Open Access Article
Open Access ArticleEncapsulation of fish oil and essential fatty acids by spray drying
Afroza
Sultana
a,
Shuji
Adachi
b and
Hidefumi
Yoshii
 *c
*c
aDepartment of Food Processing and Engineering, Chattogram Veterinary and Animal Sciences University, Chattogram-4225, Bangladesh
bFaculty of Bioenvironmental Sciences, Kyoto University of Advanced Science, Kameoka, Kyoto 621-8555, Japan
cDepartment of Food Science and Human Nutrition, Setsunan University, 45-1 Nagaotouge-cho, Hirakata 573-0101, Japan. E-mail: hidefumi.yoshii@setsunan.ac.jp
First published on 25th August 2023
Abstract
Fish oil and essential fatty acids are considered significant in functional foods. Polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and docosahexaenoic acid (22
5) and docosahexaenoic acid (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) in fish oil are prone to oxidative damage. The encapsulation of fish oil by spray drying is a useful method to stabilize fish oil by reducing contact with oxygen. In this review, kinetic analyses of oxidation of fish oil and fatty acids in bulk and emulsion systems and encapsulated fish and krill oils in spray-dried powders were reviewed. Oxidation kinetics of PUFAs could be expressed with the autoxidation equation using the fraction of unoxidized PUFA. The surface-oil ratio could be correlated with the equation 1 − (1 − 2(de/dp))3 using the ratio of oil-droplet diameter, de, to powder diameter, dp. The morphology of the spray-dried powder containing vacuoles affected the surface-oil ratio. The activation energy (Ea) could be correlated with the reconstituted oil droplet diameter of squalene powders. In the estimation of fish oil in spray-dried powder, this review showed that the model of deterioration kinetics of fish oil in the powder is very useful.
6) in fish oil are prone to oxidative damage. The encapsulation of fish oil by spray drying is a useful method to stabilize fish oil by reducing contact with oxygen. In this review, kinetic analyses of oxidation of fish oil and fatty acids in bulk and emulsion systems and encapsulated fish and krill oils in spray-dried powders were reviewed. Oxidation kinetics of PUFAs could be expressed with the autoxidation equation using the fraction of unoxidized PUFA. The surface-oil ratio could be correlated with the equation 1 − (1 − 2(de/dp))3 using the ratio of oil-droplet diameter, de, to powder diameter, dp. The morphology of the spray-dried powder containing vacuoles affected the surface-oil ratio. The activation energy (Ea) could be correlated with the reconstituted oil droplet diameter of squalene powders. In the estimation of fish oil in spray-dried powder, this review showed that the model of deterioration kinetics of fish oil in the powder is very useful.
Sustainability spotlightThe technological development of “encapsulation” to form functional foods such as fish oil with a high content of polyunsaturated fatty acids (PUFAs) in a stable form is expected to promote the No. 3 purpose, “Ensure healthy lives and promote well-being for the super-aging society,” in SDGs. It is very important to realize “healthy lives for all at all ages” by using stable functional foods. This review paper summarized especially the degradation kinetics of PUFAs in liquids and the emulsified spray-dried powder. We believe that it will be very useful for advancing the basic technology for safe intake of functional food in order to maintain the health of elderly people. |
Introduction
In recent years, the population of elderly people has been increasing rapidly. To this end, the food industry has developed functional foods to regulate the physiological characteristics of people, such as blood fluidity, blood pressure, and intestinal activity. Popular functional foods include dietary fibres, lactic acid bacteria, and n-3 (omega-3) polyunsaturated fatty acids (PUFAs), which are considered essential fatty acids. n-3 PUFAs, such as EPA (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and DHA (22
5) and DHA (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), are useful for preventing disorders such as cardiovascular disease,1 inflammation,2 cancer,3 and hypertension.4 As fatty acids, PUFAs play an important role in human nutrition and disease prevention. Many studies have reported that a high intake of n-3 fatty acids results in a low mortality of coronary artery disease.5 A review on EPA and DHA intake and death from coronary artery diseases has been reported previously.6 Numerous reviews and meta-analyses have examined the evidence concerning the cardiovascular effects of n-3 PUFAs.7,8 The global active pharmaceutical ingredient market was valued at USD 187.76 billion in 2020 and is expected to grow at a compound annual growth rate of 6.6% from 2021 to 2028 (Active Pharmaceutical Ingredients Market Report, 2021).
6), are useful for preventing disorders such as cardiovascular disease,1 inflammation,2 cancer,3 and hypertension.4 As fatty acids, PUFAs play an important role in human nutrition and disease prevention. Many studies have reported that a high intake of n-3 fatty acids results in a low mortality of coronary artery disease.5 A review on EPA and DHA intake and death from coronary artery diseases has been reported previously.6 Numerous reviews and meta-analyses have examined the evidence concerning the cardiovascular effects of n-3 PUFAs.7,8 The global active pharmaceutical ingredient market was valued at USD 187.76 billion in 2020 and is expected to grow at a compound annual growth rate of 6.6% from 2021 to 2028 (Active Pharmaceutical Ingredients Market Report, 2021).
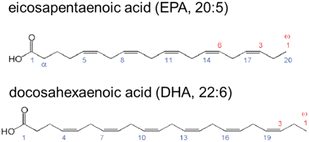
The main sources of n-3 fatty acids are fish oils, krill oil, and marine oils; they can also be found in plant products, such as flaxseeds and nuts. Fish oil is a good source of long-chain n-3 PUFAs. EPA and DHA have double bond structures that include 5 and 6 double bonds in a carbon chain of 20 and 22, respectively, with n-3 indicating the position of the first double bond in the acyl chain, as shown in Fig. 1.
Fish oils, which are an important source of n-3 fatty acids, are suitable for direct incorporation into foods; however, it is difficult to protect them from oxidation owing to their high degree of unsaturation. Anchovy and sardine oils are major sources of EPA and DHA, and currently, these two species account for 80% of n-3 products in the market.9 These oils were reported to have the highest concentrations of EPA and DHA (over 18% and 12%, respectively).10 Durmus (2018)11 reported that the fatty acid composition of seafood species included 10.69–39.57% PUFAs, 1.72–10.73% EPA, and 4.07–31.44% DHA. All seafood species had high EPA and DHA levels and a significantly higher n-3 PUFA content than the n-6 PUFA content. As EPA and DHA are important functional compounds for human health, the Japanese fisheries company, Maruha Nichiro Corporation, produces high DHA-content oil. This oil is produced by the enzymatic hydrolysis of raw tuna oil using lipase to obtain an undecomposed reaction product containing concentrated free fatty acids and DHA. Fatty acids are removed from this oil mixture to obtain a triacylglycerol fraction. This product is decolourised with active soil and deodorised by steam distillation to produce DHA-45, which is a triacylglycerol comprising 45% DHA and 4.6% EPA.12 Dahiya et al. (2023)13 reviewed the current status and future prospects of bioactive molecules delivered through sustainable encapsulation techniques for food fortification. Eratte et al. (2018)14 reviewed the microencapsulation of n-3 oil through complex coacervation.
However, there are very few papers on the oxidation kinetics of PUFAs in spray-dried powders.
These functional food materials such as fish oil, krill oil or PUFAs are very prone to oxidation. In this paper, kinetic analyses of oxidation of fish oil and fatty acids in bulk and encapsulated fish and krill oils in spray-dried powders are reviewed.
Autoxidation kinetics of PUFAs and their esters
PUFAs, such as EPA and DHA, are sensitive to oxidative damage. Lipid oxidation in foods was reported by Simic et al. (1996).15 Hammond and White (2011)16 reviewed a brief history of studies on lipid oxidation. Bolland and Gee (1946)17 presented the basis of the basic autoxidation scheme, as shown in eqn (1)–(3); they presented the kinetics of the oxidative processes during lipid oxidation and oxidation mechanism, including initiation, propagation, and termination. (i) The initiation step comprises the monomolecular step of hydroperoxide formation and peroxyl radical scavenging by antioxidants; (ii) the propagation step includes autocatalytic, monomolecular, and bimolecular or branching reactions; and (iii) the termination stage is primarily characterised by hydroperoxide decomposition and increased formation of secondary oxidation products.Initiation:
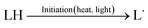 | (1) |
Propagation:
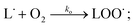 | (2) |
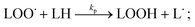 | (3) |
Termination:
 | (4) |
 | (5) |
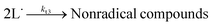 | (6) |
Lipid oxidation comprises complex reactions in which the aforementioned three processes are intricately intertwined; hence, it is difficult to analyse the rate of lipid oxidation.
The autoxidation of PUFAs or their esters is a complicated process, and the aforementioned kinetics are typically considered for describing the individual steps. Özilgen and Özilgen (1990)18 proposed a kinetic model to describe the entire oxidation process of lipids. Adachi, Ishiguro, & Matsuno (1995)19 proposed an autocatalytic-type equation for describing the entire oxidation process of n-6 PUFAs and the first half of the oxidation process of n-3 PUFAs based on the kinetic equation proposed by Bolland and Gee (1946)17 for the propagation step of lipid oxidation. The oxidation rate of PUFA was assumed to be proportional to the product of the concentrations of unoxidised and oxidised PUFAs.
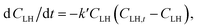 | (7) |
| dY/dt = − kY(1 − Y) | (8) |
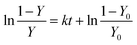 | (9) |
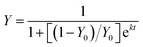 | (10) |
The parameter Y0, which reflects the initial state of the PUFA to be oxidised, is close, but not equal to 1. Eqn (8) describes the entire oxidation process of PUFAs and their esters. Fig. 2 shows the autoxidation processes of ethyl arachidonate (EtARA) and ethyl docosahexaenoate (EtDHA) at 50 °C and 75% relative humidity. The inset of Fig. 2 shows the applicability of eqn (9) to the oxidation process. The equation was applicable to the entire oxidation process of EtARA and the first half of the oxidation process of EtDHA. The latter half of the oxidation process of EtDHA cannot be described by eqn (8), but it can be simply expressed by first-order kinetics. The kinetic equations were also applicable to the autoxidation processes of other PUFAs and their acylglycerols.20
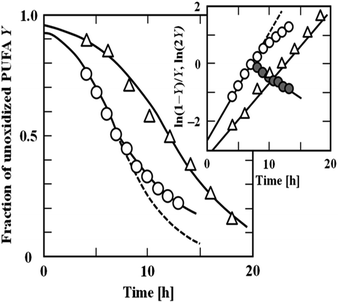 | ||
| Fig. 2 Changes in the fraction of unoxidized (△) ethyl arachidonate (EtARA) and (○) ethyl docosahexaenoate (EtDHA) during autoxidation at 50 °C and 75% relative humidity. Inset: applicability of eqn (9) to the autoxidation processes of EtARA and EtDHA. The closed symbol (●) indicates that the latter half of the oxidation of EtDHA obeyed the first-order kinetics (Adachi et al., 1995).19 “Reproduced from ref. 19 with permission from JAOCS., copyright 1995”. | ||
PUFAs increase in weight as they are oxidised, and the autoxidation of PUFAs is exothermic. The autoxidation processes of the ethyl esters of PUFAs were monitored using thermogravimetry, calorimetry, and gas chromatography to investigate the stoichiometric coefficient of the PUFA and oxygen.21 The change in weight (Δw) during the oxidation is related to the fraction of the unoxidised PUFA (Y), as shown in eqn (11).
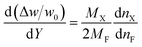 | (11) |
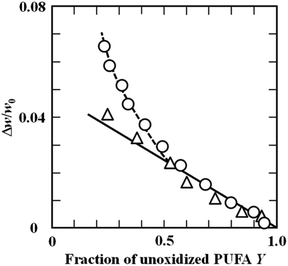 | ||
| Fig. 3 Relationships between the relative weight gain Δw/w0 and fraction of the unoxidised substrate (Y) for (△) ethyl EtARA and (○) EtDHA at 50 °C (Adachi et al., 1995).21 “Reproduced from ref. 21 with permission from Food Sci Technol Int, copyright 1995”. | ||
The oxidation behaviours of unsaturated fatty acids were represented by the autocatalytic reaction using Y, except when Y of n-3 unsaturated fatty acids is above 0.5. This formula could also be applied to powder using the model in which the free energy of activation for the rate constant is assumed to obey a Gaussian distribution (Ishido et al., 2002).22
Spray drying as a method to encapsulate fish oil and essential fatty acids
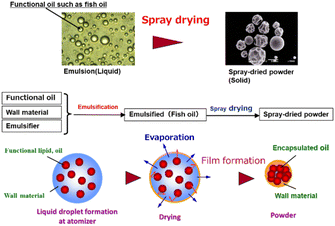
Spray drying is a technique to obtain powders with a submicron-to-micron size range containing oil-droplets such as functional food oil from the emulsion or slurry solution. Spray drying could form functional food powders, pharmaceuticals, and food powders such as soup or juice. Fig. 4 shows the encapsulation processes of fish oil by spray drying. The first important operation factor is the inlet air temperature, which affects the moisture content in the powder, powder recovery efficiency, and powder morphology. The second important operation factor in spray drying is the rotation speed of the rotary atomizer or the gas pressure of the one- or two-fluid nozzle. Atomizer conditions affect the powder particle size and also the oil-droplet size. High shear-stress at the atomizer affects the aggregation behaviour of the oil-droplets in the emulsion.Wall materials
The selection of wall materials is an important step in forming a stable emulsified fish-oil powder. Wall materials play a crucial role in stabilizing fish oil and form good flowable powder. To get oxidative stable fish oil, the wall material should have low oxygen diffusivity and a dense coating film as an oxygen-transfer barrier. In order to obtain a dense film, a low-molecular-weight sugar might be preferable to form a dry-film quickly and easily during spray drying. However, low-molecular-weight sugars have low glass transition temperature and a low powder recovery efficiency in spray drying. When sucrose was used as the wall material, the starch coating process was attached to a spray dryer.23 Maltodextrin as an additive to the sugar wall material is used to control the glass transition temperature. Xiao et al. (2022)24 reviewed maltodextrin as a wall material for microcapsules. The dextrose equivalent (DE) of MD affected the morphology of spray-dried powder. Siemons et al. (2020)25 showed DE of MD determined particle morphology during single sessile drying.Low-molecular-weight sugar and maltodextrin do not have antioxidant ability. Proteins as wall materials could not form dense films, but whey protein or caseinates have antioxidant and also emulsifying abilities. The selection of wall materials should be determined by whether or not a stable fish oil powder can be obtained.
Preparation of emulsions
An emulsification process is used to facilitate the powder formation of fish oil; in this process, fish oil is mixed with a solution comprising an emulsifier and a wall material, followed by a spray drying process, in which the emulsified solution is atomised into droplets in hot air and spray dried, as shown in Fig. 4.The emulsification process is aimed at reducing the oil droplet size and producing a stable emulsion solution. The emulsification process is crucial because it affects the powder properties significantly. In particular, the oil droplet size in an emulsion is an important factor that determines the proportion of fish oil in the spray-dried powder and the proportion of unencapsulated fish oil represented by the surface-oil ratio. Therefore, the selection of an emulsifier is important for the formation of fish oil powder. In the food industry, emulsified functional oil is prepared using a high-pressure homogenizer using about 20 MPa. In the formation of emulsified fish oil, whey protein or caseinate protein at about 3–6 wt% is used as an emulsifier. But octenyl succinic acid anhydride modified starch (OSA-starch) is often used instead of protein emulsifiers due to the increasing number of allergic patients recently.
Drying
The liquid droplets of the emulsion in contact with hot air assist in evaporating the water from the emulsion. In this process, many factors, such as droplet size, type, solid content, inlet air temperature, and emulsion supply rate, affect the powder size and moisture content of the spray-dried powder after drying.The properties of encapsulated fish oil and essential fatty acids
Encapsulation efficiency (EE) is defined using the following equation: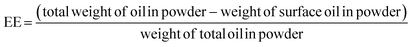 | (12) |
EE is affected by several parameters, such as the oil droplet diameter, solid and oil contents, and the processing conditions of spray drying. The surface-oil ratio is the most important parameter for estimating the shelf life of fish oil in spray-dried powders. The surface-oil ratio, s, is defined as the ratio of the amount of oil exposed on the surface of a microcapsule to the entire amount of oil within the microcapsule.
| s = (surface oil powder)/(total oil in powder) | (13) |
The surface oil is related to the susceptibility of the microencapsulated oil to oxidation.
Jafari et al. (2008)26 showed that nanooil droplets, with a diameter in the range of 210–280 nm, contain the lowest surface-oil ratio in encapsulating fish oil by spray drying. Ahn et al. (2008)27 indicated that low microencapsulation efficiency of powder may lead to a higher surface-oil ratio and indicated higher lipid oxidation in the encapsulation of sunflower oil and its powder storage at 60 °C for 30 days. Carneiro et al. (2013)28 indicated that a high encapsulation efficiency and a minimal surface-oil ratio in the spray-dried powder are essential to produce a stable powder. These studies suggest that the surface-oil ratio is crucial for evaluating the stability of encapsulated oil in spray-dried powder. Shiga et al. (2014)29 proposed a simple method for determining the flaxseed or fish oil content in microcapsules prepared by spray drying using N,N-dimethylformamide (DMF). The DMF method (because DMF is used to dissolve microcapsules) reduces solvent consumption, increases the extraction yield, and enhances the extract. The extraction of surface oil from spray-dried powders by dispersion with hexane under various conditions has been reported by our group.29–32
Furthermore, hexane is known to be more effective than petroleum ether to measure the surface-oil ratio, if the samples are stored for any time duration.33 Abd Ghani et al. (2017)34 indicated that it was difficult to remove the surface oil of nano-sized oil droplets by hexane washing of spray-dried powders because of the higher stability of smaller oil droplets. Several researchers have measured the surface-oil ratio (extractable oil) of fish oil encapsulated with wall materials such as modified starch,35,36 modified starch or glucose,37 modified starch and whey protein,26 and whey protein and sodium caseinate (NC).38 Their data indicated that a higher oil droplet diameter in the spray-dried powder corresponded to a higher surface-oil ratio. Abd Ghani et al. (2017)32 correlated the average reconstituted oil droplet diameter to the surface-oil ratio using the data reported above. No systematic investigations have been conducted on the effects of oil droplet and powder diameters on the surface-oil ratio and the encapsulated efficiency in spray-dried powders.
Fig. 5 shows an electron micrograph of the cut surface of an emulsified spray-dried fish oil powder. Many oil droplets were observed in the shell of the spray-dried powder and one large vacuole was found in the centre of the powder. Oil droplets on the surface of the vacuole can be seen in this figure.
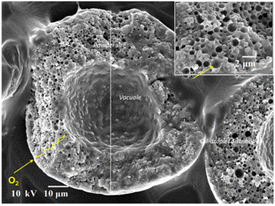 | ||
| Fig. 5 Scanning electron micrograph of the cut surface of spray-dried powder containing emulsified fish oil. | ||
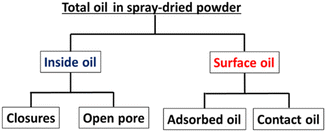
Fig. 6 shows the locations of oil presence in spray-dried powder containing emulsified oil. While encapsulating functional oil by spray drying, the surface-oil ratio is important for evaluating the stability of functional oil in spray-dried powders. The encapsulated oil is the inside oil in closure within the wall material of the spray-dried powder. The surface oil present as adsorbed oil and contact oil exists mainly on the surface of the spray-dried powder and inside the vacuole.
Physical properties
The physicochemical properties of the emulsified fish oil spray-dried powder are (1) the amount of fish oil content in the powder, (2) the surface-oil ratio, (3) average powder size and average oil droplet size in powder, (4) powder morphology, and (5) powder fluidity. The fish oil content of the emulsified fish oil spray-dried powder was generally 40–45 wt%, but recently, due to the increase in various operating costs, some powders had 60 wt% fish oil. In the emulsified middle-chain triacylglycerol oil case, spray-dried powder contains almost 80 wt% in the powder. Fish oil content and the ratio of the average oil droplet diameter to the average powder diameter greatly affected the surface-oil ratio. Spray drying, which has a small encapsulation efficiency and larger surface-oil ratio in the encapsulation, is meaningless. In general, in order to produce a stable emulsified fish oil powder, it is preferable to form a powder having a small surface-oil ratio and a larger powder diameter with smaller oil droplets. A powder structure with a low percentage of voids is also important, i.e. solid powder forming powders with a low surface-oil ratio. Using a low-molecular-weight sugar as the wall material, a solid powder could be produced. But those sugars such as sucrose have low glass transition temperature (Tg). When using a wall material with Tg, the powder recovery should be considered.Stability of encapsulated oils
Effect of oil droplet size on the oxidation of microencapsulated PUFA
Nakazawa et al. (2008)39 examined the effects of oil droplet size and the weight ratio of oil to the wall material on the oxidation of methyl linoleate (MeLA) microencapsulated with maltodextrin (MD) by spray drying. As shown in Fig. 7, the oxidation of MeLA was more retarded for the microcapsules prepared from emulsions with smaller oil droplets.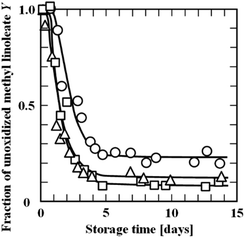 | ||
| Fig. 7 Oxidation processes at 50 °C of MeLA encapsulated with MD at a weight ratio of 0.35. Microcapsules were prepared from the O/W emulsions with median oil droplet diameters of (○) 0.02, (△) 0.6, and (□) 1 μm (Nakazawa et al., 2008).39 “Reproduced from ref. 39 with permission from J Oreo Sci., copyright 2008”. | ||
The oxidation was more suppressed for the microcapsules with a lower weight ratio of oil to the wall material. The fraction of unoxidised MeLA levelled off at Y∞ upon prolonged storage. The dependence of Y∞ on the weight ratio was analysed using two- and three-dimensional percolation models. These models were also applied to the oxidation processes of linoleic acid (LA) encapsulated with polysaccharides of different weight ratios by a single droplet drying method.40 The effects of oil fraction and oil droplet size in microcapsules on the surface-oil ratio were examined by simulating two- and three-dimensional percolation models.41 The surface-oil ratio was lower when the oil content was lower in the microcapsules, particularly with smaller oil droplets. The simulation results indicated that smaller oil droplets were more favourable for suppressing the oxidation of microencapsulated PUFAs.
The surface-oil ratios of microcapsules with different oil droplet-to-microcapsule size ratios were estimated based on the two-dimensional percolation model, assuming that the frequency function of the oil droplet-to-microcapsule size ratio can be expressed by a log-normal distribution.42 The variance in the distribution of the oil droplet-to-microcapsule size ratio had no significant effect on the surface-oil ratio in the microcapsules at any oil fraction.
Oil isolated from the microcapsule surface was hard to oxidise. The two-dimensional percolation model was also applied to statistically calculate the interior oil fraction in a microcapsule, which is defined as the ratio of the volume of oil isolated from the microcapsule surface to the whole volume of the microcapsule.43 As the entire oil fraction increased, the interior oil fraction first increased and then sharply decreased after reaching a maximum value. The reduction of the oil droplet size was effective in suppressing the oxidation owing to the higher interior oil fraction.
Fig. 8 shows the volume-based distribution of the reconstituted oil droplet diameter and particle diameter in the spray-dried powder. This figure shows the particle diameter and reconstituted oil droplet diameter for each DE of MD were almost the same (approximately 1 μm).
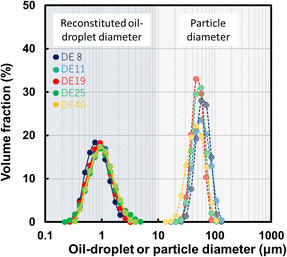 | ||
| Fig. 8 Volume-based distribution of the reconstituted oil droplet diameter and particle diameter in spray-dried powder. Closed keys (solid lines) show the reconstituted oil droplet diameter and thin-colour keys (dotted lines) show the particle diameter distribution (Abd Ghani et al., 2017).32 “Reproduced from ref. 32 with permission from J Chem Eng Soc. Jpn., copyright 2017”. | ||
However with an increase in the DE of MD, these phenomena might occur because the water diffusion coefficient increases with an increase in the DE of MD. The vacuole diameter significantly depended on the DE of the MD. This vacuole affects the surface-oil ratio because the oil droplets exist at the shell of the spray- dried powder. Abd Ghani et al. (2017)32 correlated the surface-oil ratio (s) and the ratio of the vacuole diameter (dv) to the particle diameter (dp) and obtained a linear correlation equation, s = 0.42(dv/dp).
The s can also be correlated with the ratio (E) of the oil droplet diameter (de) to the particle diameter (dp) as follows:
| s = 1 − (1 − 2E)3 | (14) |
These results indicate that a higher encapsulation efficiency may be obtained with a larger particle diameter, smaller oil droplet diameter, and smaller vacuole diameter in spray-dried powder.
The effect of fish oil content added to spray-dried powder on the surface-oil ratio was investigated using DE = 19 of MD.
Fig. 9 shows the relationship of the ratio (E) for various fish oil contents in spray-dried powder. In the region where the value of E was 10−2 or less, the surface-oil ratio was as low as 0.05, whereas in the region where E was greater than or equal to 10−2, the surface-oil ratio increased sharply.
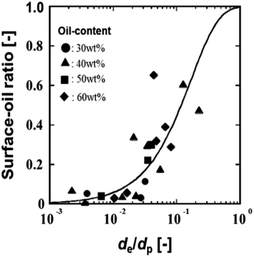 | ||
| Fig. 9 Relationship of the ratio (E) of oil droplet diameter (de) to particle diameter (dp) and surface-oil ratios for varying fish oil content in spray-dried powder. (Abd Ghani et al., 2017)32 “Reproduced from ref. 32 with permission from J Chem Eng Soc. Jpn., copyright 2017”. | ||
Stability of encapsulated oil and surface oil of emulsified fish oil and squalene in spray-dried powder
Spray-dried powder with a lower surface-oil ratio can be obtained by using a nano-emulsion based on the relationship between the surface-oil ratio and oil droplet diameter, as shown in the above section. Regarding the retention of orange oil in spray-dried powders, Risch and Reineccius (1988)44 reported that larger emulsion sizes had a longer shelf life. However, Ishido et al. (2002)22 reported that the oxidation rate of LA contained in MD was slower as the emulsion size was smaller.The changes in the peroxidation value (PV) were measured during the storage of spray-dried powder of emulsified fish oil at 105 °C using MD (DE19). The PV of the surface-oil was measured using fish oil in the hexane-washing solvent. The PV of the encapsulated oil was measured using a solubilised solvent of hexane-washed powder with DMF. The PV of the total oil was measured using a solubilised solvent of spray-dried powder with DMF.
Fig. 10 shows PV changes during the storage separately as the surface oil, the encapsulated oil, and total oil in spray-dried powder.45 The X-axis was plotted with the number scale of sampling time. Surface oils were observed to be significantly oxidised. The PVs of surface oils increased after storage at 105 °C. The PVs of the encapsulated oils were one-order of magnitude lower than the value of surface oil. As shown in Fig. 10, the surface oil oxidised initially. Small oil droplets in spray-dried powder decrease the surface-oil ratio as shown in Fig. 9. However, smaller oil droplets had larger specific surface area, which promotes oxidation. To investigate the effect of oil droplet size on the stability of squalene (SQ) in spray-dried powder of emulsified SQ, three different types of reconstituted SQ oil droplet diameters were formed using the pressure change in a high-pressure homogeniser (20, 50 and 100 MPa). Spray-drying emulsions were prepared using 40 wt% solid content, with 40 wt% SQ in the solid, 3–8% NC, 0.4 wt% lecithin, and 56.6–51.6 wt% MD (DE = 19). SQ can be analysed with gas chromatography, and the retention of squalene in the powder can be easily measured.
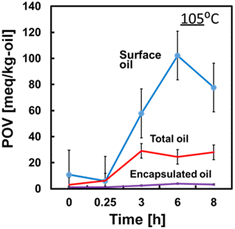 | ||
| Fig. 10 Peroxidation value changes in spray-dried powder of emulsified fish oil powder during storage at 105 °C. The blue line denotes surface oil, red line denotes total oil, and purple line denotes encapsulated oil (DE of MD = 19) (Abd Ghani et al., 2017)45 “Reproduced from ref. 45 with permission from Oxford Univ. Press., copyright 2017”. | ||
Fig. 11 shows the stability of SQ in spray-dried powder. By assuming a first-order kinetics for oxidation of SQ in the powder, the first-order kinetic constants were obtained.
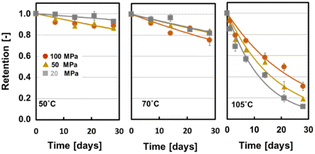 | ||
| Fig. 11 Stability of squalene in spray-dried powder at three homogenisation pressures, with high-pressure homogenisation of the emulsion at 50, 70 and 105 °C. These powders contained 5 wt% NC in the solid powder. (Yoshii et al., 2023)46 “reproduced from ref. 46 with permission from Taylor and Francis, copyright 2023”. | ||
At 105 °C, the SQ sample with smaller oil droplets in the powder was more stable than SQ with larger oil droplets. This result suggests that the radical change in the degradation rate of SQ in the powder might be a rate-limiting process in the oil droplet in the emulsified SQ powder. At 50 °C, the powder with larger oil droplets had a lower oxidation rate because of the smaller specific surface area of the oil droplets. These results revealed that the oil droplet diameter plays a major role in the stability of the encapsulated SQ.
Fig. 12 shows the Arrhenius plot of the oxidation rate constants for 3, 5, and 8 wt% of the emulsifier NC. The regression straight lines for 100 MPa and 20 MPa homogenisations intersect with each other. The dependence of oil droplet size on SQ stability differs between high and low temperatures because of the difference in the oxidation reaction mechanism at different temperatures, as described above.
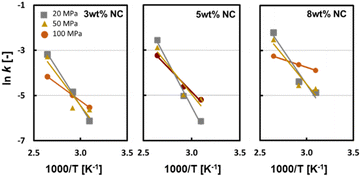 | ||
| Fig. 12 Arrhenius plots of the degradation rate constant at 50, 70, and 105 °C. The powders were prepared at □: 20 MPa, △: 50 MPa, and ○: 100 MPa. (Yoshii et al., 2023)46 “Reproduced from ref. 46 with permission from Taylor and Francis, copyright 2023”. | ||
The activation energy values were estimated using these regression lines.
Fig. 13 shows the relationship between the activation energy (Ea) [kJ mol−1] and the reconstituted oil droplet diameter (dr) [μm] of the SQ powders. Ea can be correlated with the following equation:
| Ea = 43.1dr + 22.2 | (15) |
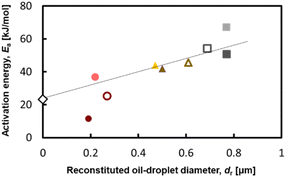 | ||
| Fig. 13 Relationship between activation energy and reconstituted oil droplet diameters of the SQ powders. Powder prepared by homogenisation at □, ●, ●: 100 MPa, △, ▲, ▲: 50 MPa and □,■,■: 20 MPa with 3 wt% NC is denoted by clear colour symbols, 5 wt% NC by light colour symbols, and 8 wt% NC by dark colour symbols. The rhombus key indicates the activation energy for the oxidation of the bulk oil of SQ. (Yoshii et al., 2023)46 “Reproduced from ref. 46 with permission from Taylor and Francis, copyright 2023”. | ||
This equation indicates that the SQ oxidation kinetics can be estimated using the oil droplet diameter in the spray-dried powder. The oil droplet diameter may affect the radical transfer rate, specific surface area of the oil droplet, and/or kinetic constant of the oil volume. The frequency factors, k0 [1/day], of SQ in these SQ systems of spray-dried powder were also correlated with the activation energies, Ea as follows:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k0 = 0.33Ea − 3.92 k0 = 0.33Ea − 3.92 | (16) |
The encapsulation process affected the stability retention of SQ in the spray-dried powder. The stability of SQ retention in the spray-dried powders, which had reconstituted oil droplet diameters of 0.69–0.77, 0.47–0.61, and 0.19–0.27 μm, was investigated at 50, 70, and 105 °C for 28 days. Larger oil droplet diameter SQ powders had a lower degradation rate at 50 and 70 °C, and smaller oil droplet diameter powders were more stable at 105 °C.
Stability of EPA and DHA in spray-dried powder of emulsified krill oil
Krill oil is a functional oil that is a source of n-3 PUFAs such as EPA and DHA. The global krill oil market is estimated to attain a value of USD 801.65 million by 2023. Sultana et al. (2021)47 investigated the stability of EPA and DHA in spray-dried powders of emulsified krill oil. Krill oil was homogenised using a saponin emulsifier (Quillayanin S-100, Maruzen Pharmaceuticals Co., Ltd, Hiroshima, Japan). The spray-dried powder of emulsified krill oil was obtained using MD (DE = 19) and an antioxidant, that is, rosemary extract oil. Fig. 14 shows the EPA retention behaviour at storage temperatures of 25, 50, and 70 °C.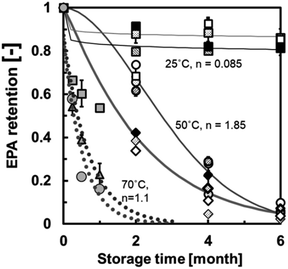 | ||
| Fig. 14 EPA retention behaviour in spray-dried powder of emulsified krill oil at storage temperatures of 25, 50, and 70 °C. Grey lines represent the samples with a solid content of 50% that were subjected to mechanical homogenisation. Dot patterns represent the samples with a solid content of 50% that were subjected to high-pressure homogenisation. Symbols filled in black represent the samples with a solid content of 60% that were subjected to mechanical homogenisation. Symbols filled in white represent the samples with a solid content of 60% that were subjected to high-pressure homogenisation. Symbols filled in grey represent krill oil (Sultana et al., 2021).47 “Reproduced from ref. 47 with permission from Elsevier, copyright 2021”. | ||
The solid lines in Fig. 14 are correlation lines calculated using the Avrami equation as follows:
| R = exp(−(kt)n) | (17) |
As mentioned above, the oxidation rate of a PUFA in a binary system was expressed using a kinetic model in which the rate was proportional to the product of the concentration of the unoxidised PUFA and the sum of the concentrations of the two oxidised PUFAs. The model was adopted to analyse the oxidation rates of EPA and DHA in microencapsulated Isada krill oil. Based on the model, the relationship between the fractions of unoxidised EPA (YE) and unoxidised DHA (YD) is expressed by the following equation (Jimenez et al., 2021)52
| YE = (YE0/YD0κ)YDκ | (18) |
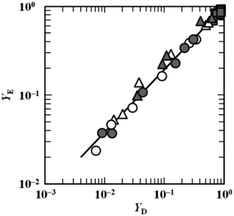 | ||
| Fig. 15 Relationship between the fraction of unoxidised DHA (YD) and unoxidised EPA (YE) in microencapsulated Isada krill oil stored at (□) 25 °C, (△) 50 °C and (○) 70 °C. The open and closed symbols indicate microcapsules with 50% and 60% solid content, respectively (Jimenez, Miyagawa, Yoshii, & Adachi, 2021).52 “Reproduced from ref. 52 with permission from J. Oreo Sci., copyright 2021”. | ||
Conclusion
The stabilities of fish oil, krill oils and PUFA in spray-dried powder were affected by the morphology of spray-dried powder and the average oil-droplet and powder particle diameters. The ratio of the diameter of the oil droplet to the particle diameter was found to be an important factor in estimating the surface-oil ratio in the spray-dried powder. The stability of PUFAs in spray-dried powder is currently being investigated using a kinetic model with basic mass transfer phenomena and chemical reactions. The Avrami equation, which is compatible with various kinetic models, is useful to estimate the stability of fish oil and krill oil in the powder using the mechanism number and degradation or oxidation kinetic constant.Author contributions
Sultana wrote the encapsulation of fish oil and krill oil part. Adachi wrote and summarized the oil oxidation kinetics and all parts of this paper. Yoshii planned this review paper and summarized mainly the stability of fish and krill oils.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Part of this research was supported by the grants from the Project of the NARO Bio-oriented Technology Research Advancement Institution (a special scheme project on vitalising management entities of agriculture, forestry, and fisheries).References
- D. S. Siscovick, T. A. Barringer, A. M. Fretts, J. H. Wu, A. H. Lichtenstein, R. B. Costello and D. Mozaffarian, Circulation, 2017, 135, 867–e884 CrossRef PubMed.
- Y. W. Chien, H. C. Peng, Y. L. Chen, M. H. Pai, H. Y. Wang, H. L. Chuang and S. C. Yang, Mediators Inflammation, 2017, 5801768 Search PubMed.
- M. Correia-da-Silva, E. Sousa 1, M. M. M. Pinto and A. Kijjoa, Semin. Cancer Biol., 2017, 46, 55–64 CrossRef CAS PubMed.
- A. M. Minihane, C. K. Armah, E. A. Miles, J. M. Madden, A. B. Clark, M. J. Caslake and P. C. Calder, J. Nutr., 2016, 146, 516–523 CrossRef CAS PubMed.
- O. Ezaki, S. Sato, M. Sakono, Y. Miyake, N. Mito and M. Umesawa, Nippon Eiyo · Shokuryo Gakkaishi, 2006, 59, 123–158 CrossRef CAS.
- D. Mozaffarian and E. B. Rimm, JAMA, 2006, 296, 1885–1899 CrossRef CAS PubMed.
- J. K. Innes and P. Calder, Int. J. Mol. Sci., 2020, 21, 1362 CrossRef CAS PubMed.
- A. P. Jain, K. K. Aggarwal and P. Y. Zhang, Eur. Rev. Med. Pharmacol. Sci., 2015, 19, 441–445 CAS.
- C. Jacobsen, Encyclopedia of Food and Health, Elsevier, 2015, pp. 686–692 Search PubMed.
- E. M. Hernandez, Functional Dietary Lipids, Woodhead Publishing Series in Food Science, Technology and Nutrition, 2016, pp. 69–101 Search PubMed.
- M. Durmus, Food Sci. Technol., 2019, 39, 454–461 CrossRef.
- R. Kroes, E. J. Schaefer, R. A. Squire and G. M. Williams, Food Chem. Toxicol., 2003, 1433–1446 CrossRef CAS PubMed.
- D. Dahiya, A. Terpou, M. Dasenaki and P. S. Nigam, Sustainable Food Technol., 2023, 1, 500–510 RSC.
- D. Eratte, K. Dowling, C. J. Barrow and B. Adhikari, Trends Food Sci. Technol., 2018, 71, 121–131 CrossRef CAS.
- M. G. Simic, S. V. Javanocic and E. Niki, ACS Symp. Ser., 1992, 500, 14–32 CrossRef CAS.
- E. M. Hammond and P. J. White, J. Am. Oil Chem. Soc., 2011, 88, 891–897 CrossRef CAS.
- J. L. Bolland and G. Gee, Trans. Faraday Soc., 1946, 42, 244–252 RSC.
- S. Özilgen and M. Özilgen, J. Food Sci., 1990, 55, 498 CrossRef.
- S. Adachi, T. Ishiguro and R. Matsuno, J. Am. Oil Chem. Soc., 1995, 72, 547–551 CrossRef CAS.
- Y. Minemoto, E. Ishido, S. Adachi and R. Matsuno, Food Sci. Technol. Res., 1999, 5, 104–107 CrossRef.
- S. Adachi, T. Ishiguro and R. Matsuno, Food Sci. Technol. Int., 1995, 1, 1–4 CrossRef CAS.
- E. Ishido, K. Hakamata, Y. Minemoto, S. Adachi and R. Matsuno, Food Sci. Technol. Res., 2002b, 81, 85–88 Search PubMed.
- S. Drusch and S. Mannino, Trends Food Sci., 2009, 20, 237–244 CrossRef CAS.
- Z. Xiao, J. Xia, Q. Zhao, Y. Niu and D. Zhao, Carbohydr. Polym., 2022, 120113 CrossRef CAS PubMed.
- I. Siemons, R. G. A. Politiek, R. M. Boom, R. G. M. van der Samn and M. A. I. Schutyser, Food Res. Int., 2020, 131, 108988 CrossRef CAS PubMed.
- S. M. Jafari, E. Assadpoor, B. Bhandari and Y. He, Food Res. Int., 2008, 41, 172–183 CrossRef CAS.
- J. H. Ahn, Y. P. Kim, Y. M. Lee, E. M. Seo, K. W. Lee and H. S. Kim, Food Chem., 2008, 107, 98–105 CrossRef CAS.
- H. C. Carneiro, R. V. Tonon, C. R. Grosso and M. D. Hubinger, J. Food Sci., 2013, 115, 443–451 CAS.
- H. Shiga, S. Adachi, S. Adachi and H. Yoshii, J. Food Eng., 2014, 15, 131–139 CrossRef.
- A. Soottitantawat, H. Yoshii, T. Furuta, M. Ohkawara and P. Linko, J. Food Sci., 2003, 68, 2256–2262 CrossRef CAS.
- V. Paramita, T. Furuta, H. Yoshii and H. Food, Sci. Technol. Res., 2010, 16, 365–372 CAS.
- A. A. Ghani, S. Adachi, K. Sato, H. Shiga, S. Iwamoto, T. L. Neoh, S. Adachi and H. Yoshii, J. Chem. Eng. Jpn., 2017, 50, 799–806 CrossRef.
- V. E. Buck and S. A. Barringer, J. AOAC Int., 2007, 90, 1729–1730 CrossRef CAS PubMed.
- A. A. Ghani, R. Francoise, H. Shiga, T. L. Neoh, S. Adachi and H. Yoshii, Food Sci. Technol. Res., 2017, 23, 503–509 CrossRef.
- S. Drusch, Y. Serfert, A. V. D. Heuvel and K. Schwarz, Food Res. Int., 2006, 39, 807–815 CrossRef CAS.
- Y. Serfert, S. Drusch and K. Schwarz, Food Chem., 2009, 113, 1106–1112 CrossRef CAS.
- S. Drusch and S. Berg, Food Chem., 2008, 109, 17–24 CrossRef CAS PubMed.
- Q. Chen, D. McGillivray, J. Wen, F. Zhong and S. Y. Quek, J. Food Eng., 2013, 117, 505–512 CrossRef CAS.
- R. Nakazawa, M. Shima, S. Adachi and S. J. Oleo, Sci, 2008, 57, 225–232 CAS.
- Y. Minemoto, S. Adachi and R. Matsuno, Biosci., Biotechnol., Biochem., 1999, 63, 866–869 CrossRef CAS PubMed.
- K. Kikuchi, S. Yamamoto, H. Shiga, H. Yoshii and S. Adachi, Japan J. Food Eng, 2013, 14, 169–175 CrossRef.
- K. Kikuchi, S. Yamamoto, H. Shiga, H. Yoshii and S. Adachi, Japan J. Food Eng., 2014, 15, 191–193 CrossRef.
- K. Kikuchi, S. Yamamoto, H. Shiga, H. Yoshii and S. Adachi, Japan J. Food Eng., 2015, 16, 303–305 CrossRef.
- S. J. Risch and G. A. Reineccius, in Flavor Encapsulation, ed. S. J. Risch and G. A. Reineccius, American Chemical Society, 1988, pp. 66–67 Search PubMed.
- A. A. Ghani, S. Adachi, H. Shiga, T. L. Neoh, S. Adachi and H. Yoshii, Biosci., Biotechnol., Biochem., 2017, 81, 705–711 CrossRef PubMed.
- H. Yoshii, S. Adachi and T. Furuta, Drying Technol., 2023, 41, 859–867 CrossRef CAS.
- A. Sultana, Y. Maki, A. Fermin, S. Adachi and H. Yoshii, Future Foods, 2021, 3, 100009 CrossRef CAS.
- H. Yoshii, S. Adachi and T. Furuta, Drying Technol., 2023, 41, 859–867 CrossRef CAS.
- R. Matsuno and S. Adachi, Japan J. Food Eng., 2023, 24, 31–36 CrossRef.
- H. Yoshii, A. Soottitantawat, X. D. Liu, T. Atarashi, T. Furuta, S. Aishima, M. Ohgawara and P. Linko, Innovative Food Sci. Emerging Technol., 2001, 2, 55–61 CrossRef CAS.
- A. A. Ghani, K. Matsumura, A. Yamauchi, H. Shiga, S. Adachi, H. Izumi and H. Yoshii, Drying Technol., 2016, 34, 1726–1734 CrossRef.
- J. A. F. Jimenez, Y. Miyagawa, H. Yoshii and S. Adachi, J. Oleo Sci., 2021, 70, 633–635 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |