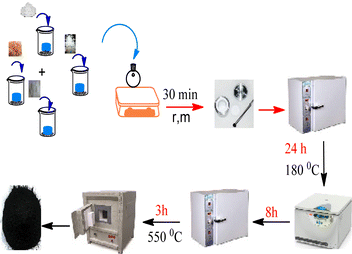
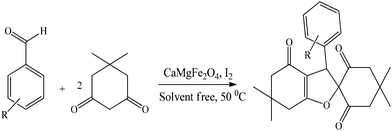
Design and preparation of hollow triple-shell CaMgFe2O4 nanospheres for green synthesis of spiro-dihydrofurans under solvent free conditions†
Somayeh
Kazempour
and
Hossein
Naeimi
 *
*
Department of Organic Chemistry, Faculty of Chemistry, University of Kashan, Kashan, 87317-51167, IR, Iran. E-mail: naeimi@kashanu.ac.ir; Fax: +98 3155912397; Tel: +98 3155912388
First published on 16th November 2022
Abstract
Magnetic hollow spheres have attracted broad interest in multidisciplinary research owing to their unique structure and excellent properties. Due to their large specific surface area, well-defined active sites, limited void space, and tunable mass transfer rate, magnetic hollow spheres can serve as excellent catalysts, supports, and reactors for various catalytic applications, including photocatalysis, heterogeneous catalysis, and electrocatalysis. In this study, hollow spheres with a multi-metallic core triple shell were prepared. The CaMgFe2O4 catalyst was characterized using FT-IR, VSM, EDX, TGA, FE-SEM, TEM and XRD techniques. The catalytic performance of the hollow CaMgFe2O4 nanospheres was evaluated for the one-pot synthesis of spiro-dihydrofurans through a condensation reaction. The results of this research suggest that hollow triple-shell CaMgFe2O4 nanospheres have potential as a reactive catalyst for this one-pot reaction.
1. Introduction
Ferrites are significant and exciting materials because of their good biocompatibility, low toxicity, catalytic properties and magnetic properties.1,2 Much attention has been paid to nano-sized magnetic ferrites due to their unrivaled properties. Nano-sized MgFe2O4, CaFe2O4 and CaMgFe2O4, possessing spinel structures, have attracted much attention due to their chemical and thermal stability and unrivaled structural, optical, magnetic, electrical and dielectric properties.3–5 The preparation methods of spinel ferrites include using a host template,6 co-precipitation7 and hydrothermal treatment.8 Hollow structures, as a class of organic/inorganic material, attract much attention in both fundamental and applied research.9 They exhibit notable features such as low density, large surface area, drug/gene storage, easy loading of functional groups, excellent permeability, and a broad range of potential applications in catalysis.10 The spaces created in these particles can be employed for a multitude of purposes, such as storing multiple products. Commonly, hollow constructions can be arranged in a variety of ways, and many other types of architecture, including spheres,11–13 fibers,14,15 and boxes,16–18 have been created. The structural characteristics of the hollow nanostructures involved include multishell,19,20 yolk–shell21 and porous materials.22–24 Researchers have recently demonstrated that elaborate hollow nano/microstructures show excellent results in a variety of emerging scientific fields including catalytic activity,25 fuel cells,26–29 proton-exchange-membrane fuel cells,30–32 super capacitors,33,34 chemical sensors35–38 and biomedicine. This is due to the simple micro structure and constrained nearby voids. Therefore, hollow nanomaterials are an excellent choice for the design of effective heterogeneous catalysts due to their unequaled morphological characteristics. Multiple studies on the catalysis of the reactions involved, such as chiral reactions39,40 and the indian film reaction,41–44 as well as catalytic mechanism, have been accomplished for hollow nanostructures due to their very regular structural features. More detailed hollow nanostructures are widely utilized in order to better meet the unique requirements of catalysis and power usage. The properties of hollow nanoparticles can be improved or even transformed by modifying their structural diversity.Spiro compounds are classified as bicyclic frameworks with at least two molecular entities (rings) attached via only one joint atom. The interesting conformational aspects and structural implications in a biological system containing this group of molecules attract attention for their importance in medicine discovery.45 If the spiro atom or any atom in either ring is not a carbon atom then the compound is considered a heterocyclic spiro compound, and one group of these is spiro-dihydrofurans.46 Spiro-dihydrofurans are frequently used as fundamental components in many kinds of natural products, emphasizing their biological significance and medicinal uses.47 Spiro-dihydrofurans have antibacterial action as well as sedative and antihypertensive properties.48,49 In reactions using basic and acidic multi-metallic catalysts, the catalysts have some limitations, including low product yields, extended reaction times, difficult reaction conditions, high catalyst loading, lack of catalyst stability, and difficulty in separating the reaction mixture.
In this paper, we present the preparation of a hollow spherical triple-shell catalyst and its application for the synthesis of spiro-dihydrofurans in the presence of iodine under solvent free conditions. This protocol indicated some advantages such as no solvent, simplicity of the reaction, easy work up, low reaction times, high efficiency and low catalyst loading.
2. Experimental
2.1. General information
All chemicals and reagents, calcium nitrate tetrahydrate (99%), magnesium nitrate hexahydrate (99%), iodine, glucose, dimedone and the respective benzaldehyde derivatives, were purchased from Merck Chemical Company. 1H NMR and 13C NMR spectra were recorded with a 400 MHz NMR spectrometer for solution NMR analysis. The spectrometer was equipped with a 5 mm probe. IR spectra were obtained with a Shimadzu instrument. By using a BANDELIN SONOPULS HD 4200 Ultrasonic homogenizer, the reaction mixture was homogenized. Electrothermal programmable digital melting point apparatus were used to measure the melting points of the products. BGMN/Profex/AutoQuan software reported the results of the X-ray diffraction study. A MIRA3 model with a voltage of 15 kV was used to conduct field emission scanning electron microscopy (FE-SEM) of the nanoparticles. Transmission electron microscopy (TEM) was carried out using a TEM PHILIPS EM 208S device. The magnetic properties of the nanoparticles were measured with a vibrating sample magnetometer (VSM, PPMS-9T) at 300 K in Iran (University of Kashan, Iran).2.2. General procedure for preparation of CaMgFe2O4
To glucose (4.95 g) in 25 mL of distilled water, Fe(NO3)3·9H2O (0.8 g), Mg(NO3)2·6H2O (0.11 g), and Ca(NO3)2·4H2O (0.1 g) were added. After stirring the reaction mixture for about 30 minutes, it was put into a stainless steel autoclave for 24 hours at 180 °C. The autoclave was slowly allowed to cool to room temperature. The precipitate was filtered, and then dried at 70 °C after washing with water and ethanol. After calcination for three hours at 550 °C, the hollow triple-shell CaMgFe2O4 spheres formed as a black solid.2.3. General procedure for synthesis of the spiro-dihydrofurans
CaMgFe2O4 (1 mg), benzaldehyde (1.0 mmol) and dimedone (2.0 mmol) were mixed in a round-bottomed flask. Then, the flask was sealed and the contents stirred magnetically for 1 min. To enable the synthesis of the desired product and to avoid the creation of side products, a small amount of iodine solid (1 mmol) was added into the obtained mixture and stirred at 50 °C. Thin-layer chromatography (TLC) was used to keep track of the reaction progress. After completion of the reaction, EtOH (5 mL) was added, and the catalyst was separated by an external magnet. The crude products were obtained, and recrystallized in ethanol to give the pure products.4′,4′,6,6-Tetramethyl-3-phenyl-3,5,6,7-tetrahydrospiro[benzofuran-2(4H),1′-cyclohexane]-2′,4,6′-trione (1a); white solid, 0.34 g, 98%; m.p.: 222–224 °C (lit. m.p. 222–224 °C);50 IR (KBr) υ = 3502, 3429, 3215, 2191, 1587, 1407 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 0.67 (s, 3H), 1.06 (s, 6H), 1.07 (s, 3H), 1.08 (s, 3H), 2.04–2.15 (m, 4H), 2.41 (d, 1H, J = 8.0 Hz), 2.57 (d, 1H, J = 8.0 Hz), 2.69 (d, H, J = 8.0 Hz), 3.63 (d, H, J = 8.0 Hz), 4.76 (s, H), 7.23–7.31 (m, 5H) ppm.
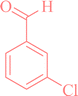
3-(3-Chlorophenyl)-4′,4′,6,6-tetramethyl-6,7-dihydro-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,4,6′(5H)-trione (2a); wormy solid, 0.38 g, 93%; m.p.: 224–227 °C (lit. m.p. 225–226 °C);50 IR (KBr) υ = 3440, 3330, 3021, 2192, 1530, 1350, 741 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 0.85 (s, 3H), 1.16 (s, 9H), 1.59 (s, 1H), 1.99 (d, H, J = 8.0 Hz), 2.15–2.25 (m, 3H), 2.56 (d, H, J = 8.0 Hz), 2.67 (s, H), 2.67 (s, H), 3.06 (d, H, J = 8.0 Hz), 4.38 (s, H), 7.04 (s, H), 7.16 (s, H), 7.26 (s, 2H) ppm.
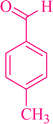
3-(p-Tolyl)-4′,4′,6,6-tetramethyl-6,7-dihydro-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,4,6′(5H)-trione (3a); white solid, 0.34 g, 93%; m.p.: 253–256–198 °C (lit. m.p. 254–256 °C);51 IR (KBr) υ = 3440, 3340, 3020, 2923, 2190, 1642, 518 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 0.85 (s, 3H), 1.14 (s, 3H), 1.17 (s, 6H), 1.62 (s, 3H), 2.01 (d, H, J = 8.0 Hz), 2.18 (t, 3H, J = 16.0 Hz), 2.31 (s, H), 2.55 (d, 3H, J = 8.0 Hz), 2.67 (s, H), 3.09 (d, H, J = 8.0 Hz), 4.44 (s, H), 7.05 (d, 2H, J = 4.0 Hz), 7.12 (d, 2H, J = 4.0 Hz) ppm.
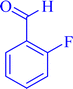
3-(2-Fluoro)-4′,4′,6,6-tetramethyl-6,7-dihydro-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,4,6′(5H)-trione (4a); yellow solid, 0.36 g, 98%; m.p.: 185–189 °C; IR (KBr) υ = 3385, 3041, 2922, 1682, 1591, 1495, 1266, 747 cm−1; 1H NMR (DMSO-d6, 400 MHz): δ = 0.85 (s, 3H), 1.18 (s, 6H), 1.20 (s, 3H), 1.58 (s, H), 2.13–2.29 (m, 4H), 2.52–2.28 (m, H), 2.69 (s, H), 3.19–3.25 (m, H), 4.95 (s, H), 6.95 (m, 2H), 7.12 (m, 2H) ppm; 13C NMR (CDCl3, 100 MHz): δ (ppm): 26.40, 28.50, 28.88, 30.28, 30.61, 34.33, 37.28, 49.79, 51.04, 51.79, 53.00, 103.15, 114.48, 123.98, 128.38, 130.01, 130.33, 133.08, 135.34, 176.70, 198.74, 199.13.
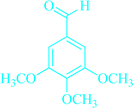
4′,4′,6,6-Tetramethyl-3-(3,4,5-trimethoxyphenyl)-6,7-dihydro-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,4,6′(5H)-trione (5a); yellow solid, 0.40 g, 93%; m.p.: 224–226 °C (lit. m.p. 224–225 °C);52 IR (KBr) υ = 3444, 3339, 2969, 2191, 1644, 1587, 755 cm−1; 1H NMR (400 MHz, DMSO-d6) 0.87 (s, 3H), 1.14 (s, 3H), 1.17 (s, 3H), 1.18 (s, 3H), 1.58 (s, H), 2.13–2.29 (m, 4H), 2.50–2.26 (m, H), 2.69 (s, H), 3.05–3.11 (m, H), 4.95 (s, H), 6.95–6.98 (m, H), 7.08–7.12 (m, 3H) ppm.
4′,4′,6,6-Tetramethyl-3-(4-dimethylaminophenyl)-3,5,6,7-tetrahydrospiro[benzofuran-2(4H),1′-cyclohexane]-2′,4,6′-trione (6a); orange solid, 0.37 g, 95%; m.p.: 251–253 °C (lit. m.p. 251–252 °C);53 IR (KBr) υ = 3394, 3328, 2965, 2189, 1642, 1501,1368, 1147, 856, 777 cm−1; 1H NMR (400 MHz, DMSO-d6): 0.86 (s, 3H), 1.13 (s, 3H), 1.19 (s, 6H), 1.59 (s, H), 2.15–2.29 (m, 4H), 2.56 (d, H, J = 8.0 Hz), 2.69 (s, H), 2.94 (s, 9H), 3.09 (d, H, J = 8.0 Hz), 4.41 (s, H), 6.63 (s, H), 7.01 (s, H) ppm.
3-(2-Bromophenyl)-4′,4′,6,6-tetramethyl-6,7-dihydro-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,4,6′(5H)-trione (7a); orange solid, 0.40 g, 95%; m.p.: 260–261 °C; IR (KBr): υ = 3415, 3330, 2190, 1654, 1585 cm−1; 1H NMR (400 MHz, DMSO-d6): 0.86 (s, 3H), 1.16 (s, 3H), 1.18 (s, 3H), 1.21 (s, 3H), 2.13–2.21 (m, 4H), 2.54 (d, H, J = 6.0 Hz), 2.69 (s, 2H), 3.52 (d, H, J = 6.0 Hz), 5.20 (s, H), 6.98 (d, H, J = 4.0 Hz), 7.14 (t, H, J = 8.0 Hz), 7.23–7.28 (m, H), 7.58 (d, H, J = 4.0 Hz) ppm; 13C NMR (CDCl3, 100 MHz): δ (ppm): 26.25, 28.46, 28.82, 30.53, 30.62, 34.31, 37.21, 49.97, 51.05, 53.87, 54.26, 103.49, 113.43, 126.67, 128.69, 128.93, 130.24, 134.90, 138.12, 176.98, 193.09, 198.43, 198.91.
3-(4-Chlorophenyl)-4′,4′,6,6-tetramethyl-6,7-dihydro-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,4,6′(5H)-trione (8a); white solid, 0.37 g, 97%; m.p.: 257–261 °C (lit. m.p. 258–260 °C);54 IR (KBr) υ = 3476, 3339, 2191, 1641, 1585, 1462, 854 cm−1; 1H NMR (400 MHz, DMSO-d6): 0.86 (s, 3H), 1.07 (s, 6H), 2.04–2.15 (m, 4H), 2.41 (d, H, J = 8.0 Hz), 2.57 (d, H, J = 10.0 Hz), 2.69 (d, H, J = 8.0 Hz), 3.64 (d, H, J = 8.0 Hz), 4.81 (s, H), 7.27 (d, 2H, J = 4.0 Hz), 7.38 (d, H, J = 4.0 Hz) ppm.
3-(4-Fluorophenyl)-4′,4′,6,6-tetramethyl-6,7-dihydro-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,4,6′(5H)-trione (9a); white solid, 0.34 g, 93%; m.p.: 248–250 °C (lit. m.p. 248–249 °C);55 IR (KBr) υ = 3380, 3034, 1506, 1517, 1462, 1315, 742 cm−1; 1H NMR (400 MHz, DMSO-d6): 0.85 (s, 3H), 1.14 (s, 3H), 1.15 (s, 3H), 1.16 (s, 3H), 1.58 (s, H), 1.96 (d, H, J = 8.0 Hz), 2.13–2.25 (m, 4H), 2.56 (d, H, J = 6.0 Hz), 2.66 (s, 1H), 3.06 (d, H, J = 8.0 Hz), 4.43 (s, H), 6.99–7.03 (m, 2H), 7.14 (d, 2H, J = 2.0 Hz) ppm.
4′,4′,6,6-Tetramethyl-3-(4-nitrophenyl)-6,7-dihydro-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,4,6′(5H)-trione (10a); white solid, 0.38 g, 96%; m.p.: 264–266 °C (lit. m.p. 265–266 °C);53 IR (KBr) υ = 3438, 3333, 2971, 2160, 1643, 1509, 7971 cm−1; 1H NMR (400 MHz, DMSO-d6): 0.85 (s, 3H), 1.14 (s, 3H), 1.17 (s, 3H), 1.19 (s, 3H), 1.93 (d, H, J = 6.0 Hz), 2.14–2.28 (m, 3H), 2.62 (d, H, J = 6.0 Hz), 2.7 (s, 2H), 3.06 (d, H, J = 6.0 Hz), 4.43 (s, H), 7.01 (t, 2H), 7.13–7.16 (m, 2H) ppm.
3-(4-Bromophenyl)-4′,4′,6,6-tetramethyl-6,7-dihydro-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,4,6′(5H)-trione (11a); white solid, 0.41 g, 96%; m.p.: 290–293 °C (lit. m.p. 290–292 °C);55 IR (KBr) υ = 3438, 3333, 2971, 2160, 1643, 1509, 797 cm−1; 1H NMR (400 MHz, DMSO-d6): 0.88 (s, 3H), 1.16 (s, 3H), 1.18 (s, 3H), 1.93 (d, H, J = 6.0 Hz), 2.16 (d, H, J = 8.0 Hz), 2.23–2.28 (m, 2H), 2.61 (d, H, J = 8.0 Hz), 2.70 (s, 2H), 3.07 (d, H, J = 8.0 Hz), 4.52 (s, H), 7.37 (d, 2H, J = 8.0 Hz), 8.20 (d, 2H, J = 8.0 Hz) ppm.
3-(2-Hydroxy,5-bromophenyl)-4′,4′,6,6-tetramethyl-6,7-dihydro-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,4,6′(5H)-trione (12a); white solid, 0.42 g, 94%; m.p.: 230 °C, IR (KBr) υ = 3438, 3333, 2971, 2160, 1643, 1509, 797 cm−1; 1H NMR (400 MHz, DMSO-d6): 0.85 (s, 3H), 1.00 (s, 3H), 1.02 (s, 3H), 1.13 (s, 3H), 1.58 (s, H), 1.99 (s, H), 2.33–2.41 (m, H), 2.48 (d, H, J = 10 Hz), 2.58 (d, H, J = 10 Hz), 4.61 (s, H), 6.91 (d, H, J = 4.0 Hz), 7.13 (s, H), 7.26 (d, H, J = 4.0 Hz), 10.04 (s, OH) ppm; 13C NMR (DMSO-d6, 100 MHz): δ (ppm): 25.68, 26.59, 28.07, 29.63, 32.08, 40.96, 50.82, 110.91, 115.92, 118.21, 128.75, 130.16, 131.03, 149.43, 157.36, 164.94, 196.10.
3. Results and discussion
3.1. Preparation and characterization of catalyst
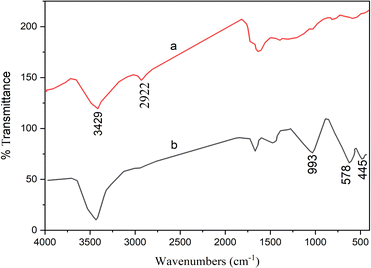
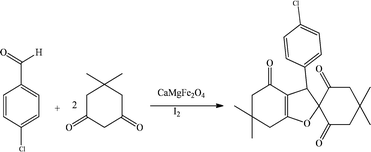
Two steps were taken in the design and preparation of the hollow triple-shell CaMgFe2O4. Mg(NO3)2·6H2O, Ca(NO3)2·4H2O and Fe(NO3)2·9H2O were combined in a solution of glucose at ambient temperature for 30 min, then heated hydrothermally at 180 °C. The formation of metal ions inside the hydrophilic carbon shell during the hydrothermal production of carbon spheres could be anticipated. After the carbon sphere templates had been eliminated thermally, hollow triple-shell CaMgFe2O4 nanospheres were created (Scheme 1). Using various analysis techniques, including FE-SEM, TEM, FT-IR, TGA (thermal gravimetric analysis), VSM, and XRD, the catalyst was identified.Fig. 1 shows the FT-IR spectra of a hollow catalyst before and after calcination. Fig. 1(a) shows that the CaMgFe2O4 catalyst before calcination is irregular. The peak at 2922 cm−1 is related to the stretching vibration of the C–H bonds, demonstrating the presence of glucose and that the carbonization is incomplete. The peak at 3429 cm−1 could be due to the stretching vibration of the O–H bond. Fig. 1(b) shows the CaMgFe2O4 catalyst after calcination, and the peak at 993 cm−1 could be due to the stretching vibration of the Ca–O bond. The stretching vibration peaks of the Mg–O and Fe–O bonds are at 578 cm−1 and 445 cm−1, respectively.56 The lack of the C–H stretching vibration peak in Fig. 1(b), in contrast with Fig. 1(a), shows that a hollow catalyst has been made.
The XRD patterns of the hollow CaMgFe2O4 spheres are shown in Fig. 2. The peaks in Fig. 2(a and b) are assigned to the catalyst before calcination and after calcination, respectively. Additionally, the presence of peaks at 2θ = 26.2, 33 and 36.6 proves that the catalyst is regular.
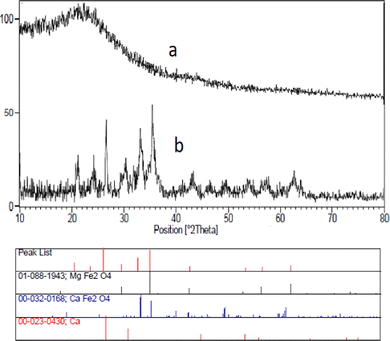 | ||
| Fig. 2 XRD patterns of the hollow triple-shell CaMgFe2O4 nanospheres (a) before, and (b) after calcination. | ||
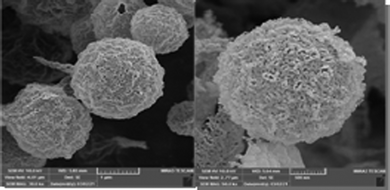
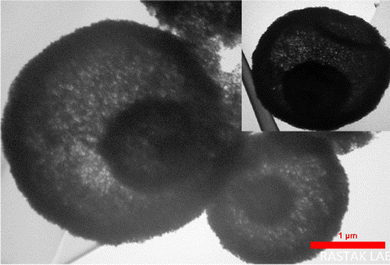
Fig. 3 shows the field emission scanning electron microscopy images for the CaMgFe2O4 catalyst. The FE-SEM images indicate that the catalyst is made up of regular nanospheres. The transmission electron microscopy image shows that the catalyst has irregular, hollow nanospheres and a triple-shell structure20 (Fig. 4).
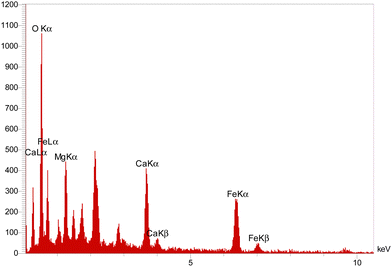
The EDX analysis of the hollow triple-shell CaMgFe2O4 nanospheres is shown in Fig. 5. This demonstrates the presence of Mg, Ca, O and Fe in the nanospheres. These results are consistent with the FT-IR data.
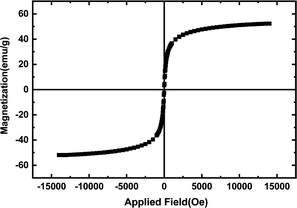
The magnetic properties of the materials were studied using a magnetometer. Fig. 6 shows the magnetization curves of the hollow CaMgFe2O4 nanospheres. The magnetization valence of the hollow CaMgFe2O4 nanospheres is 55.1 emu per g.
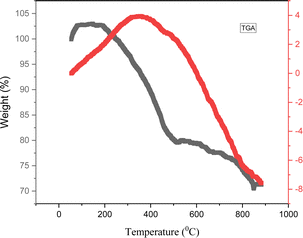
Fig. 7 shows the thermal stability of the hollow CaMgFe2O4 nanospheres from 25 to 900 °C analyzed using thermal gravimetric analysis. Weight loss of the sample was observed at 200 °C, which is related to the removal of residual water from the washing step.
3.2. Investigation of catalytic activity
The use of the hollow triple-shell CaMgF2O4 as a catalyst for the synthesis of spiro-dihydrofuran derivatives was studied (Table 1). The treatment of dimedone and benzaldehyde was chosen as a model reaction. The effects of temperature, reaction solvent, and nanocatalyst concentration on the reaction speed were carefully studied (Table 1). Low yields of the products were observed when toluene, CH3CN, DMF, and DMSO were used as solvents as shown in Table 1, entries 3–6. In the investigation of the model reaction for the synthesis of spiro-dihydrofuran, it was found that the best reaction conditions were 50 °C under solvent-free conditions (Table 1, entry 8). Additionally, the reaction was carried out using this catalyst without iodine, and no spiro-dihydrofuran product was obtained (Table 1, entry 9).| Entry | Solvent | T (°C) | Catalyst (mg) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 4-Cl–benzaldehyde (1 mmol), dimedone (2 mmol), I2 (1 mmol). b Isolated yield. c In the absence of I2. | |||||
| 1 | EtOH | Reflux | 3 | 240 | 92 |
| 2 | MeOH | Reflux | 3 | 200 | 94 |
| 3 | CH3CN | Reflux | 3 | 280 | — |
| 4 | DMSO | 140 | 3 | 120 | — |
| 5 | DMF | 140 | 3 | 45 | — |
| 6 | Toluene | Reflux | 3 | 420 | — |
| 7 | H2O/EtOH | Reflux | 4 | 260 | 90 |
| 8 | Solvent free | 50 °C | 1 | 80 | 98 |
| 9c | Solvent free | 50 °C | 1 | 270 | — |
After the reaction conditions had been optimized, other kinds of catalyst for this reaction were investigated. The findings are displayed in Table 2. In comparison to other catalysts such as MgFe2O4, CaFe2O4 and CaMgFe2O4 nanocatalysts, Et3N and morpholine, and catalyst-free conditions, it was found that the reaction occurred in the presence of the CaMgFe2O4 hollow catalyst with the maximum product yield. Moreover, the reaction without catalyst in the presence of iodine did not give any product and when other catalysts were used, low product yields were achieved at long reaction times (Table 2, entry 6 and 1–5, respectively). Therefore, the hollow triple-shell CaMgFe2O4 spheres were selected as a better catalyst than the other catalysts because they can produce a large amount of the product in this reaction.
The present work was developed and we tested for the usual synthesis of a variety of spiro-dihydrofurans after optimization of the reaction conditions, such as temperature, catalyst loading, and solvent. The results of the investigation using different types of aromatic aldehydes in the reaction with dimedone are shown in Table 3. When aliphatic aldehydes were used in the reaction, the desired product was not achieved (Table 3, entry 13).
| Entry | Aldehyde | Product | Time (min) | Yieldb (%) | m.p. (°C) |
|---|---|---|---|---|---|
| a Reaction conditions: aromatic aldehyde (1 mmol), dimedone (2 mmol), iodine (1 mmol) and CaMgFe2O4 (1 mg). b Isolated yield. | |||||
| 1 |
|
1a | 85 | 98 | 222–224 |
| 2 |
|
2a | 80 | 98 | 224–227 |
| 3 |
|
3a | 90 | 93 | 253–256 |
| 4 |
|
4a | 80 | 98 | 185–189 |
| 5 |
|
5a | 85 | 93 | 224–226 |
| 6 |
|
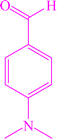
6a | 85 | 95 | 251–253 |
| 7 |
|
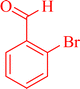
7a | 90 | 95 | 260–262 |
| 8 |
|
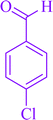
8a | 80 | 97 | 257–261 |
| 9 |
|
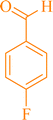
9a | 95 | 93 | 248–250 |
| 10 |
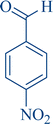
|
10a | 95 | 96 | 264–266 |
| 11 |
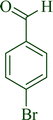
|
11a | 95 | 96 | 290–293 |
| 12 |
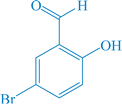
|
12a | 90 | 94 | 230–231 |
| 13 |
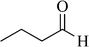
|
— | 250 | — | — |
Table 3 demonstrates that different aromatic aldehydes, including those with electron-donating and electron-withdrawing substituents, effectively interacted with dimedone. The results show that the reaction was completely general and therefore, in the majority of cases, the corresponding spiro-dihydrofurans with various substituents were formed in high yields at low reaction times.
Table 4 shows a comparison of the present method with various reported methods for the synthesis of spiro-dihydrofurans. It was found that in the current work, the product yields, reaction times and catalyst amounts for the reactions were better than in the other studies (Table 4, entry 6 vs. of the entries 1–5).
| Entry | Conditions | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|
| 1 | Cu(II)–Glycerol/MCM-41 (7 mg), BrCN, Et3N | 10 | 95 | 57 |
| 2 | I2 (1.65 eq.), DMAP (2.5 eq.), ball milling, r.t. | 60 | 92 | 53 |
| 3 | Electrolysis, NaBr (1 mmol), EtOH | — | 90 | 50 |
| 4 | KOH (1 eq.), dioxane (1.5 mL) | 12 | 86 | 58 |
| 5 | NH4OAc (1.5 eq.), I2 (1 eq.), 50 °C | 480 | 74 | 55 |
| 6 | Hollow CaMgFe2O4 (1 mg), I2 | 80 | 98 | This work |
3.3. Proposed reaction mechanism
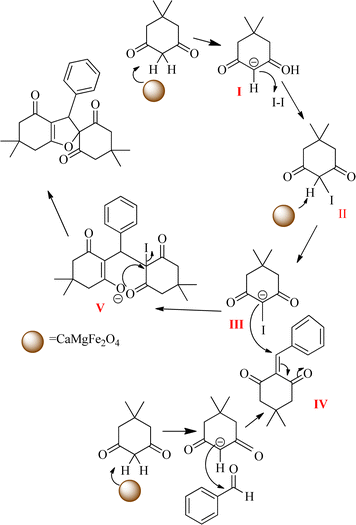
Scheme 2 shows a proposed mechanism for the synthetic process of spiro-dihydrofurans. In this reaction, iodine plays an essential role, because it is the best leaving group, leading to the cyclization of intermediate V. In the first step, the acidic hydrogen of dimedone is separated by the active base catalyst to form intermediate I, which reacts with iodine to obtain intermediate II. Hydrogen is removed from II to form III. Then, treatment of intermediate I with benzaldehyde (the Knoevenagel reaction) takes place to form IV. Finally, intermediates III and IV react together to obtain V, and after cyclization the target spiro-dihydrofuran product is obtained.3.4. Catalyst reusability
After the reaction was completed, the catalyst was separated using an external magnet, then washed with ethanol and dried. The recycled catalyst was reused in the reaction eight times. Fig. 8 shows the performance of the hollow triple-shell CaMgFe2O4 nanospheres after reuse eight times in the reaction without loss of activity.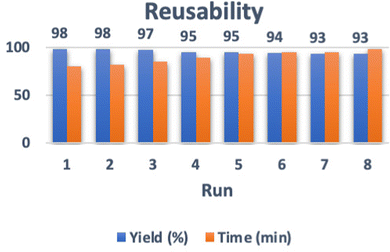 | ||
| Fig. 8 Reusability of the hollow triple-shell CaMgFe2O4 nanospheres after reuse eight times in the reaction. | ||
Additionally, a TEM image of the hollow triple-shell CaMgFe2O4 nanospheres after reuse in the reaction 8 times is shown in Fig. 9. As can be seen in this figure, the structure of the catalyst after 8 times of reuse in the reaction was stable and regular.
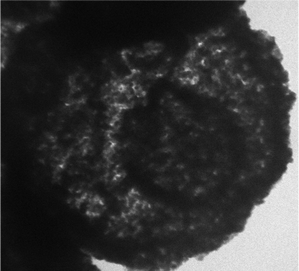 | ||
| Fig. 9 TEM of the hollow triple-shell CaMgFe2O4 nanospheres after eight times of reuse in the one-pot reaction. | ||
4. Conclusion
In this study, a hollow triple-shell CaMgF2O4 structure was designed and prepared through a hydrothermal method. The structure was a powerful catalyst for the synthesis of spiro-dihydrofurans in high yields and short reaction times. These catalytic systems are easily separated by an external magnet from the products, use solvent-free conditions, and need simple work up and low catalyst loading. Characterization of the catalyst showed that it has good magnetic properties, uniform structure and high thermal and chemical stability.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the University of Kashan for supporting this work by Grant No. 159148/73.References
- Y. Zhang, Z. Yang, D. Yin, Y. Liu, C. Fei, R. Xiong, J. Shi and G. Yan, J. Magn. Magn. Mater., 2010, 322, 3470 CrossRef CAS.
- M. Gharibshahian, O. Mirzaee and M. S. Nourbakhsh, J. Magn. Magn. Mater., 2017, 425, 48 CrossRef CAS.
- N. H. Sulaiman, M. J. Ghazali, J. Yunas, A. Rajabi, B. Y. Majlis and M. Razali, Ceram. Int., 2018, 44, 46 CrossRef CAS.
- R. Eisavi and K. Naseri, RSC Adv., 2021, 11, 13061 RSC.
- H. Naeimi and S. Mohammadi, ChemistrySelect, 2020, 5, 2627 CrossRef CAS.
- N. S. Kommareddi, M. Tata, V. T. Jhon, G. L. McPherson and M. F. Herman, Chem. Mater., 1996, 8, 801 CrossRef CAS.
- M. F. F. Lelis, A. O. Porto, C. M. Goncalves and J. D. Fabris, J. Magn. Magn. Mater., 2003, 278, 263 CrossRef.
- S. Komarneni, M. C. D. Arrigo, C. Leonelli, G. C. Pellacani and H. Katsuki, J. Am. Ceram. Soc., 1998, 81, 3041 CrossRef CAS.
- X. W. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 3987 CrossRef CAS.
- Z. G. Feng, Y. S. Li, D. C. Niu, L. Li, W. R. Zhao, H. R. Chen, J. H. Gao, M. L. Ruan and J. L. Shi, Chem. Commun., 2008, 2629 RSC.
- S. Seisno, K. Suga, T. Nakagawa and T. A. Yamamoto, J. Magn. Magn. Mater., 2017, 427, 276 CrossRef CAS.
- W. Wei, Z. Wang, J. Xu, L. Zong, K. Zhao, H. Wang, H. Li and R. Yu, Sci. Bull., 2017, 62, 326 CrossRef CAS.
- X. Zhao, J. Huang, L. Feng, L. Cao, J. Li and L. Zhou, J. Alloys Compd., 2017, 718, 7 CrossRef CAS.
- C. F. Wan, T. Yang, G. G. Lipscomb, D. J. Stookey and T. S. Chung, J. Membr. Sci., 2017, 538, 96 CrossRef CAS.
- L. Garcia-Fernandez, B. Wang, M. C. GarciaPayo, K. Li and M. Khayet, Desalination, 2017, 420, 226 CrossRef CAS.
- C. Jia, X. Zhang and P. Yang, Appl. Surf. Sci., 2017, 430, 457 CrossRef.
- X. He, X. Li and M. Zhu, J. Power Sources, 2016, 333, 10 CrossRef CAS.
- X. Chen, Y. Huang, H. Huang, M. Wang and K. Wang, Mater. Lett., 2015, 149, 33 CrossRef CAS.
- H. Wang, H. Tang, R. Wang and D. Y. Wang, Mater. Chem. Front., 2017, 1, 414 RSC.
- M. Rajabzadeh, R. Khalifeh, H. Eshghi and M. Bakavoli, J. Catal., 2018, 360, 261 CrossRef CAS.
- A. D. Pandey, R. Guettel, M. Leoni, F. Schueth and C. Weidenthaler, J. Phys. Chem. C, 2010, 114, 19386 CrossRef CAS.
- (a) S. Mohammadi and H. Naeimi, Appl. Catal., A, 2020, 602, 117720 CrossRef CAS; (b) H. Naeimi, V. Nejadshafiee and M. R. Islami, Microporous Mesoporous Mater., 2016, 227, 23 CrossRef CAS.
- J. H. Kim, B. Fang, S. B. Yoon and J. S. Yu, Appl. Catal., B, 2009, 88, 368 CrossRef CAS.
- W. Zhao, M. Lang, Y. Li, L. Li and J. Shi, J. Mater. Chem., 2009, 19, 2778 RSC.
- X. Pan, Z. Fan, W. Chen, Y. Ding, H. Luo and X. Bao, Nat. Mater., 2007, 6, 507 CrossRef CAS.
- Y. Yao, M. T. McDowell, I. Ryu, H. Wu, N. Liu, L. Hu, W. D. Nix and Y. Cui, Nano Lett., 2011, 11, 2949 CrossRef CAS PubMed.
- J. M. Jeong, B. G. Choi, S. C. Lee, K. G. Lee, S. J. Chang, Y. K. Han, Y. B. Lee, H. U. Lee, S. Kwon, G. Lee, C. S. Lee and Y. S. Huh, Adv. Mater., 2013, 25, 6250 CrossRef CAS.
- J. Luo, X. Xia, Y. Luo, C. Guan, J. Liu, X. Qi, C. F. Ng, T. Yu, H. Zhang and H. J. Fan, Adv. Energy Mater., 2013, 3, 737 CrossRef CAS.
- X. Zhao, R. Yu, H. Tang, D. Mao, J. Qi, B. Wang, Y. Zhang, H. Zhao, W. Hu and D. Wang, Adv. Mater., 2017, 29, 1703870 CrossRef.
- S. J. Han, Y. K. Yun, K. W. Park, Y. E. Sung and T. Hyeon, Adv. Mater., 2003, 15, 1922 CrossRef CAS.
- E. Pizzutilo, J. Knossalla, S. Geiger, J. P. Grote, G. Polymeros, C. Baldizzone, S. Mezzavilla, M. Ledendecker, A. Mingers, S. Cherevko, F. Schueth and K. J. J. Mayrhofer, Adv. Energy Mater., 2017, 7, 1700835 CrossRef.
- D. S. He, D. He, J. Wang, Y. Lin, P. Yin, X. Hong, Y. Wu and Y. Li, J. Am. Chem. Soc., 2016, 138, 1494 CrossRef CAS.
- C. Y. Cao, W. Guo, Z. M. Cui, W. G. Song and W. Cai, J. Mater. Chem., 2011, 21, 3204 RSC.
- H. Du, L. Jiao, Q. Wang, J. Yang, L. Guo, Y. Si, Y. Wang and H. Yuan, Nano Res., 2013, 6, 87–98 CrossRef CAS.
- J. Zhang, X. Liu, S. Wu, M. Xu, X. Guo and S. Wang, J. Mater. Chem., 2010, 20, 6453 RSC.
- Y. Qin, F. Zhang, Y. Chen, Y. Zhou, J. Li, A. Zhu, Y. Luo, Y. Tian and J. Yang, J. Phys. Chem. C, 2012, 116, 11994 CrossRef CAS.
- L. Wang, H. Dou, Z. Lou and T. Zhang, Nanoscale, 2013, 5, 2686 RSC.
- W. T. Koo, S. J. Choi, N. H. Kim, J. S. Jang and I. D. Kim, Sens. Actuators, B, 2016, 223, 301 CrossRef CAS.
- J. Gao, J. Liu, S. Bai, P. Wang, H. Zhong, Q. Yang and C. Li, J. Mater. Chem., 2009, 19, 8580 RSC.
- R. Liu, R. Jin, J. An, Q. Zhao, T. Cheng and G. Liu, Chem. – Asian J., 2014, 9, 1388 CrossRef CAS.
- J. Shi, L. Chen, N. Ren, Y. Zhang and Y. Tang, Chem. Commun., 2012, 48, 8583 RSC.
- K. Engstrom, E. V. Johnston, O. Verho, K. P. J. Gustafson, M. Shakeri, C. W. Tai and J. E. Backvall, Angew. Chem., Int. Ed., 2013, 52, 14006 CrossRef.
- Z. Jia, K. Wang, B. Tan and Y. Gu, ACS Catal., 2017, 7, 3693 CrossRef CAS.
- X. Li, Z. Guo, C. Xiao, T. W. Goh, D. Tesfagaber and W. Huang, ACS Catal., 2014, 4, 3490 CrossRef CAS.
- R. Pradhan, M. Patra, A. K. Behera, B. K. Mishra and R. K. Behera, Tetrahedron, 2006, 62, 779e828 CrossRef.
- D. Bora, A. Kaushal and N. Shankaraiah, Eur. J. Med. Chem., 2021, 215, 113263 CrossRef CAS PubMed.
- (a) A. D. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef PubMed; (b) P. Chauhan, S. Mahajan and D. Enders, Chem. Commun., 2015, 51, 12890 RSC; (c) A. Kimata, H. Nakagawa, R. Ohyama, T. Fukuuchi, S. Ohta, T. Suzuki and N. Miyata, J. Med. Chem., 2007, 50, 5053 CrossRef CAS; (d) M. Kojima, H. Hosoda, Y. Date, M. Nakazato, H. Matsuo and K. Kangawa, Nature, 1999, 402, 656 CrossRef PubMed.
- (a) M. S. Che, P. A. Barve and V. J. Suryanarayan, Heterocycl. Chem., 2007, 44, 49 CrossRef; (b) I. Schlemminger, B. Schmidt, D. Flockerzi, H. Tenor and C. Zitt, A. Hatzelmann, D. Marx, C. Braun, R. Kuelzer, A. Heuser, H. P. Kley, G. J. Sterk, WO2010055083, 2008; (c) B. Schmidt, C. Scheufler, J. Volz, M. P. Feth, R. P. Hummel, A. Hatzelmann, C. Zitt, A. Wohlsen, D. Marx, H. P. Kley, D. Ockert, A. Heuser, J. A. M. Christiaans, G. J. Sterk and W. M. P. B. Menge, WO2008138939, 2010.
- (a) V. Kumar, K. Kaur, G. K. Gupta and A. K. Sharma, Eur. J. Med. Chem., 2013, 69, 735 CrossRef CAS PubMed; (b) S. Fustero, M. Sánchez Roselló, P. Barrio and A. Simon Fuentes, Chem. Rev., 2011, 111, 6984 CrossRef CAS; (c) E. Narender Reddy, B. Chiranjeevi, K. Sudha Sravanti, U. Ramesh, N. Jagadeesh Babu and A. Krishnaiah, Bioorg. Med. Chem. Lett., 2015, 25, 2918 CrossRef.
- Y. Changsheng, W. Ying, L. Tuanjie, Y. Chenxia, L. Liang and W. Chao, Tetrahedron, 2013, 69, 10593 CrossRef.
- K. Ashok, M. Nagaraju, B. Chiranjeevi and A. Krishnaiah, Synlett, 2018, 1037–1042 Search PubMed.
- T. Xing Chao, Y. Yan, T. Man-Su, J. Bo, L. Chao and T. Shu Jiang, J. Heterocycl. Chem., 2014, 51, 436 CrossRef.
- G. W. Wang and J. Gao, Org. Lett., 2009, 11, 2385 CrossRef CAS PubMed.
- D. P. Sahu, S. K. Giri, V. Varshney and S. Kumar, Synth. Commun., 2009, 39, 3406 CrossRef CAS.
- H. Kazuki, N. Satomi, N. Hiroshi, S. Keisuke and Y. Suzuka, Tetrahedron, 2020, 76, 131165 CrossRef.
- H. Shafie and K. Niknam, New J. Chem., 2021, 45, 11697 RSC.
- H. Batmani, N. Noroozi Pesyan, F. Havasi and M. Aalinejad, Appl. Organomet. Chem., 2019, 33, 4997 CrossRef.
- X.-C. Tu, Y. Yu, M.-S. Tu, B. Jiang, C. Li and S. J. Tu, J. Heterocycl. Chem., 2014, 51, 436 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nj04507a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |