements, respectively, which reveals a multiaxial ferroelectric with 4 equivalent polarization directions, corresponding to 2 equivalent ferroelectric axes. Interestingly, most organic–inorganic hybrid ferroelectrics exhibit uniaxial ferroelectric properties. The multi-axis property of 1 will simplify the preparation of polycrystalline films.
 | ||
| Fig. 5 The equatorial plane projection (a) and spatial symmetry operation diagram (b) of 1-HTP and 1-RTP. | ||
The polarization electric (P–E) field hysteresis loop test provides direct evidence to prove whether the crystalline material has ferroelectric properties. In order to confirm the ferroelectric properties of compound 1, the polarization electric (P–E) field hysteresis loop was measured. A well-shaped P–E hysteresis loop was obtained using the Sawyer–Tower circuit method (Fig. 4c), and the measured saturation polarization value (Ps) was about 0.24 μC cm−2, which is much higher than that of molecular ferroelectrics [(CH3)3NCH2CHCH2][FeCl4] (0.18 μC cm−2),7 (formamidine)3[Bi2I9] (0.04 μC cm−2),44 and (tetramethyl-phosphonium)3[Sb2Cl9] (0.05 μC cm−2).45 The spontaneous polarization of compound 1 is smaller than that of [(CH3)4N][FeCl4] (3.3 μC cm−2),46 (C6H5CH2CH2NH3)2[CdI4] (0.36 μC cm−2),22 and (DMA)4[PbI6] (0.36 μC cm−2),24 but its Curie temperature (Tc = 341.8 K) is much higher than theirs. Our subsequent work will focus on exploring molecular ferroelectrics with high phase transition temperature, large spontaneous polarization, and high piezoelectric properties.
To further confirm the ferroelectric behavior of compound 1, its films were measured by piezoelectric response force (PFM) microscopy, which is the most powerful method for characterizing ferroelectric materials.47 Vertical and lateral PFM imaging can detect the out-of-plane and in-plane components of polarization, respectively. Fig. 6a and b, respectively, show the lateral amplitude and phase diagrams of compound 1, and Fig. 6c and d, respectively, show the corresponding vertical amplitude and phase diagrams at the same position. The lateral and vertical phase diagrams show distinct non-180° domains and are separated by domain walls, exhibiting a multi-axial property. At the same time, a typical butterfly loop (Fig. 6e) and a hysteresis loop (Fig. 6f) further confirm that compound 1 films have good ferroelectric switching properties.
 | ||
| Fig. 6 The lateral and vertical PFM amplitude (a and c) and phase (b and d) mapping of compound 1. An amplitude–voltage butterfly loop (e) and a phase–voltage hysteresis loop (f) of compound 1. | ||
The most essential characteristic of ferroelectrics is that they can realize spontaneous polarization under the action of an applied electric field. In order to verify the switchable spontaneous polarization of compound 1, PFM tip polarization experiments were carried out to clearly show the domain switchable process in compound 1 thin films, with a selected region of 4 μm × 4 μm.48 After +60 V tip bias is applied at the yellow point to polarize the film surface for 1 second, as shown in Fig. S5,† the polarization direction of the region marked with the yellow dashed ellipse is redirected (Fig. S5b†). Then, an opposite tip bias of −60 V is applied at the green dot for 1 second, and the direction of polarization of the region marked with the green dashed ellipse is redirected (Fig. S5c†). These PFM measurements strongly demonstrated the presence of stable and switchable polarization in compound 1 films. The switchable spontaneous polarization makes it a potential storage material.
Conclusions
In conclusion, we have successfully synthesized and characterized a multifunctional organic–inorganic hybrid metal halide ferroelectric (C8H14NO)[FeCl4]. By using single crystal structure measurement and variable temperature PXRD, it is revealed that the compound undergoes a reversible structural phase transition. Notably, it shows multiaxial ferroelectricity (Aizu notation: 4/mmmFmm2) and ferroelectric–paraelectric phase transition at 341.8 K. This discovery provides a new way to explore molecular ferroelectrics with multiaxial properties and high phase transition temperature, especially in electrical–thermal double switch and other applications.Author contributions
Yun-Zhi Tang, Yu-Hui Tan and Fang-Xin Wang conceived the experiment and co-wrote the manuscript. Fang-Xin Wang, Hao Zhang and Yu-Kong Li performed the experiments. All the authors discussed the results.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 21761013, 21671086, 21461010, and 21471070) and the Science and Technology Bureau project of Ganzhou.References
- W. Zhang and R. G. Xiong, Ferroelectric metal-organic frameworks, Chem. Rev., 2012, 112, 1163–1195 CrossRef CAS PubMed.
- J. Li, Y. Liu, Y. Zhang, H. L. Cai and R. G. Xiong, Molecular ferroelectrics: where electronics meet biology, Phys. Chem. Chem. Phys., 2013, 15, 20786–20796 RSC.
- J. C. Zhuang, W. J. Wei, N. Song, Y. Z. Tang, Y. H. Tan, D. C. Han and Y. K. Li, A Narrow Bandgap of 2D Ruddlesden-Popper Bilayer Perovskite with Giant Entropy Change and Photoluminescence, Chem. – Eur. J., 2021, 27, 15716–15721 CrossRef CAS PubMed.
- W. J. Xu, K. Romanyuk, J. M. G. Martinho, Y. Zeng, X. W. Zhang, A. Ushakov, V. Shur, W. X. Zhang, X. M. Chen, A. Kholkin and J. Rocha, Photoresponsive Organic-Inorganic Hybrid Ferroelectric Designed at the Molecular Level, J. Am. Chem. Soc., 2020, 142, 16990–16998 CrossRef CAS PubMed.
- H. Zhang, Y. H. Tan, Y. Z. Tang, X. W. Fan, X. L. Peng, R. R. Han, Y. K. Li and F. X. Wang, Two Manganese(II)-Based Hybrid Multifunctional Phase Transition Materials with Strong Photoluminescence, High Quantum Yield, and Switchable Dielectric Properties: (C6NH16)2MnBr4and (C7NH18)2MnBr4, Inorg. Chem., 2022, 61, 10454–10460 CrossRef CAS PubMed.
- T. T. Ying, H. J. Shi, S. P. Chen, Y. Z. Tang, Y. H. Tan, S. F. Wang, Z. Sun, F. X. Wang and M. Y. Wan, Large Spontaneous Polarization Ferroelectric Property, Switchable Second-Harmonic Generation Responses, and Magnetism in an Fe-Based Compound, Inorg. Chem., 2023, 62, 6189–6195 CrossRef CAS PubMed.
- T. Ying, Y. Tan, Y. Tang, X. Fan, F. Wang, M. Wan, J. Liao and Y. Huang, High-Tc Quadratic Nonlinear Optical and Dielectric Switchings in Fe-Based Plastic Crystalline Ferroelectric, Inorg. Chem., 2022, 61, 20608–20615 CrossRef CAS PubMed.
- Z. Yang, S. Jiao, Z. Tang, X. Sun, D. Li, P. Chen, Y. Lu, W. Zhang, H. Cai and X. Wu, Lead-Free Hybrid Organic-Inorganic Ferroelectric: (3,3-Difluoropyrrolidinium)2ZnCl4.H2O, Inorg. Chem., 2023, 62, 4181–4187 CrossRef CAS PubMed.
- X. L. Xu, L. B. Xiao, J. Zhao, B. K. Pan, J. Li, W. Q. Liao, R. G. Xiong and G. F. Zou, Molecular Ferroelectrics-Driven High-Performance Perovskite Solar Cells, Angew. Chem., Int. Ed., 2020, 59, 19974–19982 CrossRef CAS PubMed.
- Y.-K. Li, Y.-H. Tan, Y.-Z. Tang, X.-W. Fan, S.-F. Wang, T.-T. Ying and H. Zhang, Unusual high-temperature host–guest inclusion compound-based ferroelectrics with nonlinear optical switching and large spontaneous polarization behaviours, Inorg. Chem. Front., 2022, 9, 3702–3708 RSC.
- Y. K. Li, Y. Y. Lai, T. T. Ying, D. C. Han, Y. H. Tan, Y. Z. Tang, P. K. Du and H. Zhang, A multifunctional molecular ferroelectric with chiral features, a high Curie temperature, large spontaneous polarization and photoluminescence: (C9H14N)2CdBr4, Chem. Sci., 2021, 12, 13061–13067 RSC.
- Y. K. Li, Y. Liu, Y. Z. Tang, W. J. Wei, N. Song, P. K. Du and D. C. Han, Syntheses, Crystal Structures, SHG Response and Purple Luminescent Property of Tetra(isothiocyanate) Mn(II) and Substituted Benzyl Triphenylphosphonium Cations, Chin. J. Struct. Chem., 2021, 6, 739–745 Search PubMed.
- Y. Wang, C. Shi and X.-B. Han, Organic–inorganic hybrid [H2mdap][BiCl5] showing an above-room-temperature ferroelectric transition with combined order–disorder and displacive origins, Polyhedron, 2017, 133, 132–136 CrossRef CAS.
- C. Shi, L. Ye, Z. X. Gong, J. J. Ma, Q. W. Wang, J. Y. Jiang, M. M. Hua, C. F. Wang, H. Yu, Y. Zhang and H. Y. Ye, Two-Dimensional Organic-Inorganic Hybrid Rare-Earth Double Perovskite Ferroelectrics, J. Am. Chem. Soc., 2020, 142, 545–551 CrossRef CAS PubMed.
- P. Xu, H. Ye, Y. Yao, T. Zhu and J. Luo, Lead-free Double Perovskite Semiconductor with Rigid Spacer-Induced High-Tc Dielectric Switch Features, Chem. – Eur. J., 2023, 29, e202300667 CrossRef CAS PubMed.
- L. Hua, J. Wang, Y. Liu, W. Guo, Y. Ma, H. Xu, S. Han, J. Luo and Z. Sun, Improper High-Tc Perovskite Ferroelectric with Dielectric Bistability Enables Broadband Ultraviolet-to-Infrared Photopyroelectric Effects, Adv. Sci., 2023, 10, e2301064 CrossRef PubMed.
- L. Chen, W. Q. Liao, Y. Ai, J. Li, S. Deng, Y. Hou and Y. Y. Tang, Precise Molecular Design Toward Organic-Inorganic Zinc Chloride ABX(3) Ferroelectrics, J. Am. Chem. Soc., 2020, 142, 6236–6243 CrossRef CAS PubMed.
- H. Y. Zhang, X. J. Song, H. Cheng, Y. L. Zeng, Y. Zhang, P. F. Li, W. Q. Liao and R. G. Xiong, A Three-Dimensional Lead Halide Perovskite-Related Ferroelectric, J. Am. Chem. Soc., 2020, 142, 4604–4608 CrossRef CAS PubMed.
- Y.-P. Gong, X.-X. Chen, G.-Z. Huang, W.-X. Zhang and X.-M. Chen, Ferroelasticity, thermochromism, semi-conductivity, and ferromagnetism in a new layered perovskite: (4-fluorophenethylaminium)2[CuCl4], J. Mater. Chem. C, 2022, 10, 5482–5488 RSC.
- D. C. Han, Y. H. Tan, W. C. Wu, Y. K. Li, Y. Z. Tang, J. C. Zhuang, T. T. Ying and H. Zhang, High-Temperature Phase Transition Containing Switchable Dielectric Behavior, Long Fluorescence Lifetime, and Distinct Photoluminescence Changes in a 2D Hybrid CuBr4 Perovskite, Inorg. Chem., 2021, 60, 18918–18923 CrossRef CAS PubMed.
- H. Zhou, K. Yang, Y. Liu, Y. Tang, W. Wei, Q. Shu, J. Zhao and Y. Tan, In situ [2 + 3] cycloaddition synthesis, crystal structures, strong SHG responses and fluorescence properties of three novel Zn coordination polymers, Chin. Chem. Lett., 2020, 31, 841–845 CrossRef CAS.
- B. Huang, L. Y. Sun, S. S. Wang, J. Y. Zhang, C. M. Ji, J. H. Luo, W. X. Zhang and X. M. Chen, A near-room-temperature organic-inorganic hybrid ferroelectric: [C6H5CH2CH2NH3]2[CdI4], ChemComm, 2017, 53, 5764–5766 RSC.
- Z. Gao, Y. Wu, Z. Tang, X. Sun, Z. Yang, H.-L. Cai and X. S. Wu, Ferroelectricity of trimethylammonium bromide below room temperature, J. Mater. Chem. C, 2020, 8, 5868–5872 RSC.
- Y. Wang, Z. Tang, C. Liu, J. Jiang, W. Liu, B. Zhang, K. Gao, H.-L. Cai and X. Wu, Room temperature ferroelectricity and blue photoluminescence in zero dimensional organic lead iodine perovskites, J. Mater. Chem. C, 2021, 9, 223–227 RSC.
- D. W. Fu, H. L. Cai, S. H. Li, Q. Ye, L. Zhou, W. Zhang, Y. Zhang, F. Deng and R. G. Xiong, 4-Methoxyanilinium perrhenate 18-crown-6: a new ferroelectric with order originating in swinglike motion slowing down, Phys. Rev. Lett., 2013, 110, 257601 CrossRef PubMed.
- H. Y. Ye, Q. Zhou, X. Niu, W. Q. Liao, D. W. Fu, Y. Zhang, Y. M. You, J. Wang, Z. N. Chen and R. G. Xiong, High-Temperature Ferroelectricity and Photoluminescence in a Hybrid Organic-Inorganic Compound: (3-Pyrrolinium)MnCl3, J. Am. Chem. Soc., 2015, 137, 13148–13154 CrossRef CAS PubMed.
- C.-F. Wang, N. Wang, L. Liu, L.-p. Miao, H.-Y. Ye, Y. Zhang and C. Shi, Enantiomeric hybrid high-temperature multiaxial ferroelectrics with a narrow bandgap and high piezoelectricity, Chin. Chem. Lett., 2023, 34, e108051 CrossRef.
- H. Y. Zhang, Y. Y. Tang, P. P. Shi and R. G. Xiong, Toward the Targeted Design of Molecular Ferroelectrics: Modifying Molecular Symmetries and Homochirality, Acc. Chem. Res., 2019, 52, 1928–1938 CrossRef CAS PubMed.
- C. K. Yang, Y. T. Ding, J. Wang, Y. Rao, W. Q. Liao, Y. F. Xie, W. N. Zou and R. G. Xiong, Directional Intermolecular Interactions for Precise Molecular Design of a High-Tc Multiaxial Molecular Ferroelectric, J. Am. Chem. Soc., 2019, 141, 1781–1787 CrossRef CAS PubMed.
- Y. Zhang, W. Zhang, S. H. Li, Q. Ye, H. L. Cai, F. Deng, R. G. Xiong and S. D. Huang, Ferroelectricity induced by ordering of twisting motion in a molecular rotor, J. Am. Chem. Soc., 2012, 134, 11044–11049 CrossRef CAS PubMed.
- P. González-Izquierdo, O. Fabelo, L. Cañadillas-Delgado, G. Beobide, O. Vallcorba, J. Salgado-Beceiro, M. Sánchez-Andújar, C. Martin, J. Ruiz-Fuentes, J. E. García, M. T. Fernández-Díaz and I. de Pedro, ((R)-(-)-3-Hydroxyquinuclidium)[FeCl4]; a plastic hybrid compound with chirality, ferroelectricity and long range magnetic ordering, J. Mater. Chem. C, 2021, 9, 4453–4465 RSC.
- Y. M. You, H. Y. Ye, Y. Zhang, Q. H. Zhou, X. H. Niu, J. L. Wang, P. F. Li, D. W. Fu, Z. M. Wang, S. Gao, K. L. Yang, J. M. Liu, J. Y. Li, Y. F. Yan and R. G. Xiong, An organic-inorganic perovskite ferroelectric with large piezoelectric response, Science, 2017, 357, 306–309 CrossRef CAS PubMed.
- X. N. Hua, W. Q. Liao, Y. Y. Tang, P. F. Li, P. P. Shi, D. Zhao and R. G. Xiong, A Room-Temperature Hybrid Lead Iodide Perovskite Ferroelectric, J. Am. Chem. Soc., 2018, 140, 12296–12302 CrossRef CAS PubMed.
- W. J. Xu, P. F. Li, Y. Y. Tang, W. X. Zhang, R. G. Xiong and X. M. Chen, A Molecular Perovskite with Switchable Coordination Bonds for High-Temperature Multiaxial Ferroelectrics, J. Am. Chem. Soc., 2017, 139, 6369–6375 CrossRef CAS PubMed.
- J. C. Zhuang, Y. H. Zhang, N. Song, Y. H. Tan, Y. Z. Tang, Y. L. Huang, H. Zhang and Y. K. Li, Neutral 1D Perovskite-Type ABX3 Phase Transition Material with a Narrowband Emission and Semiconductor Property, Chem. – Asian J., 2022, 17, e202101134 CrossRef CAS PubMed.
- Y. Ai, P. F. Li, M. J. Yang, Y. Q. Xu, M. Z. Li and R. G. Xiong, An organic plastic ferroelectric with high Curie point, Chem. Sci., 2022, 13, 748–753 RSC.
- J. Li, C. Xu, W.-Y. Zhang, P.-P. Shi, Q. Ye and D.-W. Fu, Smart and efficient opto-electronic dual response material based on two-dimensional perovskite crystal/thin film, J. Mater. Chem. C, 2020, 8, 1953–1961 RSC.
- T.-T. Ying, Y.-Z. Tang, Y.-H. Tan, Y.-R. Zhao, J.-Y. Wang, M.-Y. Wan, F. X. Wang, X. W. Fan and Y. K. Li, Organic Single-Component Enantiomers with High Phase Transition Temperatures and Dielectric Switching Properties, Cryst. Growth Des., 2022, 22, 7501–7507 CrossRef CAS.
- Y. Zheng, J. Xu and X. H. Bu, 1D Chiral Lead Halide Perovskites with Superior Second–Order Optical Nonlinearity, Adv. Opt. Mater., 2022, 10, e2101545 CrossRef.
- B. Li, Y. Yu, M. Xin, J. Xu, T. Zhao, H. Kang, G. Xing, P. Zhao, T. Zhang and S. Jiang, Second-order nonlinear optical properties of copper-based hybrid organic-inorganic perovskites induced by chiral amines, Nanoscale, 2023, 15, 1595–1601 RSC.
- B. Zhao, Y. Yang, S. Zhao, Y. Shen, X. Li, L. Li, C. Ji, Z. Lin and J. Luo, A new phase-matchable nonlinear optical silicate: Rb2ZnSi3O8, J. Mater. Chem. C, 2017, 5, 11025–11029 RSC.
- S. K. Kurtz and T. T. Perry, A Powder Technique for the Evaluation of Nonlinear Optical Materials, J. Appl. Phys., 1968, 39, 3798–3813 CrossRef CAS.
- W.-J. Wei, H.-Q. Gao, Y. Yang, M. Fang, X.-D. Wang, X. Chen, W.-L. Qin, G.-Q. Chen, R.-X. Li, Y.-Z. Tang and Y. Wei, Zero-Dimensional Molecular Ferroelectrics with Significant Nonlinear Effect and Giant Entropy, Chem. Mater., 2022, 34, 6323–6330 CrossRef CAS.
- P. Szklarz, A. Gągor, R. Jakubas, P. Zieliński, A. Piecha-Bisiorek, J. Cichos, M. Karbowiak, G. Bator and A. Ciżman, Lead-free hybrid ferroelectric material based on formamidine: [NH2CHNH2]3Bi2I9, J. Mater. Chem. C, 2019, 7, 3003–3014 RSC.
- M. Wojciechowska, A. Gągor, A. Piecha-Bisiorek, R. Jakubas, A. Ciżman, J. K. Zaręba, M. Nyk, P. Zieliński, W. Medycki and A. Bil, Ferroelectricity and Ferroelasticity in Organic Inorganic Hybrid (Pyrrolidinium)3[Sb2Cl9], Chem. Mater., 2018, 30, 4597–4608 CrossRef CAS.
- J. Harada, N. Yoneyama, S. Yokokura, Y. Takahashi, A. Miura, N. Kitamura and T. Inabe, Ferroelectricity and Piezoelectricity in Free-Standing Polycrystalline Films of Plastic Crystals, J. Am. Chem. Soc., 2018, 140, 346–354 CrossRef CAS PubMed.
- X. G. Chen, Y. Y. Tang, H. P. Lv, X. J. Song, H. Peng, H. Yu, W. Q. Liao, Y. M. You and R. G. Xiong, Remarkable Enhancement of Piezoelectric Performance by Heavy Halogen Substitution in Hybrid Perovskite Ferroelectrics, J. Am. Chem. Soc., 2023, 145, 1936–1944 CrossRef CAS PubMed.
- Z. H. Wei, Z. T. Jiang, X. X. Zhang, M. L. Li, Y. Y. Tang, X. G. Chen, H. Cai and R. G. Xiong, Rational Design of Ceramic-Like Molecular Ferroelectric by Quasi-Spherical Theory, J. Am. Chem. Soc., 2020, 142, 1995–2000 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional data. CCDC 2243166. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qi00964e |
| This journal is © the Partner Organisations 2023 |