Sustainable production of prodigiosin from rice straw derived xylose by using isolated Serratia marcescens (CMS 2): statistical optimization, characterization, encapsulation & cost analysis
Received
30th June 2023
, Accepted 1st October 2023
First published on 2nd October 2023
Abstract
Prodigiosin, a pigment renowned for its multifaceted utility in biomedical, nutraceutical, and food domains, encounters impediments stemming from its expensive production process, limited accessibility, and modest yield. To address this challenge, the present investigation centered on the cultivation of naturally isolated Serratia marcescens CMS2, and the primary objective was to fine-tune fermentation conditions employing response surface methodology (RSM) to bolster prodigiosin synthesis. This endeavor leveraged xylose derived from rice straw as an economical carbon source. The cost-effective growth medium was enriched with peanut de-oiled cake, resulting in a remarkable 1.9-fold amplification in prodigiosin output. Under optimized conditions of pH (6.5), substrate concentration (1.5%), inoculum size (1.25%) and agitation rate (150 rpm), 0.5048 color value units per mg of prodigiosin were obtained. The purified prodigiosin underwent a comprehensive characterization utilizing various analytical techniques such as UV-vis spectroscopy, FT-IR spectroscopy, UPLC (ultra-performance liquid chromatography), TLC (thin-layer chromatography), and GC-MS (gas chromatography-mass spectrometry). The UV-vis spectra unveiled a pronounced absorption peak at 535 nm, while GC-MS analysis disclosed a distinctive peak at 324.96 m/z, accompanied by its derivatives. A significant breakthrough was realized when prodigiosin was encapsulated with polysaccharides, resulting in heightened water solubility and an expansion of its potential applications within the food industry. By implementing the cost-effective growth medium developed in this study, a substantial economic benefit of $578.41 was achieved, with the total scalable cost amounting to 8986.84 INR for 1 mg of prodigiosin. These findings position prodigiosin as an economically viable option in a competitive market landscape.
Sustainability spotlight
By promoting environmentally responsible waste management, we may unlock the latent potential of the enormous volume of agro-waste as a priceless resource, and counteract the harmful impacts of synthetic dye consumption and disposal. We used rice straw to make prodigiosin pigment, by a microbial route, demonstrating an innovative approach to managing waste, utilizing resources, and finding prospective sources for making useful products. Our study aligns seamlessly with the United Nations’ SDG12 goals of promoting responsible consumption and production. Through dedicated research and innovation, we can reduce the environmental impact of waste and contribute to a more sustainable future. By recognizing the intrinsic value of waste, we can pave the way for a greener and responsible approach for resource utilization, benefiting the environment and society.
|
1. Introduction
Prodigiosin is natural colorant and potential replacement for synthetic food colours. The use of such a natural colorant will reduce the health risks associated with synthetic food dyes and provide a sustainable and eco-friendly alternative. It can be produced by various bacterial species and characterized by a common pyrrolyl pyrromethene skeleton. It has been reported to exhibit anti-bacterial,1 anti-fungal,2 anti-proliferative, anti-protozoal,3 anti-metastatic4 and immunosuppressive properties.5 Therefore, the demand for the prodigiosin pigment is driven by its potential applications in various fields, including nutraceuticals, cosmetics, and the food industry as a natural alternative to synthetic colorants and health benefits.6 A recent study using bioinformatics tools showed a good docking score of prodigiosin with human epidermal growth factor-2 (HER-2), mitogen-activated protein kinase (MEK) and S6 kinase protein (S6K) −44.25 kcal mol−1, −44.99 kcal mol−1, and −40.91 kcal mol−1 respectively.7 Through co-polymerization of prodigiosin with natural polysaccharides like starch, cellulose, and chitosan as well as with some proteins like albumin, legume, and gelatin, which are broadly used for the creation of nanostructures for the delivery of various drugs derived from their non-toxicity, stability, small size, and biodegradability, different bioactivities can be achieved for many biomedical applications. Incorporating naturally occurring bioactive substances into nanoencapsulated systems or nanoemulsions, where they may be released under regulated conditions and produce a particular bioactivity, is another promising strategy; it is also used as a pH indicator, self-induced fluorescence agent and acts as a controlling agent as well in biofilm making.8 A review showed the application of prodigiosin in the agriculture sector and reported insecticidal activity of prodigiosin against Plutella xylostella which showed 96% mortality rate with 8 μg g−1 diet, and thus, it can be used as an insecticide in the agriculture industry.9 As a result, there is a growing demand for natural pigments, particularly those derived from microorganisms such as Serratia marcescens, Streptomyces coelicolor, Pseudoalteromonas sp. and Vibrio species.10
The microbial production of such pigments faces numerous challenges such as low yield and instability with the high cost of synthetic fermentative media being a significant obstacle. To overcome this challenge, an optimal scenario would be to use inexpensive and eco-friendly substrates for microbial fermentation, which could result in a more sustainable and responsible industry. Agricultural residues such as corn, wheat straw, pulp peel, rice straw, and sugarcane bagasse which are rich in cellulose, hemicellulose, and lignin are considered low cost and promising substrates for the generation of valuable products. Studies have shown that prodigiosin production can be enhanced by optimizing the growth conditions, such as pH, temperature, and nutrient concentration. The addition of certain oil seed cakes can also increase the yield of prodigiosin.11 Further research and development are needed to overcome these challenges by optimizing the production process, using low-cost substrates and developing a more stable form of prodigiosin.
The aim of the current research is to gain an understanding of the process by which Serratia marcescens synthesizes prodigiosin, utilizing rice straw hydrolysate (RSH) and peanut de-oiled cake (PDOC) as a cost-effective substrate. To our knowledge, no prior studies have reported the statistical analysis using the response surface methodology (RSM) utilizing xylose, a reducing sugar derived from rice straw, as a source of carbon for the manufacture of prodigiosin by using Serratia marcescens. RSM was used to create experiments, draw models, assess the impacts of various variables, and determine the ideal parameters of a multivariable system in order to produce meaningful responses.12 The study employed RSM to optimize the factors that influence pigment growth, and evaluated the extraction and purification of the pigment using a range of analytical techniques including UPLC, TLC, GC-MS, UV-vis spectroscopy, and FT-IR. Additionally, pigment properties were analysed, including its antioxidant and antimicrobial activities. Furthermore, the utilization of agricultural waste for prodigiosin production is a sustainable solution for waste management while also offering a potential source for the production of valuable products.
2. Materials and methods
2.1 Materials
All chemicals and media used in this study were purchased from Sigma-Aldrich and were of HPLC grade. Rice straw was taken from a local field located in CIAB, Mohali, and peanuts were purchased from a local market in Mohali (Punjab). Peanut de-oiled cake was extracted in our own lab. Standard prodigiosin was purchased from Merck, Germany.
2.2 Isolation and identification of the culture
CIAB (30.667927463597213, 76.72196820411781), Mohali's local field, was used to aseptically collect a soil sample that was then placed in a sterile plastic bag for storage. 1 g of the soil sample was dissolved in 100 ml of water to create a stock solution, which was then serially diluted with nutrient agar (NA) media in order to identify any potential pigment-producing bacteria connected with the sample. To encourage the growth of distinct coloured bacterial colonies, inoculated plates were then placed in an incubator (Memmert, Germany) and kept at 30 °C for 7 days. The colonies were visually estimated, counted, and isolated for further studies.13 The probable pigment producing isolates were purified using the streak plate technique, and for further identification and characterization, the culture was sent to the Institute of Microbial Technology (IMTECH) in Chandigarh, India.
2.3 Pretreatment of lignocellulosic feedstock
Rice straw was subjected to acidic pretreatment with sulphuric acid (H2SO4) as described by Singh et al.14; biomass was pretreated with 1.5% (v/v) diluted H2SO4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/v) for 24 h. The pH of pretreated rice straw hydrolysate (RSH) was 0.8; it was neutralized with barium hydroxide till pH 7 was attained and detoxified with 1.5% activated charcoal. The sugar content of hydrolysate before and after pretreatment was evaluated using HPLC (Agilent, HiPlex, Santa Clara, California, USA).
10 w/v) for 24 h. The pH of pretreated rice straw hydrolysate (RSH) was 0.8; it was neutralized with barium hydroxide till pH 7 was attained and detoxified with 1.5% activated charcoal. The sugar content of hydrolysate before and after pretreatment was evaluated using HPLC (Agilent, HiPlex, Santa Clara, California, USA).
2.4 Preparation of inoculum for pigment production
Seed culture of the Serratia marcescens isolate was prepared as follows: ‘a few loops full of bacterial growth from an overnight culture on brain-heart infusion medium were inoculated into a 100 ml Erlenmeyer shake flask which contained 20 ml of chemically defined liquid medium. The flask was then incubated for 24 h in an orbital shaker incubator at 30 °C and 200 rpm which was then used as a seed culture for inoculating the production medium at a level of 2% (v/v) under the same incubation conditions for 48 h. During the incubation, the samples were taken for the analyses of prodigiosin production, biomass and substrate concentration. Each run was conducted either in triplicate or duplicate.
2.5 Pigment production via submerged fermentation
A bacterial culture of Serratia marcescens CMS 2 was grown overnight on a nutrient agar medium and a loop full of it was transferred to a 100 ml Erlenmeyer shake flask consisting of 20 ml of liquid medium. The initial liquid medium consisted of xylose (1%), yeast extract (0.3%), and peptone (0.5%) and had a pH of 7 that was adjusted using 1 M NaOH. The media was sterilized through autoclaving at 121 °C for 15 minutes.15 The flask was then placed in an orbital shaker incubator at 30 °C and 200 rpm for 24 h. This culture was then employed as a seed for the production medium at a level of (2%) under the same fermentation conditions for 72 h.
2.6 Statistical analysis of fermentation process parameters by using RSM for pigment production
Initially, optimization of submerged fermentation process parameters like temperature, pH, substrate concentration, inoculum size and rpm was performed through practical experimentation using RSH. The statistical analysis of experimental data was performed by using Design Expert software (version 12.0.11.0, Stat-Ease, Inc., Minneapolis, USA). After identifying critical factors through screening, the Box–Behnken design (BBD) was implemented to obtain a quadratic model. BBD enabled the study of effective parameters with minimal experiments and interaction between the factors and their responses. Each factor was assigned low (−1) and high (+1) levels such as 5 (−1) and 8 (+1) for pH, 0.5 (−1) and 2.5 (+1) for substrate concentration (%), 0.5 (−1) and 2 (+1) for inoculum size (% v/v) and 100 (−1) and 200 (+1) for rpm. They were coded as A, B, C, and D, respectively. All 29 runs were carried out at 30 °C in submerged fermentation using media containing 0.5% peptone, 0.3% yeast extract and rice straw hydrolysate (RSH) as the substrate. The second-order regression equation was used to obtain optimum process variable values and for graphical analysis. The adequacy of the model was verified using analysis of variance (ANOVA).16 Finally, the statistical model was validated at the lab scale under predicted conditions for pigment production.
2.7 Extraction of the pigment
A slightly modified version of the technique was utilized to extract prodigiosin from cell biomass.17 Initially, the fermented broth underwent centrifugation for 15 minutes at 4 °C and 8000 rpm. The pigment was extracted from the cell pellet by resuspending it in 96% ethanol acidified with 0.1 N hydrochloric acid (HCl), and subsequently incubated for 30 minutes at room temperature. Following this, the mixture was centrifuged again at 8000 rpm and 4 °C for 15 minutes until the pellet became colourless, and the resulting supernatant was concentrated at 30 °C by using a rotary vacuum evaporator (Rotavapor RV.10, IKA Staufen, Germany).
2.8 Color value estimation of the pigment
The color of the pigment was evaluated by measuring the absorbance of the extracted pigment at a wavelength of 535 nm using an ultraviolet-visible double beam spectrophotometer (UV-1900i SHIMADJU, Japan) with the dilution factor taken into account. The absorbance values obtained were converted to pigment units using a specific equation,18 which provided an indication of the amount of color generated during the fermentation process.
| Color value = optical density (O.D) × dilution × volume of extract/amount of sample (mg) |
2.9 Strategies to enhance pigment production
To enhance the color value of the pigment, modifications were made to the rice straw hydrolysate (RSH) media employed in the RSM. Peptone was substituted with PDOC (extracted using an oil extractor) at a concentration of 10 g L−1, while yeast extract concentration was increased from 5 g L−1 to 10 g L−1.19 Both the fermentative broths were subjected to the optimized conditions established by the RSM and incubated for 72 h. After the incubation period, the pigment was extracted using acidified ethanol and the color value of the pigment was determined by measuring absorbance at 535 nm. Furthermore, the pigment was concentrated using a rotary evaporator and concentration of the pigment was measured by taking the weight of the dried pigment.
2.10 Effect of RSH sugar concentration on cell biomass and pigment production
In this study, the ability of the bacterial strain Serratia marcescens CMS2 to utilize sugars present in RSH for both cell growth and pigment production was evaluated. To accomplish this, 2 ml of the sample from the modified broth was collected every 24 h for a duration of up to 96 h. The cell mass was obtained through centrifugation, while the pigment was extracted from the collected cell mass and the color value was quantified. The resulting data for cell mass and the color value of the pigment were transformed into units of mg mL−1 and color value units per mg, respectively, and plotted against time (h). The concentration of sugars (mg mL−1) was determined using HPLC.
2.11 Purification of the pigment
After prodigiosin was extracted, the pigment was purified using silica gel column chromatography.10 Silica with a pore size of 60 Å and mesh size of 200–400 (Sigma-Aldrich) was used to separate non-colored impurities from the pigment. The concentrated sample was applied to the column and a solvent system made up of n-hexane and ethyl acetate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0; v/v to 0
0; v/v to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; v/v) was run through the column with a flow rate of 1 ml min−1. The red-colored fractions that eluted out were collected, and prodigiosin was confirmed by using the absorption spectra. The red-colored fractions that contained prodigiosin were collected and concentrated by rotary evaporation at 30 °C.
2; v/v) was run through the column with a flow rate of 1 ml min−1. The red-colored fractions that eluted out were collected, and prodigiosin was confirmed by using the absorption spectra. The red-colored fractions that contained prodigiosin were collected and concentrated by rotary evaporation at 30 °C.
2.12 Characterization of the pigment
2.12.1 Preliminary identification of the pigment.
To conduct the initial identification of prodigiosin, the pigment dissolved in absolute ethanol was split into two portions. One portion was made acidic by adding a small amount of concentrated HCl, while the other was made alkaline by adding few drops of concentrated ammonia solution.20
2.12.2 UV-vis spectral analysis.
Absorption spectra of the pigment were analysed at pH levels of 2, 7, and 9 within the wavelength range of 200–800 nm by using a UV-vis spectrophotometer with ethanol serving as the blank. The purified pigment, dissolved in ethanol, had a pH of 7.0. However, to achieve pH values of 9.0 and 2.0, 0.1 N NaOH and 0.1 N HCl were respectively used for the pH adjustments.21
2.12.3 Thin layer chromatography (TLC).
To check the purity of the pigment, TLC was performed by the utilization of a solvent system consisting of chloroform and methanol (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and carried out on silica gel 60 F254 TLC-cards (Sigma-Aldrich) with dimensions of 20 × 20 cm.10 The standard and sample were compared and identified with the help of the retention factor (Rf).
1) and carried out on silica gel 60 F254 TLC-cards (Sigma-Aldrich) with dimensions of 20 × 20 cm.10 The standard and sample were compared and identified with the help of the retention factor (Rf).
| Rf = distance travelled by the compound/distance travelled by the solvent. |
2.12.4 Ultra performance liquid chromatography (UPLC) analysis.
Prodigiosin concentration was measured using an LC-GC-M system (Agilent 6300 Series Ion Trap LC/GC-MS system, Agilent Technologies, Santa Clara, CA, USA). A XDB- C18 (5 μm, 4.6 × 150 mm, Agilent Technologies) column was used for high-performance liquid chromatography analysis. Mobile phase A was mixed with water (95%) and acetonitrile (5%) containing formic acid (0.2%), and mobile phase B was mixed with water (5%) and acetonitrile (95%) containing formic acid (0.2%). The analysis was conducted using the following method: 10–100% B for 0–20 min, 100% B for 20–25 min, and 100–10% B for 25–30 min at a flow rate of 0.5 ml min−1.
Prodigiosin purity was measured using an Agilent Zorbax eclipse plus C18 analytical column (4.6 × 100 mm, 5-Micron) at 25 °C; the UPLC (Waters, Milford, MA, USA) analysis involved injecting 10 μl of the sample and separating the components with a flow rate of 1 ml min−1. Mobile phase A was acetonitrile, and methanol containing formic acid (0.1%) was mobile phase B. The analysis was conducted using the following method: 25% of solvent A and 75% of solvent B for 10 minutes.
2.12.5 Gas chromatography-mass spectrometry (GC-MS) analysis.
The extracted pigment was dissolved in 5 ml of methanol solvent and GC-MS was employed to perform pigment characterization. The molecular mass of the pigment was obtained using GC-MS (TRACE 1300/5975C, Thermo Fisher Scientific S.p.A. Milan, Italy) with helium as the carrier gas flowing at a rate of 1.2 ml min−1 in split mode (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) and a mass spectroscopy (GC-MS) detector. The oven temperature was maintained as follows: an initial temperature of 120 °C was held for 1 minute, and then increased at a rate of 10 °C min−1 up to 280 °C, and finally it was held at 280 °C for 5 minutes. The instrumental parameters were as follows: the mass transfer line temperature was set to 280 °C, electron energy was 70 eV, ion source temperature was 250 °C, and the mass range scanned was 50–1000 m/z.
10) and a mass spectroscopy (GC-MS) detector. The oven temperature was maintained as follows: an initial temperature of 120 °C was held for 1 minute, and then increased at a rate of 10 °C min−1 up to 280 °C, and finally it was held at 280 °C for 5 minutes. The instrumental parameters were as follows: the mass transfer line temperature was set to 280 °C, electron energy was 70 eV, ion source temperature was 250 °C, and the mass range scanned was 50–1000 m/z.
2.12.6 Fourier transform infrared (FT-IR) analysis.
The purified red pigment was subjected to Fourier transform infrared (FT-IR) spectroscopic analysis on an Agilent, Cary 660 using an attenuated total reflection (ATR) accessory. An empty ATR cell was run as a blank for background correction, and the sample was scanned at a resolution of 4 cm−1 from 4000 to 400 cm−1. In order to confirm the presence of prodigiosin, the obtained peaks were compared with those in the literature.
2.12.7 Nuclear magnetic resonance (NMR).
For nuclear magnetic resonance (NMR) analysis pertaining to the characterization of prodigiosin, a Bruker Advance 300 instrument tuned to 500 MHz was utilised. Besides the utilisation of tetramethylsilane (TMS) as an internal standard, completely dry NMR tubes with a diameter of 5 mm were utilised22 in order to hold the sample while it was dissolved in DMSO.
2.12.8 Determination of antioxidant activity by the DPPH (2,2-diphenyl-1-picrylhydrazyl) method.
The DPPH radical scavenging technique was used to determine the antioxidant activity of the extracted pigment.23 4 mg of DPPH was dissolved in 100 ml methanol, which was used as a control. To obtain the stock solution (3 mg/3 ml), the pigment was mixed with methanol. The stock solution was diluted with methanol to achieve concentrations of 50–300 μg ml−1 for the test samples, with 100% methanol serving as a blank. 1 ml each of diluted test samples were added to micro centrifuge tubes containing 1 ml of 0.1 M methanolic solution of DPPH. The solution was then left in the dark for 30 minutes to allow for a reaction. The measurement of absorbance at a wavelength of 517 nm was conducted using a UV-vis spectrometer, with ascorbic acid serving as the reference standard. The following formula was used to calculate percentage inhibition:
| % DPPH inhibition = [(Abs control − Abs sample)/Abs control] × 100 |
2.13 Economic analysis
The aim of this study includes an extensive cost-benefit analysis of the entire prodigiosin production process to determine its economic feasibility for industrial scale-up and commercialization. Prior literature suggests that any process must be economically feasible to be considered for further industrial development.24 Thus, a comprehensive cost-benefit analysis was conducted in accordance with strict technical requirements for industrial implementation, incorporating all the input and output parameters considered in this study. The input parameters, including total production cost and packaging cost, were converted to their INR equivalent and then into current USD. The difference between input and output costs suggested the net economic benefit of producing prodigiosin using a specific type of media, thus determining its potential for commercialization and scaling up for industrial use.
2.14 Encapsulation of the pigment
Pigment encapsulation was achieved through slight modification of the process,25 which involves the dissolution of maltodextrin (5% w/v) and citrus peel pectin (1% w/v) in 100 ml of distilled water. This mixture was then combined with an ethanolic solution of prodigiosin pigment (in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of pigment to pectin and maltodextrin) and subjected to stirring at a temperature of 70 °C until the ethanol was completely evaporated. The resulting suspension underwent homogenization for 10 minutes utilizing a sonicator (Elmasonic, S100H, Germany) after which it was freeze-dried. To evaluate the solubility of the freeze-dried particles, 5 mg were added to 5 ml of solvents including HCl (0.1 M), NaOH (0.1 M), acetone, n-hexane, ethyl acetate, methanol, ethanol and water. The morphology of the particles was observed using a scanning electron microscope (SEM) (Nikon, H600L) after attaching the powdered samples to carbon conductive tape and sputter-coating them with gold. Digital images were acquired using an excitation voltage of 10 kV.
1 ratio of pigment to pectin and maltodextrin) and subjected to stirring at a temperature of 70 °C until the ethanol was completely evaporated. The resulting suspension underwent homogenization for 10 minutes utilizing a sonicator (Elmasonic, S100H, Germany) after which it was freeze-dried. To evaluate the solubility of the freeze-dried particles, 5 mg were added to 5 ml of solvents including HCl (0.1 M), NaOH (0.1 M), acetone, n-hexane, ethyl acetate, methanol, ethanol and water. The morphology of the particles was observed using a scanning electron microscope (SEM) (Nikon, H600L) after attaching the powdered samples to carbon conductive tape and sputter-coating them with gold. Digital images were acquired using an excitation voltage of 10 kV.
2.15 Statistical analysis
The experimental outcomes presented are the mean values of multiple replications. Data were subjected to analysis of variance by ANOVA (Table 3) with statistical significance (p < 0.05), followed by comparison utilising the least significant difference (LSD) test. All statistical analyses, including ANOVA, Duncan's test and t-test, were executed using software (IBM-SPSS, Version-28, Armonk, New York (NY), USA).
3. Results and discussion
3.1 Isolation and identification of the culture
Serially diluted soil sample plated on nutrient agar plate produced a wide range of pigmented colonies. Out of these, a red coloured colony was isolated on NA plates and preserved at 4 °C. The pigment-producing strain was identified as Serratia marcescens ATCC 13880 (GenBank Accession No. OR497520) based on a combination of biochemical tests, morphological characteristics, and analysis of the 16S rRNA gene sequence. The isolated strain displayed a remarkable level of similarity, with a sequence similarity of 99.72% as shown in Fig. 1.
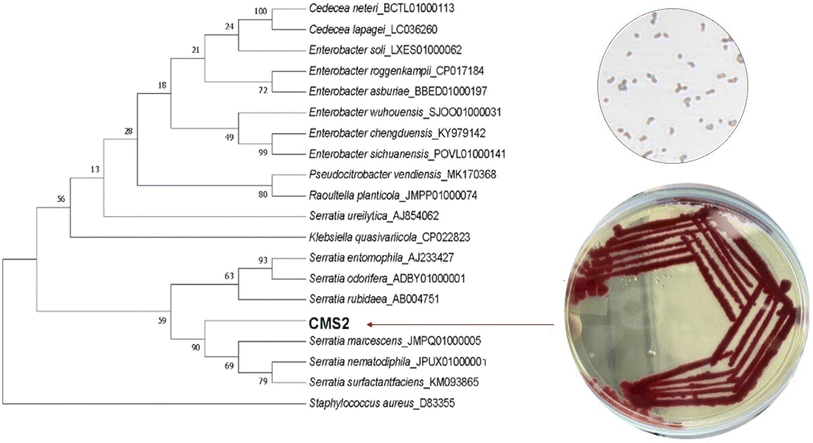 |
| | Fig. 1 Phylogenetic tree of isolated strain CMS2 based on 16S rRNA gene sequences. | |
3.2 Reducing sugar content in pretreated rice straw hydrolysate
Low-cost lignocellulosic rice straw was hydrolysed by acidic pretreatment. The sugar content (mg ml−1) of RSH before and after pretreatment was calculated using HPLC26 and is depicted in Table 1.
Table 1 Sugar concentration (mg ml−1) of RSH before and after pretreatmenta
| Sugar |
Before pretreatment RSH (mg ml−1) |
After pretreatment RSH (mg ml−1) |
|
Data are expressed in mean ± SD and significantly different (p < 0.05).
|
| Glucose |
01.449 ± 0.041 |
00.985 ± 0.001 |
| Xylose |
15.481 ± 1.054 |
13.147 ± 0.950 |
| Arabinose |
02.551 ± 0.008 |
02.005 ± 0.006 |
Table 2 Total production cost of prodigiosin/mg from an isolated strain
| S.no |
Expenditure |
Production cost (INR) |
| 1 |
A. Raw material cost |
|
| (a) Rice straw |
00.03 |
| (b) Peanut de oiled cake |
04.05 |
| (c) Yeast extract |
25.55 |
| (d) Activated charcoal |
10.33 |
| (e) Barium hydroxide |
17.55 |
| (f) Silica gel |
06.45 |
| B. Solvent for extraction and purification |
|
| (a) Ethanol |
240.00 |
| (b) Hexane |
473.33 |
| (c) Ethyl acetate |
928.88 |
| (d) Hydrochloride acid |
88.88 |
| (e) Sulphuric acid |
06.94 |
| 2 |
Utility cost (a) power/electricity (Autoclave, incubator, centrifuge, rotary vacuum evaporator, laminar flow, and extractor) |
4950 |
| 3 |
Total cost of production (1 + 2) |
1801.99 + 4950 = 6751.99 |
| 4 |
Packaging cost 10% |
7427.16 |
| 5 |
Average yield lost cost 10% |
742.71 |
| 6 |
Net profit ratio 10% |
816.98 |
| 7 |
Cost of the developed product/mg |
8986.84 |
3.3 Statistical optimization
In this study, standard ANOVA was used to investigate the interactive effects of key factors. The optimal values with a 95% confidence level of the tested variables were c. Similar results for pH, substrate concentration and rpm were reported by Vijayalakshmi & Jagathy, 2016 for prodigiosin production by Serratia marcescens.27 The F value and p value were used to determine the significance of each coefficient and the values of p higher than F, but <0.05 shows the significant model terms. The response surface quadratic model of ANOVA showed that if the p < 0.0001, and the model was significant (Table 3). The fitness of the model was analysed by using the determination coefficient (R2) where, R2 = 0.9470, adjusted R2 = 0.8941 and the coefficient of variance was 14.80%, and the adequate precision value was found to be 17.8516 which means an adequate signal.
Table 3 ANOVA table for pigment production for the coded form of variables
| Source |
Sum of squares |
df |
Mean square |
F-value |
p-value |
|
| Model |
0.8359 |
14 |
0.0597 |
17.88 |
<0.0001 |
Significant |
|
A-pH |
0.0581 |
1 |
0.0581 |
17.40 |
0.0009 |
|
|
B-Substrate concentration |
0.0001 |
1 |
0.0001 |
0.0342 |
0.8560 |
|
|
C-Inoculum size% |
0.0127 |
1 |
0.0127 |
3.80 |
0.0717 |
|
|
D-rpm |
0.0118 |
1 |
0.0118 |
3.53 |
0.0813 |
|
|
AB
|
0.0064 |
1 |
0.0064 |
1.92 |
0.1879 |
|
|
AC
|
0.0271 |
1 |
0.0271 |
8.10 |
0.0129 |
|
|
AD
|
0.0471 |
1 |
0.0471 |
14.10 |
0.0021 |
|
|
BC
|
0.0062 |
1 |
0.0062 |
1.87 |
0.1931 |
|
|
BD
|
0.2030 |
1 |
0.2030 |
60.78 |
<0.0001 |
|
|
CD
|
0.0062 |
1 |
0.0062 |
1.85 |
0.1958 |
|
|
A
2
|
0.4188 |
1 |
0.4188 |
125.42 |
<0.0001 |
|
|
B
2
|
0.0040 |
1 |
0.0040 |
1.20 |
0.2910 |
|
|
C
2
|
0.0051 |
1 |
0.0051 |
1.52 |
0.2379 |
|
|
D
2
|
0.0020 |
1 |
0.0020 |
0.5932 |
0.4540 |
|
The regression equation for the levels of color value produced was:
| Color value/mg = +0.5042 − 0.0696A − 0.0031B − 0.0325C − 0.0313D − 0.0400AB − 0.0822AC − 0.1085AD − 0.0395BC − 0.2253BD − 0.0393CD − 0.2541A2 + 0.0249B2 − 0.0280C2 − 0.0175D2 |
where
A,
B,
C, and
D represent pH, substrate concentration, inoculum size and rpm, respectively. RPM plays a significant role and values from 100–200 rpm (
Table 4) showed that pigment production increased with increasing rpm. The two joint (binomial) coefficient
A2 showed the highest value other than other binomial coefficient, this was highly significance. Monomial coefficient
B showed the highest
p-value
i.e. 0.8560 which showed the insignificance of this factor. In two factor interaction, the maximum
F value (60.78) was obtained for the BD with
p-value <0.0001, and
AD also showed two factor significance, which means that interaction between the two factors are significant for this model.
Table 4 The Box–Behnken design for the study of the effect of process parameters on bio-pigment production
|
|
Factor 1 |
Factor 2 |
Factor 3 |
Factor 4 |
Response 1 |
| Run |
pH |
Substrate concentration (%) |
Inoculum size (%) |
Stirring (rpm) |
Color Value Unit (CVU per mg) |
| 1 |
6.5 |
1.5 |
1.25 |
150 |
0.495 |
| 2 |
6.5 |
1.5 |
2 |
100 |
0.473 |
| 3 |
6.5 |
2.5 |
2 |
150 |
0.416 |
| 4 |
6.5 |
1.5 |
0.5 |
200 |
0.451 |
| 5 |
8 |
1.5 |
1.25 |
100 |
0.29 |
| 6 |
6.5 |
1.5 |
2 |
200 |
0.245 |
| 7 |
8 |
0.5 |
1.25 |
150 |
0.187 |
| 8 |
6.5 |
0.5 |
0.5 |
150 |
0.508 |
| 9 |
8 |
1.5 |
0.5 |
150 |
0.269 |
| 10 |
8 |
1.5 |
1.25 |
200 |
0.034 |
| 11 |
6.5 |
1.5 |
1.25 |
150 |
0.519 |
| 12 |
5 |
0.5 |
1.25 |
150 |
0.253 |
| 13 |
6.5 |
1.5 |
1.25 |
150 |
0.502 |
| 14 |
8 |
1.5 |
2 |
150 |
0.117 |
| 15 |
5 |
1.5 |
0.5 |
150 |
0.234 |
| 16 |
6.5 |
0.5 |
1.25 |
100 |
0.344 |
| 17 |
6.5 |
2.5 |
1.25 |
200 |
0.3 |
| 18 |
5 |
2.5 |
1.25 |
150 |
0.371 |
| 19 |
6.5 |
1.5 |
1.25 |
150 |
0.496 |
| 20 |
5 |
1.5 |
2 |
150 |
0.411 |
| 21 |
6.5 |
1.5 |
0.5 |
100 |
0.522 |
| 22 |
6.5 |
2.5 |
1.25 |
100 |
0.75 |
| 23 |
5 |
1.5 |
1.25 |
200 |
0.393 |
| 24 |
6.5 |
1.5 |
1.25 |
150 |
0.509 |
| 25 |
5 |
1.5 |
1.25 |
100 |
0.215 |
| 26 |
6.5 |
0.5 |
1.25 |
200 |
0.795 |
| 27 |
6.5 |
2.5 |
0.5 |
150 |
0.575 |
| 28 |
8 |
2.5 |
1.25 |
150 |
0.145 |
| 29 |
6.5 |
0.5 |
2 |
150 |
0.507 |
Furthermore, 3D response surface and counter plots presented in Fig. 2 demonstrate the combined effect of A, B, C, and D on production of the pigment. In Fig. 2a, it can be observed that there was an increase in the color value with increase in A up to 6.5, and a further increase up to 8 resulted in suppression of the pigment. A study also reported that there was increase in pigment production when pH increases from 5 to 7.28 In Fig. 2b, an increase in D from 100–200 rpm and 6.5 pH resulted in maximum pigment production, while inoculum size and substrate concentration together do not have a significant effect on pigment (Fig. 2d).
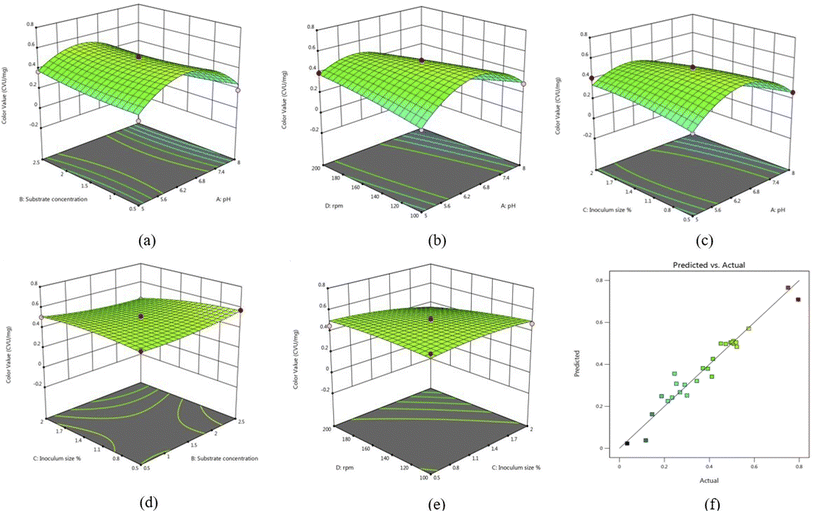 |
| | Fig. 2 Three-dimensional response surface plots showing the interaction between (a) pH and substrate concentration (b) rpm and pH (c) inoculum size and pH (d) inoculum size and substrate concentration (e) rpm and inoculum size (f) model (predicted) versus actual values in the production of prodigiosin. | |
3.4 Enhanced pigment production using peanut de oiled cake
When a combination of POC (10 g L−1) and yeast extract (10 g L−1) was utilized as a nitrogen source, the resulting color value at 72 h was 1.55 CVU per mg, and prodigiosin production was 6100 mg L−1; a medium containing yeast extract (10 g L−1) and peptone (10 g L−1) resulted in 0.815 CVU per mg and prodigiosin production was 3210.52 mg L−1. Several scientific investigations have demonstrated that the utilization of specific carbon and nitrogen sources can affect prodigiosin production. For instance, Lin et al., 2019 indicated that the combination of beef extract and peanut powder resulted in 2.8 times higher prodigiosin production than that of beef extract and peanut powder alone.19 In our research, we observed that prodigiosin production was increased by 1.9 times by adding yeast extract and POC as the nitrogen source, and xylose, derived from RSH, as the carbon source. This difference in pigment production can be attributed to the presence of inhibitors, such as hydroxyl-methyl furfural, phenolics, acetic acid, and furfural, that were formed during the acid pretreatment of rice straw.26
3.5 Effect of RSH sugar concentration on cell biomass and pigment production
The study revealed that as the incubation period increases, the microorganisms start utilizing sugars to produce biomass and the pigment, leading to a decline in the sugar concentrations. Initially, at 0 h, glucose and arabinose were present in minimal quantities, while the xylose concentration was 15.38 mg ml−1 which decreased to 4.6 mg ml−1 at 96 h. The maximum color value was observed at 72 h (1.55 CVU per mg). On the other hand, the quantity of cell mass demonstrated an upward trend with an increase in incubation time till 96 h as shown in Fig. 3.
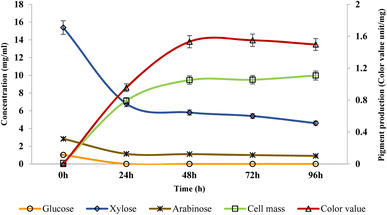 |
| | Fig. 3 Effect of RSH sugar concentration on biomass and pigment production. | |
3.6 Extraction and purification of the prodigiosin pigment
Following its synthesis by the bacterium, the pigment was extracted from the cellular biomass (Fig. 4a) to obtain a solution of red color (Fig. 4b). Subsequently, the pigment underwent a purification process involving column chromatography, wherein eight fractions were collected, and their absorption patterns were analysed. Fractions 6, 7, and 8 exhibited a peak at 535 nm, signifying the presence of the desired pigment that is prodigiosin, while the remaining fractions were impurities due to reduction of absorbance as shown in Fig. 4c. The results obtained in this study align with the findings reported.10 The eluted fractions of red color were subsequently collected and concentrated using a rotary evaporator at a temperature of 30 °C.
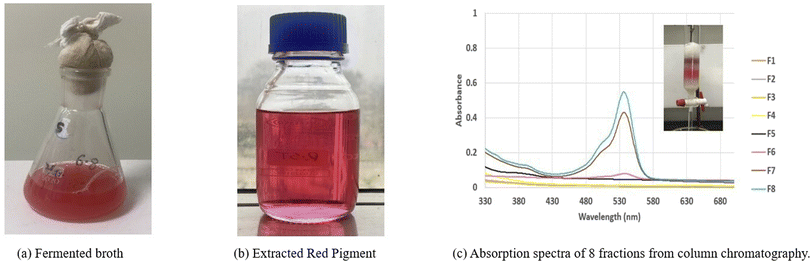 |
| | Fig. 4 (a) Fermented broth, (b) extracted red pigment and, (c) absorption spectra of 8 fractions eluted by column chromatography. | |
3.7 Confirmation of prodigiosin production and UV-vis spectral analysis
When subjected to varying pH levels, the pigment exhibited noticeable color changes, turning pink at pH 2 and yellow at pH 9, and remaining red at pH 7. This outcome validated the identification of the extracted pigment as prodigiosin. Upon dissolution of the red pigment in ethanol under pH 7 conditions, its absorption spectrum exhibited a maximal absorption peak at 535 nm. Further investigation of the pigment under different pH conditions revealed distinct maximal absorption values at 540 nm and 465 nm for pH 2 and pH 9, respectively. The absorption maximum's shift observed under alkaline pH conditions can be ascribed to the deprotonation of N atoms in pyrrole rings caused due to the alkaline solution.24
3.8 Thin-layer chromatography (TLC) and ultra-performance liquid chromatography (UPLC) analysis
A volume of 2 μl of purified pigment and the prodigiosin standard was placed onto a TLC card and separated using a solvent mixture composed of chloroform and methanol in a proportion of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Fig. 5B indicates that the standard and purified pigment displays a single spot with an Rf value of 0.61. Since the Rf value of the pigment and standard match, it can be concluded that the purified pigment is prodigiosin. Almost comparable results were reported29 with Rf = 0.59. UPLC analysis confirmed the appearance of prodigiosin in the sample, where only a solitary peak was observed with a retention time of 0.884 minutes at a wavelength of 535 nm (Fig. 5C). The sample was compared to the prodigiosin standard with a retention time of 0.843 minutes. The sample was 97.40% pure, according to quantitative UPLC analysis.
1. Fig. 5B indicates that the standard and purified pigment displays a single spot with an Rf value of 0.61. Since the Rf value of the pigment and standard match, it can be concluded that the purified pigment is prodigiosin. Almost comparable results were reported29 with Rf = 0.59. UPLC analysis confirmed the appearance of prodigiosin in the sample, where only a solitary peak was observed with a retention time of 0.884 minutes at a wavelength of 535 nm (Fig. 5C). The sample was compared to the prodigiosin standard with a retention time of 0.843 minutes. The sample was 97.40% pure, according to quantitative UPLC analysis.
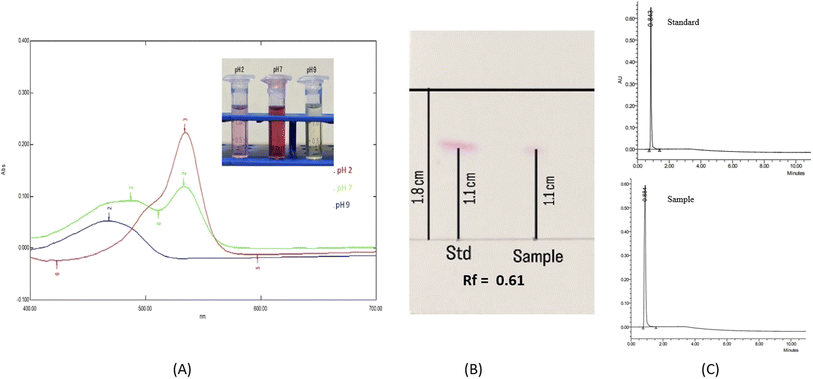 |
| | Fig. 5 (A) Absorption spectra of the red pigment dissolved in ethanol at pH values of 2, 7 and 9, (B) thin layer chromatogram of standard prodigiosin and the purified pigment and (C) UPLC analysis of the sample and standard prodigiosin. | |
3.9 GC-MS analysis of the pigment
The pigments were identified as prodigiosin through a mass spectrophotometry investigation, as indicated in Fig. 6. Analysis of the GC-MS chromatogram revealed that m/z values of the sample and standard prodigiosin were 324.96 and 324.90, respectively. The peak corresponds to the ions generated through the gas-induced dissociation of the parent molecule. Therefore, prodigiosin derivatives were detected, with GC-MS analysis showing peaks at 296.9 for 2-methyl-3-propyl-prodiginine, 310.92 for 2-methyl-3-butyl-prodiginine and 338.25 for 2-methyl-3-hexyl-prodiginine. Setiyano et al., 2020, reported the presence of similar derivative compounds.30 The peak at 310.92 represents an ion formed due to the methyl cleavage of prodigiosin's methoxy group.31
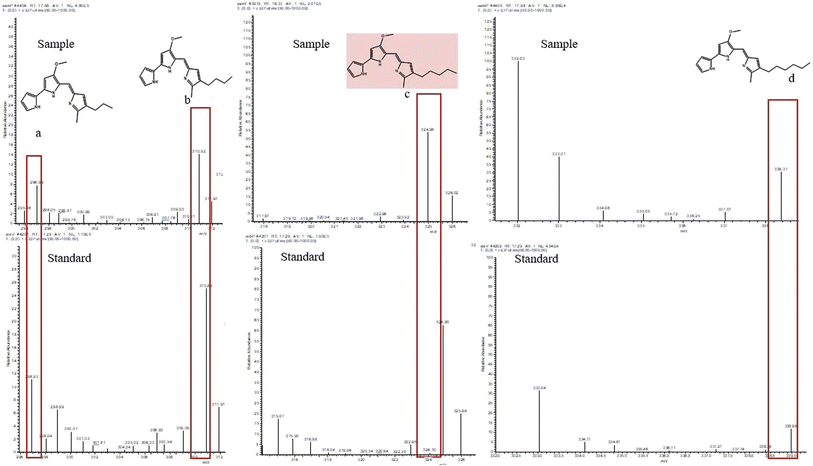 |
| | Fig. 6 Mass spectrometry of the prodigiosin standard and purified pigment produced by S. marcescens and their derivatives (a) 2-methyl-3-propyl prodiginine, (b) 2-methyl-3-butyl prodiginine, (c) prodigiosin, and (d) 2-methyl-3-hexyl-prodiginine. | |
3.10 FT-IR analysis
FT-IR analysis was performed on a purified sample (Fig. 7) of prodigiosin, and disclosed various functional groups corresponding to specific wave numbers. The analysis revealed the presence of strong bands at 2925.02 cm−1 (N–H stretching), 2855.2 cm−1 (C–H stretching), and 1711.87 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching). These functional groups of prodigiosin were consistent with the previous findings.24,32
O stretching). These functional groups of prodigiosin were consistent with the previous findings.24,32
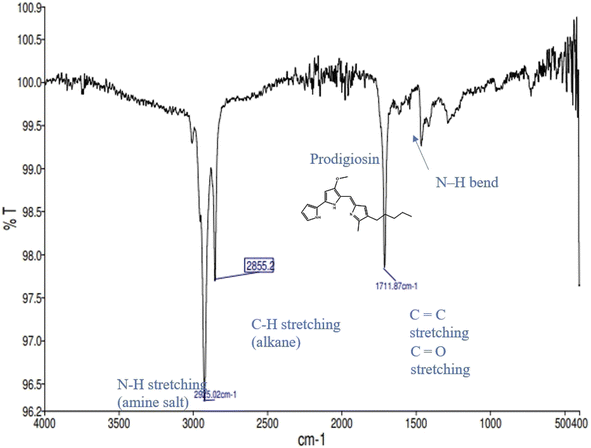 |
| | Fig. 7 Analysis of the extracted and purified prodigiosin pigment. | |
3.11 NMR analysis
Nuclear magnetic resonance spectroscopy (H-NMR) (CDCL3): δ (ppm) spectrum of prodigiosin represents the peaks corresponding to chemical shifts at 5.29–5.22 (m, 2H![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.24 (s, 3H, OCH3), 2.44–2.42 (t, 2H, CH2), 2.68–2.09 (s, 3H, CH3), 1.24–1.16 (m, 6H, CH2) and 0.60–0.87 (t, 3H, CH3) (Fig. 8). However, when comparing the spectra of pure prodigiosin, the occurrence of NH at 11.89 ppm was plainly apparent.22
CH), 3.24 (s, 3H, OCH3), 2.44–2.42 (t, 2H, CH2), 2.68–2.09 (s, 3H, CH3), 1.24–1.16 (m, 6H, CH2) and 0.60–0.87 (t, 3H, CH3) (Fig. 8). However, when comparing the spectra of pure prodigiosin, the occurrence of NH at 11.89 ppm was plainly apparent.22
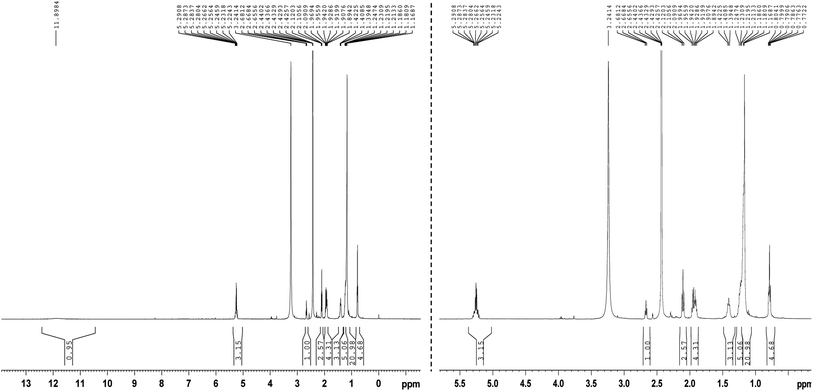 |
| | Fig. 8 NMR analysis of the purified prodigiosin pigment. | |
3.12 Antioxidant activity of the prodigiosin pigment
The primary aim of the DPPH radical scavenging technique was to determine the antioxidant activity of the extracted prodigiosin pigment, by measuring the percentage of inhibition. The data show a gradual increase in the antioxidant activity of prodigiosin with an increase in concentration as illustrated in Fig. 9. The results indicate that at concentrations of 50, 100, 150, 200, 250, and 300 μg ml−1 of pigment, the inhibition percentage was 77.7%, 80.95%, 84.52%, 95.2%, 95.6%, and 96.4%, respectively. However, it was observed that the antioxidant activity of prodigiosin exhibited a lower level compared to that of the standard ascorbic acid. Another study also showed a DPPH scavenging activity of around 60.05% with 10 μg ml−1 concentration of red pigment prodigiosin. Electron spin resonance (ESR) spectroscopy was also used, and revealed that 5 μg ml−1 and 10 μg ml−1 of prodigiosin showed a DPPH scavenging activity of 60% and 99%. respectively. The production of DPPH radicals that are then neutralised by H+ from an antioxidant may be the cause of the signal reduction in ESR. The protonated H+ of the C-ring in prodigiosin reacts with the DPPH molecule's N radical, and the deviation was recorded in the ESR spectrum. This proved the molecule's capacity to scavenge radicals.33.
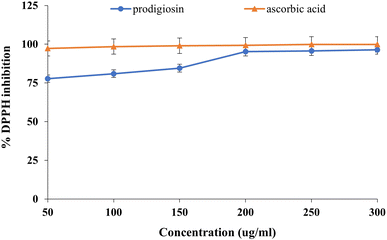 |
| | Fig. 9 Antioxidant activity of prodigiosin and standard ascorbic acid. | |
3.13 Economic analysis
In this study, an evaluation of the cost-effectiveness of prodigiosin production was conducted utilizing Serratia marcescens in media containing RSH, POC, and yeast extract, yielding 6100 mg L−1. Table 2 outlines the various input costs, including chemicals, raw materials, and electricity costs, which were used to determine the overall cost of prodigiosin production per mg. Based on a thorough review of literature reports, downstream processing charges were determined to be 65% of the total prodigiosin production cost. The analysis revealed that the total cost of sale for 1 mg of prodigiosin produced utilizing the cost-effective media was 8986.846 INR. Comparing this price to the commercial cost of prodigiosin from the international market, which is currently INR 56,422.88 per mg (Santa Cruz Biotechnology), the economic gain was calculated by subtracting the cost of the developed product from the commercial cost. The study found that the economic gain was $578.41(1USD = 82.01INR). Therefore, producing prodigiosin using a cost-effective substrate (RSH and POC) can result in significant economic benefits.
3.14 Solubility and morphology of the encapsulated pigment
A pigment to polysaccharide (pectin and maltodextrin) ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) was used to encapsulate the prodigiosin pigment. Although prodigiosin is hydrophobic in nature, the hydrophilic nature of the polysaccharides (coating material) facilitates the prodigiosin pigment to solubilize in water after lyophilization. It was also observed in the study that after its encapsulation it was soluble in both acidic and alkaline solutions but not in hexane, ethanol, methanol, or ethyl acetate (Fig. 9B). While the morphology of the encapsulated pigment was confirmed by SEM, the image shows the smooth spheres (Fig. 9C) of pectin and the maltodextrin coated pigment. Our findings are highly consistent with a prior study.25
1 (v/v) was used to encapsulate the prodigiosin pigment. Although prodigiosin is hydrophobic in nature, the hydrophilic nature of the polysaccharides (coating material) facilitates the prodigiosin pigment to solubilize in water after lyophilization. It was also observed in the study that after its encapsulation it was soluble in both acidic and alkaline solutions but not in hexane, ethanol, methanol, or ethyl acetate (Fig. 9B). While the morphology of the encapsulated pigment was confirmed by SEM, the image shows the smooth spheres (Fig. 9C) of pectin and the maltodextrin coated pigment. Our findings are highly consistent with a prior study.25
4. Conclusion
The present study substantiates the utilization of agricultural wastes as a substrate for prodigiosin pigment production using the self-isolated strain, Serratia marcescens CMS2. Statistical analysis was performed using response surface methodology and the addition of peanut de-oiled cake in sustainable media increases the yield of prodigiosin. Upon optimization, 0.5048 color value units per mg of prodigiosin were obtained and after complete purification and characterization, prodigiosin was confirmed with a purity of 97.40%. Furthermore, the purified pigment was encapsulated using polysaccharides for use as a delivery vehicle in food systems due to its water solubility. A significant economic benefit of $578.41 was realized by using the cost-effective growth medium created in this study, with a total scaled cost of 8986.84 INR for 1 mg of prodigiosin. These results establish that the developed product is cost effective and has the potential to be applicable in the commercial markets of pharmaceuticals, food and cosmetics.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors would like to express their gratitude to the Center of Innovative and Applied Bioprocessing (DBT-CIAB) for provided support and motivation throughout this research study. Additionally, the authors would like to thank Mr Umesh Singh for his help and technical assistance throughout the study. We also extend our gratitude to the CEO, CIAB, and staff members for their constant support.
References
- C. Sudhakar, C. Shobana, T. Selvankumar and K. Selvam, Prodigiosin production from Serratia marcescens strain CSK and their antioxidant, antibacterial, cytotoxic effect and in silico study of caspase-3 apoptotic protein, Biotechnol. Appl. Biochem., 2022, 69(5), 1984–1997 CrossRef CAS PubMed.
- R. Srimathi, R. Priya, M. Nirmala and A. Malarvizhi, Isolation, identification, optimization of prodigiosin pigment produced by Serratia marcescens and its applications, Int. J. Latest Eng. Manag. Res., 2017, 02(09), 11–21 Search PubMed.
- K. Papireddy, M. Smilkstein, J. X. Kelly, A. Shweta, S. M. Salem, M. Alhamadsheh, S. W. Haynes, G. L. Challis and K. A. Reynolds, Antimalarial activity of natural and synthetic prodiginines, J. Med. Chem., 2011, 54(15), 5296–5306 CrossRef CAS PubMed.
- J. Zhang, Y. Shen, J. Liu and D. Wei, Antimetastatic effect of prodigiosin through inhibition of tumor invasion, Biochem. Pharmacol., 2005, 69(3), 407–414 CrossRef CAS PubMed.
- R. D'Alessio, A. Bargiotti, O. Carlini, F. Colotta, M. Ferrari, P. Gnocchi, A. Isetta, N. Mongelli, P. Motta, A. Rossi and M. Rossi, Synthesis and immunosuppressive activity of novel prodigiosin derivatives, J. Med. Chem., 2000, 43(13), 2557–2565 CrossRef PubMed.
- H. Vaidya, N. Upasani and P. S. Wagh, Microbial Pigments: Natural Colorants and their Industrial Applications, Int. J. Curr. Microbiol. Appl. Sci., 2021, 05, 2319–7706 Search PubMed.
- T. Paul, P. Bhardwaj, A. Mondal, T. K. Bandyopadhyay, N. Mahata and B. Bhunia, Identification of Novel Protein Targets of Prodigiosin for Breast Cancer Using Inverse Virtual Screening Methods, Appl. Biochem. Biotechnol., 2023, 1–9 Search PubMed.
- R. G. Araújo, N. R. Zavala, C. Castillo-Zacarías, M. E. Barocio, E. Hidalgo-Vázquez, L. Parra-Arroyo, J. A. Rodríguez-Hernández, M. A. Martínez-Prado, J. E. Sosa-Hernández, M. Martínez-Ruiz and W. N. Chen, Recent advances in prodigiosin as a bioactive compound in nanocomposite applications, Molecules, 2022, 27(15), 4982 CrossRef PubMed.
- T. H. Nguyen, S. L. Wang and V. B. Nguyen, Recent advances in eco-friendly and scaling-up bioproduction of prodigiosin and its potential applications in agriculture, Agronomy, 2022, 12(12), 3099 CrossRef CAS.
- A. H. Faraag, A. I. El-Batal and H. H. El-Hendawy, Characterization of prodigiosin produced by Serratia marcescens strain isolated from irrigation water in Egypt, Nat. Sci., 2017, 15(5), 55–68 Search PubMed.
- A. Bhagwat and U. Padalia, Optimization of prodigiosin biosynthesis by Serratia marcescens using unconventional bioresources, J. Genet. Eng. Biotechnol., 2020, 18, 1–9 CrossRef PubMed.
- J. Chen, X. Lan, R. Jia, L. Hu and Y. Wang, Response Surface Methodology (RSM) Mediated Optimization of Medium Components for Mycelial Growth and Metabolites Production of Streptomyces alfalfae XN-04, Microorganisms, 2022, 10(9), 1854 CrossRef CAS PubMed.
- N. Anzum, F. I. Khan, M. Z. Hossain, M. N. Islam and M. L. Saha, Isolation and Identification of Pigment Producing Bacteria from the Ratargul Swamp Forest Soil, Dhaka Univ. J. Biol. Sci., 2022, 31(1), 1–8 CrossRef.
- S. Singh, D. Kaur, S. K. Yadav and M. Krishania, Process scale-up of an efficient acid-catalyzed steam pretreatment of rice straw for xylitol production by C. Tropicalis MTCC 6192, Bioresour. Technol., 2021, 320, 124422 CrossRef CAS PubMed.
- P. L. Haddix and R. M. Shanks, Prodigiosin pigment of Serratia marcescens is associated with increased biomass production, Arch. Microbiol., 2018, 200, 989–999 CrossRef CAS PubMed.
- S. J. Mohammed, K. J. Kadhum and K. waleed Hameed, Classical and statistical optimization of medium composition for promoting prodigiosin produced by local isolate of Serratia
marcescens, Al-Khawarizmi Eng. J., 2018, 14(4), 92–102 CrossRef.
- M. T. Rokade and A. S. Pethe, Isolation, Identification and Optimization Study of Prodigiosin from Serratia marcesces, Print Biosci. Discov., 2017, 8(3), 388–396 Search PubMed.
- R. Sehrawat, P. S. Panesar, T. L. Swer and A. Kumar, Response surface methodology (RSM) mediated interaction of media concentration and process parameters for the pigment production by Monascus purpureus MTCC 369 under solid state fermentation, Pigm. Resin Technol., 2017, 46(1), 14–20 CrossRef CAS.
- C. Lin, X. Jia, Y. Fang, L. Chen, H. Zhang, R. Lin and J. Chen, Enhanced production of prodigiosin by Serratia marcescens FZSF02 in the form of pigment pellets, Electron. J. Biotechnol., 2019, 40, 58–64 CrossRef CAS.
- N. N. Gerber and M. P. Lechevalier, Prodiginine (prodigiosin-like) pigments from Streptomyces and other aerobic Actinomycetes, Can. J. Microbiol., 1976, 22(5), 658–667 CrossRef CAS PubMed.
- N. Darshan and H. K. Manonmani, Prodigiosin inhibits motility and activates bacterial cell death revealing molecular biomarkers of programmed cell death, AMB Express, 2016, 6(1), 50 CrossRef CAS PubMed.
- D. Vijay, N. S. Alshamsi, Z. Moussa and M. K. Akhtar, Extraction of the Anticancer and Antimicrobial Agent, Prodigiosin, from Vibrio gazogenes PB1 and Its Identification by 1D and 2D NMR, Molecules, 2022, 27(18), 6030 CrossRef CAS PubMed.
- M. A. Othman, F. I. El-Zamik, M. I. Hegazy and A. S. Salama, Isolation and identification of egyptian strains of Serratia marcescens producing antibacterial and antioxidant prodigiosin pigment, Zagazig J. Agric. Res., 2019, 46(5), 1573–1582 CrossRef.
- T. Paul, T. K. Bandyopadhyay, A. Mondal, O. N. Tiwari, M. Muthuraj and B. Bhunia, A comprehensive review on recent trends in production, purification, and applications of prodigiosin, Biomass Convers. Biorefin., 2020, 1–23 CAS.
- S. Namazkar and W. A. Ahmad, Spray-dried prodigiosin from Serratia marcescens as a colorant, Biosci. Biotechnol. Res. Asia, 2013, 10(1), 69–76 CrossRef CAS.
- N. Panjiar, A. J. Mattam, S. Jose, S. Gandham and H. R. Velankar, Valorization of xylose-rich hydrolysate from rice straw, an agroresidue, through biosurfactant production by the soil bacterium Serratia nematodiphila, Sci. Total Environ., 2020, 729, 138933 CrossRef CAS PubMed.
- K. Vijayalakshmi and K. Jagathy, Production of prodigiosin from Serratia marcescens and its antioxidant and anticancer potential, Int. J. Adv. Res. Biol. Sci., 2016, 3, 75–88 CAS.
- V. S. Gondil, M. Asif and T. C. Bhalla, Optimization of physicochemical parameters influencing the production of prodigiosin from Serratia nematodiphila RL2 and exploring its antibacterial activity, 3 Biotech, 2017, 7, 1–8 CrossRef PubMed.
- L. L. JC, C. C. Maciel, H. S. Xavier, C. A. Alves da Silva and G. M. Campos-Takaki, Production and Toxicological Evaluation of prodigiosin from Serratia marcescens UCP/WFCC1549 on mannitol solid medium, Int. J. Appl. Res. Nat. Prod., 2014, 7(2), 32–38 Search PubMed.
- E. Setiyono, M. A. Adhiwibawa, R. Indrawati, M. N. Prihastyanti, Y. Shioi and T. H. Brotosudarmo, An Indonesian marine bacterium, Pseudoalteromonas rubra, produces antimicrobial prodiginine pigments, ACS omega, 2020, 5(9), 4626–4635 CrossRef CAS PubMed.
- S. Morgan, M. J. Thomas, K. M. Walstrom, E. C. Warrick and B. J. Gasper, Characterization of prodiginine compounds produced by a Vibrio species isolated from salt flat sediment along the Florida Gulf Coast, Fine Focus, 2017, 35–51 Search PubMed.
- Z. Alijani, J. Amini, M. Ashengroph and B. Bahramnejad, Antifungal activity of Serratia rubidaea mar61-01 purified prodigiosin against colletotrichum nymphaeae, the causal agent of strawberry anthracnose, J. Plant Growth Regul., 2022, 1 Search PubMed.
- K. V. Arivizhivendhan, M. Mahesh, R. Boopathy, S. Swarnalatha, R. Regina Mary and G. Sekaran, Antioxidant and antimicrobial activity of bioactive prodigiosin produces from Serratia marcescens using agricultural waste as a substrate, J. Food Sci. Technol., 2018, 55, 2661–2670 CrossRef CAS PubMed.
Footnote |
| † Contributed equally. |
|
| This journal is © The Royal Society of Chemistry 2023 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/v) for 24 h. The pH of pretreated rice straw hydrolysate (RSH) was 0.8; it was neutralized with barium hydroxide till pH 7 was attained and detoxified with 1.5% activated charcoal. The sugar content of hydrolysate before and after pretreatment was evaluated using HPLC (Agilent, HiPlex, Santa Clara, California, USA).
10 w/v) for 24 h. The pH of pretreated rice straw hydrolysate (RSH) was 0.8; it was neutralized with barium hydroxide till pH 7 was attained and detoxified with 1.5% activated charcoal. The sugar content of hydrolysate before and after pretreatment was evaluated using HPLC (Agilent, HiPlex, Santa Clara, California, USA).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0; v/v to 0
0; v/v to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; v/v) was run through the column with a flow rate of 1 ml min−1. The red-colored fractions that eluted out were collected, and prodigiosin was confirmed by using the absorption spectra. The red-colored fractions that contained prodigiosin were collected and concentrated by rotary evaporation at 30 °C.
2; v/v) was run through the column with a flow rate of 1 ml min−1. The red-colored fractions that eluted out were collected, and prodigiosin was confirmed by using the absorption spectra. The red-colored fractions that contained prodigiosin were collected and concentrated by rotary evaporation at 30 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and carried out on silica gel 60 F254 TLC-cards (Sigma-Aldrich) with dimensions of 20 × 20 cm.10 The standard and sample were compared and identified with the help of the retention factor (Rf).
1) and carried out on silica gel 60 F254 TLC-cards (Sigma-Aldrich) with dimensions of 20 × 20 cm.10 The standard and sample were compared and identified with the help of the retention factor (Rf).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) and a mass spectroscopy (GC-MS) detector. The oven temperature was maintained as follows: an initial temperature of 120 °C was held for 1 minute, and then increased at a rate of 10 °C min−1 up to 280 °C, and finally it was held at 280 °C for 5 minutes. The instrumental parameters were as follows: the mass transfer line temperature was set to 280 °C, electron energy was 70 eV, ion source temperature was 250 °C, and the mass range scanned was 50–1000 m/z.
10) and a mass spectroscopy (GC-MS) detector. The oven temperature was maintained as follows: an initial temperature of 120 °C was held for 1 minute, and then increased at a rate of 10 °C min−1 up to 280 °C, and finally it was held at 280 °C for 5 minutes. The instrumental parameters were as follows: the mass transfer line temperature was set to 280 °C, electron energy was 70 eV, ion source temperature was 250 °C, and the mass range scanned was 50–1000 m/z.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of pigment to pectin and maltodextrin) and subjected to stirring at a temperature of 70 °C until the ethanol was completely evaporated. The resulting suspension underwent homogenization for 10 minutes utilizing a sonicator (Elmasonic, S100H, Germany) after which it was freeze-dried. To evaluate the solubility of the freeze-dried particles, 5 mg were added to 5 ml of solvents including HCl (0.1 M), NaOH (0.1 M), acetone, n-hexane, ethyl acetate, methanol, ethanol and water. The morphology of the particles was observed using a scanning electron microscope (SEM) (Nikon, H600L) after attaching the powdered samples to carbon conductive tape and sputter-coating them with gold. Digital images were acquired using an excitation voltage of 10 kV.
1 ratio of pigment to pectin and maltodextrin) and subjected to stirring at a temperature of 70 °C until the ethanol was completely evaporated. The resulting suspension underwent homogenization for 10 minutes utilizing a sonicator (Elmasonic, S100H, Germany) after which it was freeze-dried. To evaluate the solubility of the freeze-dried particles, 5 mg were added to 5 ml of solvents including HCl (0.1 M), NaOH (0.1 M), acetone, n-hexane, ethyl acetate, methanol, ethanol and water. The morphology of the particles was observed using a scanning electron microscope (SEM) (Nikon, H600L) after attaching the powdered samples to carbon conductive tape and sputter-coating them with gold. Digital images were acquired using an excitation voltage of 10 kV.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Fig. 5B indicates that the standard and purified pigment displays a single spot with an Rf value of 0.61. Since the Rf value of the pigment and standard match, it can be concluded that the purified pigment is prodigiosin. Almost comparable results were reported29 with Rf = 0.59. UPLC analysis confirmed the appearance of prodigiosin in the sample, where only a solitary peak was observed with a retention time of 0.884 minutes at a wavelength of 535 nm (Fig. 5C). The sample was compared to the prodigiosin standard with a retention time of 0.843 minutes. The sample was 97.40% pure, according to quantitative UPLC analysis.
1. Fig. 5B indicates that the standard and purified pigment displays a single spot with an Rf value of 0.61. Since the Rf value of the pigment and standard match, it can be concluded that the purified pigment is prodigiosin. Almost comparable results were reported29 with Rf = 0.59. UPLC analysis confirmed the appearance of prodigiosin in the sample, where only a solitary peak was observed with a retention time of 0.884 minutes at a wavelength of 535 nm (Fig. 5C). The sample was compared to the prodigiosin standard with a retention time of 0.843 minutes. The sample was 97.40% pure, according to quantitative UPLC analysis.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching). These functional groups of prodigiosin were consistent with the previous findings.24,32
O stretching). These functional groups of prodigiosin were consistent with the previous findings.24,32
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.24 (s, 3H, OCH3), 2.44–2.42 (t, 2H, CH2), 2.68–2.09 (s, 3H, CH3), 1.24–1.16 (m, 6H, CH2) and 0.60–0.87 (t, 3H, CH3) (Fig. 8). However, when comparing the spectra of pure prodigiosin, the occurrence of NH at 11.89 ppm was plainly apparent.22
CH), 3.24 (s, 3H, OCH3), 2.44–2.42 (t, 2H, CH2), 2.68–2.09 (s, 3H, CH3), 1.24–1.16 (m, 6H, CH2) and 0.60–0.87 (t, 3H, CH3) (Fig. 8). However, when comparing the spectra of pure prodigiosin, the occurrence of NH at 11.89 ppm was plainly apparent.22
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) was used to encapsulate the prodigiosin pigment. Although prodigiosin is hydrophobic in nature, the hydrophilic nature of the polysaccharides (coating material) facilitates the prodigiosin pigment to solubilize in water after lyophilization. It was also observed in the study that after its encapsulation it was soluble in both acidic and alkaline solutions but not in hexane, ethanol, methanol, or ethyl acetate (Fig. 9B). While the morphology of the encapsulated pigment was confirmed by SEM, the image shows the smooth spheres (Fig. 9C) of pectin and the maltodextrin coated pigment. Our findings are highly consistent with a prior study.25
1 (v/v) was used to encapsulate the prodigiosin pigment. Although prodigiosin is hydrophobic in nature, the hydrophilic nature of the polysaccharides (coating material) facilitates the prodigiosin pigment to solubilize in water after lyophilization. It was also observed in the study that after its encapsulation it was soluble in both acidic and alkaline solutions but not in hexane, ethanol, methanol, or ethyl acetate (Fig. 9B). While the morphology of the encapsulated pigment was confirmed by SEM, the image shows the smooth spheres (Fig. 9C) of pectin and the maltodextrin coated pigment. Our findings are highly consistent with a prior study.25








