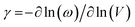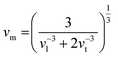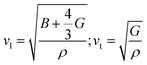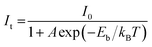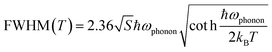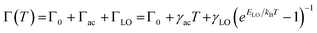Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Soft crystal lattice and large anharmonicity facilitate the self-trapped excitonic emission in ultrathin 2D nanoplates of RbPb2Br5†
Jayita
Pradhan
a,
Anustoop
Das
a,
Kaushik
Kundu
 a,
Chahat
a and
Kanishka
Biswas
a,
Chahat
a and
Kanishka
Biswas
 *ab
*ab
aNew Chemistry Unit, Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), Jakkur P. O., Bangalore 560064, India. E-mail: kanishka@jncasr.ac.in
bSchool of Advanced Materials, International Centre for Materials Science, Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), Jakkur P. O., Bangalore 560064, India
First published on 1st August 2022
Abstract
Self-trapping of excitons (STE) and concomitant useful broadband emission in low-dimensional metal halides occur due to strong electron–phonon coupling, which exhibit potential applications in optoelectronics and solid-state lighting. Lattice softness and high anharmonicity in the low-dimensional structure can lead to transient structural distortion upon photoexcitation that should promote the spatial localization or trapping of charge carriers, which is essential for STE. Herein, we report the ligand-assisted reprecipitation synthesis of ultrathin (∼3.5 nm) two-dimensional (2D) metal halide, RbPb2Br5 nanoplates (NPLs), which demonstrate highly Stokes shifted and broadband emission covering most parts of the visible to near IR range (500–850 nm) with a long-lived photoluminescence (PL) lifetime. The excitation wavelength independent emission and emission wavelength independent excitation spectra along with the analogous PL decay kinetics of bulk and NPLs suggest the intrinsic nature of broadband emission. The experimental low sound velocity (∼1090 m s−1) and associated low bulk and shear moduli (10.10 and 5.51 GPa, respectively) indicate the large anharmonicity and significantly soft lattice structure, which trigger the broadband STE emission in 2D NPLs of RbPb2Br5. Strong electron-longitudinal optical (LO) phonon coupling results in broadband STE emission in 2D RbPb2Br5 NPLs.
Introduction
Metal halides have fascinated researchers due to their countless stimulating properties and potential applications in optoelectronics, photodetection and photovoltaics.1–4 The ease of their colloidal synthesis in the form of intensely luminescent nanocrystals along with their tuneable optoelectronic properties has attracted researchers from diverse fields.3–6 Recently, low-dimensional all-inorganic metal halides with two-dimensional (2D) layered structures such as APb2X5 (e.g. A = Cs; X = Cl, Br, I) have been examined as a noteworthy case.7–9 Among them, CsPb2Br5 with a layered structure turned into an emergent material, in which a Cs cation is sandwiched between [Pb2Br5]− polyhedra,9 and exhibited high photoluminescence quantum yield (PLQY), colour tunability, and enhanced stability in humid environments and at high temperatures.7,8 However, the limited compositional variability of AB2X5 halide nanostructures is inadequate for exploring their optoelectronic properties in detail. Only a few potential alternatives are available to replace Cs in the all-inorganic AB2X5-type structures.10,11 Rb, as a neighbouring alkali metal of Cs, is suitable to form AB2X5-type metal halides. However, layered RbPb2Br5 has rarely been investigated except for a few electronic structural studies on its single crystals.11,12 Simple synthesis and fundamental understanding of the optical properties of RbPb2Br5 in the form of 2D nanoplates/nanosheets or nanocrystals are still elusive.Dimensionality reduction facilitates the disconnection of the metal halide octahedra and the lattice becomes progressively softer. In such systems, self-trapped excitons (STEs) form due to local structural distortion upon photoexcitation.13 Strong electron–phonon coupling causes elastic lattice deformation and consequent STE results in broadband emission with a large Stokes shift, which showed promise for solid state lighting.14 Thereby, it would be exciting to study the optical properties and electron–phonon coupling in 2D layered RbPb2Br5 both in the form of bulk and ultrathin nanostructures.
Herein, we present simple ligand-assisted reprecipitation (LARP) synthesis of 2D ultrathin few-layer nanoplates (NPLs) of layered metal halide, RbPb2Br5, which displays broadband self-trapped excitonic (STE) emission. We have further synthesized controlled bulk polycrystals of RbPb2Br5 by solvent free mechanochemistry. Photoluminescence excitation (PLE) and PL spectra of RbPb2Br5 NPLs and bulk polycrystals are independent of emission and excitation wavelengths, which reveal that broadband emission originates from relaxation of the same excited state. We have observed a significant Stokes shifted (∼400 nm) emission with a long PL lifetime in the microsecond (μs) range. The temperature-dependent (15–350 K) PL studies confirm the strong electron-longitudinal optical (LO) phonon coupling in 2D RbPb2Br5 NPLs. Large lattice anharmonicity and low sound velocity (∼1090 m s−1) indicate the structure to be elastically very soft with significantly low bulk and shear moduli. The soft crystal lattice and strong electron–phonon coupling trigger the broadband STE emission in 2D NPLs of RbPb2Br5.
Results and discussion
We have synthesized nanoplates (NPLs) of RbPb2Br5via the ligand-assisted reprecipitation (LARP) method at room temperature (Scheme S1a, ESI†). Typically, a dimethyl sulfoxide (DMSO) solution consisting of RbBr and PbBr2 at a stoichiometric molar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) along with organic ligands [e.g., oleic acid (OA) and oleyamine (OLAm)] was swiftly injected into chloroform under vigorous stirring. After 20 s, the as-synthesized product was collected and washed with chloroform for further measurement. Further, we have synthesized micrometre sized particles (∼1 g) of RbPb2Br5 by implementing solid-state mechanochemical grinding with appropriate stoichiometric ratios of RbBr and PbBr2 (Scheme S1b, ESI†).
2) along with organic ligands [e.g., oleic acid (OA) and oleyamine (OLAm)] was swiftly injected into chloroform under vigorous stirring. After 20 s, the as-synthesized product was collected and washed with chloroform for further measurement. Further, we have synthesized micrometre sized particles (∼1 g) of RbPb2Br5 by implementing solid-state mechanochemical grinding with appropriate stoichiometric ratios of RbBr and PbBr2 (Scheme S1b, ESI†).
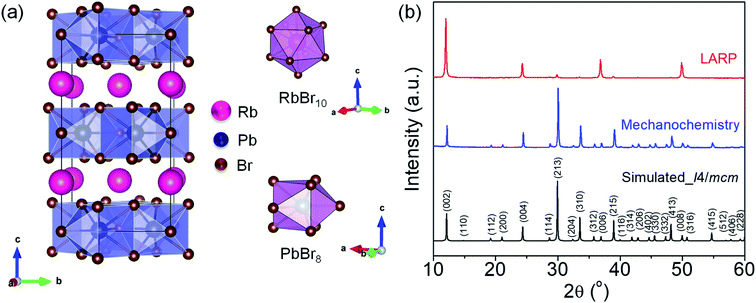
RbPb2Br5 adopts a tetragonal crystal structure with the I4/mcm space group, which consists of intercalated Rb+ ions sandwiched in between the 2D layers of [Pb2Br5]− polyhedral units (Fig. 1a). In the tetragonal unit cell, the RbBr10 polyhedron exhibits a bicapped square antiprism unit, while the PbBr8 polyhedron shows a bicapped trigonal prism coordination (Fig. 1a).15 The powder X-ray diffraction (PXRD) patterns of the as-synthesized bulk and NPL samples have been indexed to the tetragonal phase of RbPb2Br5 (space group I4/mcm) (Fig. 1b).15 The PXRD pattern of the as-synthesized NPLs obtained from the LARP route shows diffraction peaks corresponding to mainly (002), (004), (006) and (008) planes, which clearly indicates the 2D growth along the <110> direction. The characteristic layered peak appears at 2θ = 11.66° corresponding to the (002) plane in both RbPb2Br5 bulk polycrystals and NPLs. We have studied the environmental and the thermal stabilities of the mechanochemically synthesized bulk RbPb2Br5 powders. The bulk polycrystals display excellent crystallinity and stability even after 250 days of being kept under ambient conditions (Fig. S1a, ESI†), whereas thermogravimetric analysis (TGA) reveals good thermal stability up to 465 °C (Fig. S1b, ESI†). Additionally, we have checked the thermal stability of the NPLs which reveals an analogous stability profile to that of the bulk polycrystals (Fig. S1c, ESI†). In the case of NPLs, we noticed an early weight loss of ∼2% between 25 °C and 235 °C (inset, Fig. S1c, ESI†). This is attributed to the removal of surface capping organic ligands (oleic acid and oleylamine).16,17
We have studied the PXRD patterns of the as-synthesized RbPb2Br5via the LARP method with and without the addition of capping ligands (OA and OLAm), and the comparison of the PXRD patterns reveals that the directional growth takes place only in the presence of OA and OLAm (Fig. S2, ESI†). The presence of capping ligands has been further verified by Fourier transform infrared (FTIR) spectroscopy (Fig. S3, ESI†). The FTIR spectrum of the as-synthesized NPLs exhibits the symmetric and asymmetric stretching vibrations of –CH2 and –CH3 moieties in the range of 2850–2950 cm−1, which can be assigned to the oleyl group [i.e., CH3(CH2)7–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–(CH2)8−] from both OA and OLAm.18,19 The –CH2 bending modes at ∼1460 and ∼1374 cm−1 have also appeared from the hydrocarbon chains of OA and OLAm. Additionally, the spectrum of NPL solution shows a typical vibrational signature of the carboxylic acid group (νC
CH–(CH2)8−] from both OA and OLAm.18,19 The –CH2 bending modes at ∼1460 and ∼1374 cm−1 have also appeared from the hydrocarbon chains of OA and OLAm. Additionally, the spectrum of NPL solution shows a typical vibrational signature of the carboxylic acid group (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at 1722 cm−1 along with the νN–H stretching mode at ∼3300 cm−1 from the amine moiety. The peak broadening of N–H stretching mode indicates the successful coordination of OLAm to the surface of nanostructures (Fig. S3, ESI†).18
O) at 1722 cm−1 along with the νN–H stretching mode at ∼3300 cm−1 from the amine moiety. The peak broadening of N–H stretching mode indicates the successful coordination of OLAm to the surface of nanostructures (Fig. S3, ESI†).18
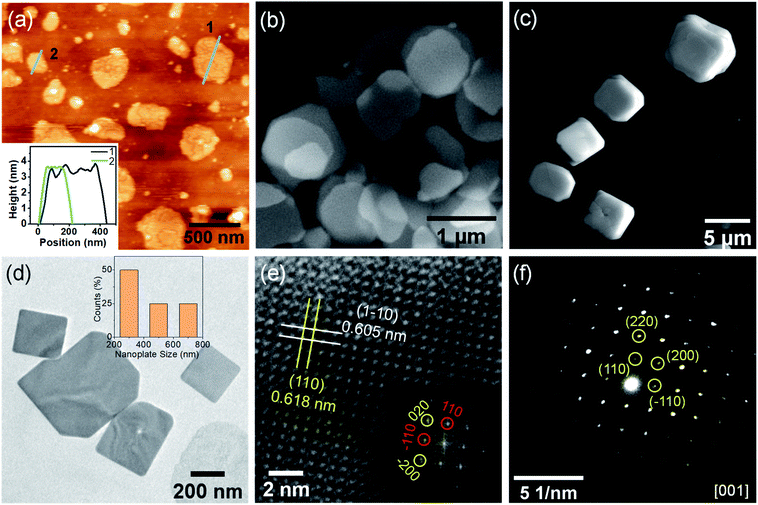
The thickness of the NPLs was measured by atomic force microscopy (AFM) and the height profile measurements reveal a thickness of ∼3.5 nm of the NPLs (Fig. 2a), which corresponds to the 3–4 layers of RbPb2Br5. Further, the morphology of NPLs was explored by field emission scanning electron microscopy (FESEM), which further reveals thin 2D nanoplate morphology (Fig. 2b). The lateral dimension of NPLs ranges between ∼300–750 nm. The FESEM image of bulk RbPb2Br5 polycrystals shows the truncated cuboidal morphology with a particle size of 5–8 μm (Fig. 2c). Furthermore, the morphology of the LARP-synthesized sample without capping agent reveals a similar truncated cuboid with a particle size of 1.5–3 μm (Fig. S4, ESI†). Thereby, the results confirm that the capping ligands facilitate the directional growth of 2D ultrathin NPLs during LARP synthesis.
The transmission electron microscopy (TEM) image of the as-synthesized RbPb2Br5 obtained by the LARP method in the presence of OA and OLAm confirms the 2D NPL morphology (Fig. 2d), which is consistent with the directional growth revealed by the PXRD pattern (Fig. 1b). The particle size distribution histogram shows a broad size distribution of the NPLs (Fig. 2d, inset). High-resolution TEM (HRTEM) analysis reveals the single-crystalline nature of the NPLs (Fig. 2e). The tetragonal I4/mcm symmetry demands interplanar spacings for the (hkl) and (h-kl) family of lattice planes to be identical. Interestingly, the HRTEM analysis reveals that d110 and d1–10 are non-equivalent by 0.13 Å. The corresponding FFT analysis also exhibits a deviation of 0.11 Å between (110) and (−110) interplanar lattice spacings. Additionally, the (200) and (020) planes show a deviation of 0.08 Å in the interplanar distances (Fig. 2e, inset). Furthermore, we have carried out TEM analysis for bulk RbPb2Br5 (Fig. S5, ESI†) and found similar results. A careful inspection of the HRTEM image of the bulk sample reveals that the d110 and d1–10 are non-equivalent by 0.09 Å. The corresponding FFT analysis exhibits a deviation in (110) and (−110) interplanar lattice spacings by 0.07 Å (Fig. S5b, ESI†). These results show that the lattice parameters a and b are no longer equal on a local scale for NPLs and for bulk samples and signify the presence of intrinsic local distortion in the RbPb2Br5 lattice.20 This local distortion in the average tetragonal structure may give rise to the strong lattice anharmonicity,21,22 which will have an impact on the luminescence properties. The selected area electron diffraction (SAED) pattern of the RbPb2Br5 NPLs additionally confirms the single crystalline nature (Fig. 2f). The diffraction spots associated with the (110), (220) and (200) crystal planes can be indexed to the [001] zone axis of tetragonal RbPb2Br5.
To understand the lattice dynamics in 2D RbPb2Br5 NPLs, we have performed Raman spectroscopic investigation at room temperature. The distinctive vibrational modes appear from the PbBr8 polyhedron in RbPb2Br5 NPLs (Fig. 3a). Two strong Raman signals at ∼74 and 130 cm−1 are likely to emerge from the vibrations in the PbBr8 unit.23 Deconvolution of the peaks reveals the splitting of each peak into two components, which appear at 62.8, 73.6, 130.4 and 141.5 cm−1. This can be ascribed to the different types of Pb–Br bonding strength in the PbBr8 polyhedron corresponding to two different bond lengths, 3.15 Å (two longer bonds) and 2.87 Å (six shorter bonds), respectively.24 While the covalent bond distance between Pb and Br is about 2.66 Å, close to the sum of covalent radii of Pb (1.46 Å) and Br (1.20 Å),25 the Pb–Br ionic interaction distance is found to be 3.25 Å.26 The Rb–Br distance is 3.59 Å in RbPb2Br524 which is close to the sum of the ionic radii of Rb+ (1.66 Å) and Br− (1.96 Å).26 Thereby, RbPb2Br5 exhibits a chemical bonding hierarchy which induces anharmonicity and softness in the crystal lattice.
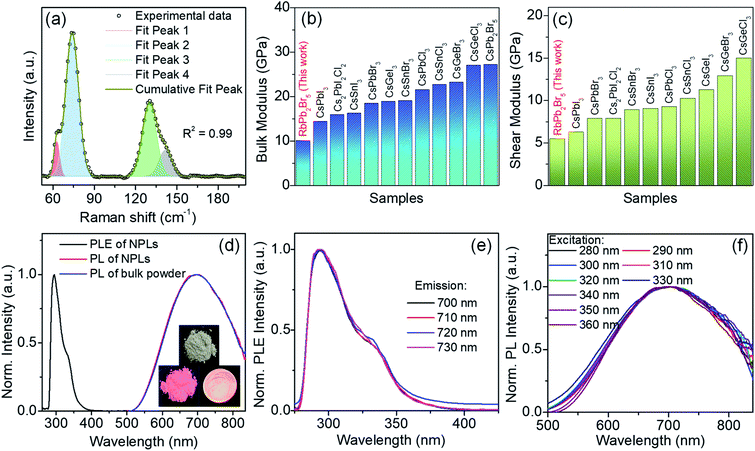 | ||
| Fig. 3 (a) Raman spectrum of RbPb2Br5 NPLs. Comparison of (b) bulk modulus and (c) shear modulus of RbPb2Br5 NPLs with several other all-inorganic metal halide perovskites such as CsPbX3 (X = Cl, Br, I),26 CsGeX3,26 CsSnX3 (X = Br, Cl),26 Cs2PbI2Cl2,27 CsSnI3,28 and CsPb2Br5.29 (d) PLE spectrum (black line) of RbPb2Br5 NPLs and PL spectra of NPLs (red line) and bulk polycrystals (blue line) of RbPb2Br5 at room temperature. Inset shows the images of bulk polycrystals under ambient light (top), UV light irradiation at 365 nm on bulk polycrystals and a drop cast film of NPLs (bottom). Wavelength-dependent (e) excitation and (f) emission spectra of RbPb2Br5 NPLs at room temperature. | ||
Quantitatively, the anharmonicity can be evaluated using the Grüneisen parameter (γ), which is defined as:27
which exhibits a low value of 1090 m s−1. The high value of γ in RbPb2Br5 NPLs represents the presence of high lattice anharmonicity.27 The elastic moduli of the sample were estimated by using the following equations:
where B and G are the bulk and shear moduli respectively. ρ refers to the density of the sample. The estimated bulk and shear moduli (10.10 and 5.51 GPa, respectively) are found to be lower compared to other all-inorganic halide perovskites revealing a significantly softer lattice (Fig. 3b and c).29,31–33 The presence of lattice anharmonicity and a soft crystal structure is important for a system to show STE emission.34
The optical properties of the RbPb2Br5 bulk polycrystals and NPL solution in chloroform were investigated by electronic absorption and PL spectroscopy. Bulk RbPb2Br5 polycrystals show a band gap of 3.51 eV (Fig. S6a, ESI†). The absorption spectrum of RbPb2Br5 NPL solution shows both excitonic and band edge absorption features at 3.55 eV (350 nm) and 4.27 eV (290 nm), respectively, with a long absorption tail in the lower energy region (Fig. S6b, ESI†).14,35,36 The probable presence of sub-bandgap state transition might be responsible for the extended absorption tail in the lower energy region, which may have originated from the surface defects of the NPLs.37,38 The excitonic peak is a typical observation in 2D halide perovskites.35 The PLE spectrum of the NPLs exhibits a distinct peak at 294 nm corresponding to the absorption onset (Fig. 3d). At room temperature, a broadband emission ranging from ∼500 nm to the near infrared region (∼850 nm) is observed for both the NPL solution and bulk polycrystals centred at ∼695 nm, when excited at 370 nm (Fig. 3d). The broad emission centred at ∼695 nm is consistent with the pinkish white colour of both the bulk polycrystals and NPL thin film when irradiated with a 365 nm UV lamp (inset of Fig. 3d). Significant Stokes shifted PL (340–400 nm) with a large full width at half-maximum (FWHM) of 275 nm is observed for both RbPb2Br5 NPLs and bulk polycrystals (Fig. 3d, and S7, ESI†). The large Stokes shift of over 340 nm is desirable for light-emitting applications as it greatly reduces the self-absorption.14,39 In general, such broadband and highly Stokes shifted emission does not arise from direct band-to-band recombination. For instance, in alkali halides and organic–inorganic hybrid halide crystals the broadband emission is due to the strong electron–phonon interaction in the deformable lattice and associated self-trapped excitonic (STE) recombination.14,34,40–43 The large Stokes shift is caused by the energy loss in the process of excited state structural distortion.34
The as-synthesized RbPb2Br5 NPL sample shows an absolute PLQY of ∼13%, which is reasonably higher compared to that of the earlier reported broadband STE-emitting Pb based 2D halide perovskites (Table S2, ESI†). Similarly, few other all-inorganic STE-emitting halide materials, Cs4SnBr6 and Cs2AgBiCl6 exhibited PLQY in the range of ∼5 to 15%.41,44 The identical shape and features of the PLE spectra collected across the broad emission range as well as the excitation independent PL spectra indicate the broadband emission is originating from the relaxation of the same excited state (Fig. 3e and f and S7a and b, ESI†).45 Furthermore, the identical PL emissions from both bulk and NPL samples rule out the involvement of defect states, which also indicates the existence of STE states.
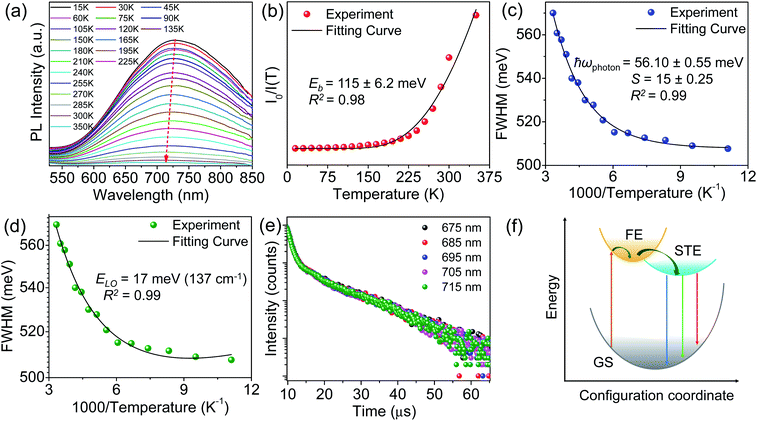
To probe the underlying mechanism of broadband emission in RbPb2Br5, temperature-dependent PL spectra were measured for the NPLs (Fig. 4a). Temperature-dependent PL spectra measured in the range of 15–350 K show a monotonous decrease in PL intensity and an increase in FWHM with increasing temperature indicating the increased probability of nonradiative relaxation and stronger electron–phonon coupling at higher temperature, respectively.36,42,46 Simultaneously, a slight blue shift in the emission peak is observed with the increase in temperature (Fig. 4a). Such a blueshift in PL for RbPb2Br5 NPLs can be ascribed to the slight shift in the relative positions of conduction and valence bands as temperature increases.47,48 No higher energy peak is observed at lower temperatures. Only one Gaussian-shaped broadband emission indicates that the self-trapping barrier is low, and the excitons are easily trapped.43 The thermal quenching behaviour is well fitted using the Arrhenius model,
To get an estimation of the electron–phonon coupling in RbPb2Br5 NPLs, we further determined the Huang–Rhys electron–phonon coupling parameter (S) and effective phonon energy (ħωphonon) using the Toyozawa model,42,52
From the temperature dependent PL spectra, we can also evaluate the effective vibrational mode that couples with the electronic transition resulting in STE emission.14 Thereby, we fitted the FWHM to the Fröhlich longitudinal optical (LO) phonon broadening model developed by Rudin et al. (Fig. 4d) to extract the LO phonon energy using the following equation,56,57
Furthermore, we have carried out time-resolved PL (TRPL) measurements to gain more insight into the excitonic recombination dynamics in RbPb2Br5 NPLs. The PL decay curve is fitted using the double exponential function with an average lifetime (τavg) of 10.03 μs (Fig. 4e and Table S4, ESI†). Additionally, we have performed the TRPL measurement of the NPL sample by monitoring across the wide emission range (675–715 nm), which shows a similar nature of decay kinetics (Fig. 4e) and infers an identical origin of broad emission. The bulk polycrystalline sample exhibited similar decay kinetics to the NPLs with an τavg of 8.82 μs (Fig. S8 and Table S4, ESI†). This observation suggests that the surface-to-bulk ratio has a minor impact on the PL decay kinetics.61 These results endorse the intrinsic characteristics of PL emission.61 Together with these findings, the assignment of the highly Stokes shifted broad PL emission in RbPb2Br5 can be ascribed to the phonon-assisted recombination of STEs. The excitation and recombination processes in RbPb2Br5 are schematically illustrated in Fig. 4f. Following the photon absorption, an electron is promoted to the excited state, and after its thermalization it gets self-trapped and localizes on Pb2+, which probably combines with another Pb2+ to form a quasi-molecule Pb23+.10,14 Simultaneously, the hole may localize on Br−, which dimerizes with adjacent Br− to form a molecular ion Br2−.10,14 This process leads to transient lattice distortion which is facilitated by a soft lattice and high anharmonicity. The self-trapped electron and hole then recombine together emitting broadband emission.
Finally, to achieve the band gap tuning in this material, the pure phase analogues of RbPb2Br5 were synthesized by the halide mixing strategy via mechanochemistry. The PXRD patterns of RbPb2Cl2Br3 and RbPb2Br4I reveal that both the materials possess similar tetragonal crystal structures to RbPb2Br5 (Fig. S9a, ESI†). The 2θ value shifts to a lower angle in RbPb2Br4I whereas it shifts to a higher angle in RbPb2Cl2Br3 in comparison to pure RbPb2Br5 because of the substitution of Br− by larger I− and smaller Cl− ions, respectively (Fig. S9b, ESI†). The halide mixing effect is also reflected in optical spectroscopy. We have been able to tune the band gap in the 2.9–3.6 eV range by adjusting the halide composition in bulk RbPb2Br5. The band gap increases to 3.62 eV with Cl− incorporation, while it decreases to 2.94 eV with I− incorporation in comparison to the pure bromide system (3.51 eV) (Fig. S10, ESI†). The PL peak position also red shifts from RbPb2Cl2Br3 (∼685 nm) to RbPb2Br5 (∼695 nm) to RbPb2Br4I (∼765 nm) (Fig. S10b, SI†). Furthermore, we have synthesized the phase pure sample of the Cs-analogue (i.e., CsPb2Br5) by mechanochemical grinding, as confirmed from its PXRD pattern (Fig. S11a, ESI†), which can also be indexed based on the tetragonal structure (space group I4/mcm).15 The solid-state electronic absorption spectrum of as synthesized CsPb2Br5 (Fig. S11b, ESI†) exhibits an absorption edge at ∼3.54 eV which is consistent with the previous reports.9,62 The PL spectrum of bulk CsPb2Br5 polycrystals (Fig. S11d, ESI†) revealed a narrow emission centred at 550 nm when excited at 350 and 380 nm.62,63 The narrow emission line for CsPb2Br5 in comparison to that for RbPb2Br5 might be ascribed to the suppression of electron–phonon coupling in CsPb2Br5.64 Thus, by changing only the A cation in between 2D [Pb2Br5]− polyhedral units, the nature of PL emission varied immensely and subsequently, this exciting observation needs further detailed investigations.
Conclusions
In summary, we have synthesized ultrathin NPLs of 2D layered RbPb2Br5 and controlled bulk polycrystals by simple ligand assisted reprecipitation and mechanochemical grinding, respectively. The NPLs display a highly Stokes shifted (∼400 nm) broadband PL emission with no overlap between their excitation and emission spectra. Furthermore, the NPLs exhibit a long lifetime component in the microsecond range. A soft crystal lattice along with high anharmonicity boosts structural distortion during photoexcitation where electrons can be easily trapped. As a result, 2D all-inorganic metal halide RbPb2Br5 exhibits strong electron–phonon coupling and STE with broadband emission. Furthermore, we have tuned the band gap in the range of ∼2.9–3.6 eV by adjusting the halide composition in the soft lattice of RbPb2Br5. Thus, our findings give a new direction towards the fundamental understanding of the emission process in low dimensional halides with the influence of chemical bonding, lattice anharmonicity and lattice dynamics which show potential prospects in a new generation of solid-state lighting and displays.Data availability
All data are available in the manuscript and in the ESI.†Author contributions
K. B. conceived the idea and designed the study. J. P., A. D., K. K. and K. B. carried out the synthesis, structural, optical, and sound velocity measurements, and other analyses. C. helped in the synthesis of NPLs. J. P. wrote the first draft; and A. D., K. K. and K. B. contributed to editing the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
K. B. acknowledges support from the Swarna-Jayanti fellowship, Science and Engineering Research Board (SERB) (SB/SJF/2019-20/06). J. P. acknowledges support from the SERB National Post-doctoral Fellowship (PDF/2021/000118). A. D. thanks CSIR for a fellowship. We thank Paribesh Acharyya for useful discussion.References
- C. C. Stoumpos and M. G. Kanatzidis, Acc. Chem. Res., 2015, 48, 2791–2802 CrossRef CAS PubMed.
- N. Pradhan, ACS Energy Lett., 2019, 4, 1634–1638 CrossRef CAS.
- Q. A. Akkerman, G. Rainò, M. V. Kovalenko and L. Manna, Nat. Mater., 2018, 17, 394–405 CrossRef CAS PubMed.
- A. Dey, J. Ye, A. De, E. Debroye, S. K. Ha, E. Bladt, A. S. Kshirsagar, Z. Wang, J. Yin, Y. Wang, L. N. Quan, F. Yan, M. Gao, X. Li, J. Shamsi, T. Debnath, M. Cao, M. A. Scheel, S. Kumar, J. A. Steele and a. et, ACS Nano, 2021, 15, 10775–10981 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- A. Swarnkar, R. Chulliyil, V. K. Ravi, M. Irfanullah, A. Chowdhury and A. Nag, Angew. Chem., Int. Ed., 2015, 54, 15424–15428 CrossRef CAS PubMed.
- K.-H. Wang, L. Wu, L. Li, H.-B. Yao, H.-S. Qian and S.-H. Yu, Angew. Chem., Int. Ed., 2016, 55, 8328–8332 CrossRef CAS PubMed.
- L. Ruan, W. Shen, A. Wang, A. Xiang and Z. Deng, J. Phys. Chem. Lett., 2017, 8, 3853–3860 CrossRef CAS PubMed.
- P. Acharyya, P. Pal, P. K. Samanta, A. Sarkar, S. K. Pati and K. Biswas, Nanoscale, 2019, 11, 4001–4007 RSC.
- I. N. Ogorodnikov, A. A. Smirnov, V. A. Pustovarov, L. I. Isaenko, A. Y. Tarasova and V. Y. Yakovlev, Phys. Solid State, 2009, 51, 1640–1648 CrossRef CAS.
- A. Y. Tarasova, L. I. Isaenko, V. G. Kesler, V. M. Pashkov, A. P. Yelisseyev, N. M. Denysyuk and O. Y. Khyzhun, J. Phys. Chem. Solids, 2012, 73, 674–682 CrossRef CAS.
- A. A. Lavrentyev, B. V. Gabrelian, V. T. Vu, N. M. Denysyuk, P. N. Shkumat, A. Y. Tarasova, L. I. Isaenko and O. Y. Khyzhun, J. Phys. Chem. Solids, 2016, 91, 25–33 CrossRef CAS.
- J. Yin, J.-L. Brédas, O. M. Bakr and O. F. Mohammed, Chem. Mater., 2020, 32, 5036–5043 CAS.
- M. D. Smith and H. I. Karunadasa, Acc. Chem. Res., 2018, 51, 619–627 CAS.
- Z. Zhang, Y. Zhu, W. Wang, W. Zheng, R. Lin and F. Huang, J. Mater. Chem. C, 2018, 6, 446–451 RSC.
- Z.-J. Li, E. Hofman, J. Li, A. H. Davis, C.-H. Tung, L.-Z. Wu and W. Zheng, Adv. Funct. Mater., 2018, 28, 1704288 CrossRef.
- J. Li, L. Xu, T. Wang, J. Song, J. Chen, J. Xue, Y. Dong, B. Cai, Q. Shan, B. Han and H. Zeng, Adv. Mater., 2017, 29, 1603885 CrossRef PubMed.
- J. Pradhan, P. Moitra, Umesh, B. Das, P. Mondal, G. S. Kumar, U. K. Ghorai, S. Acharya and S. Bhattacharya, Chem. Mater., 2020, 32, 7159–7171 CrossRef CAS.
- K. Kundu, P. Acharyya, K. Maji, R. Sasmal, S. S. Agasti and K. Biswas, Angew. Chem., Int. Ed., 2020, 59, 13093–13100 CrossRef CAS PubMed.
- T. Ghosh, M. Samanta, A. Vasdev, K. Dolui, J. Ghatak, T. Das, G. Sheet and K. Biswas, Nano Lett., 2019, 19, 5703–5709 CrossRef CAS PubMed.
- M. Dutta, K. Pal, M. Etter, U. V. Waghmare and K. Biswas, J. Am. Chem. Soc., 2021, 143, 16839–16848 CrossRef CAS PubMed.
- M. Dutta, M. V. D. Prasad, J. Pandey, A. Soni, U. V. Waghmare and K. Biswas, Angew. Chem., Int. Ed., 2022, 134, e202200071 Search PubMed.
- K. Rademaker, W. F. Krupke, R. H. Page, S. A. Payne, K. Petermann, G. Huber, A. P. Yelisseyev, L. I. Isaenko, U. N. Roy, A. Burger, K. C. Mandal and K. Nitsch, J. Opt. Soc. Am. B, 2004, 21, 2117–2129 CrossRef CAS.
- D. Becker and H. P. Beck, Z. Anorg. Allg. Chem., 2004, 630, 1924–1932 CrossRef CAS.
- B. Cordero, V. Gómez, A. E. Platero-Prats, M. Revés, J. Echeverría, E. Cremades, F. Barragán and S. Alvarez, Dalton Trans., 2008, 2832–2838 RSC.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- M. Dutta, D. Sarkar and K. Biswas, Chem. Commun., 2021, 57, 4751–4767 RSC.
- J. Feng, APL Mater., 2014, 2, 081801 CrossRef.
- H. Xie, S. Hao, J. Bao, T. J. Slade, G. J. Snyder, C. Wolverton and M. G. Kanatzidis, J. Am. Chem. Soc., 2020, 142, 9553–9563 CrossRef CAS PubMed.
- A. C. Ferreira, A. Létoublon, S. Paofai, S. Raymond, C. Ecolivet, B. Rufflé, S. Cordier, C. Katan, M. I. Saidaminov, A. A. Zhumekenov, O. M. Bakr, J. Even and P. Bourges, Phys. Rev. Lett., 2018, 121, 085502 CrossRef CAS PubMed.
- P. Acharyya, T. Ghosh, K. Pal, K. Kundu, K. Singh Rana, J. Pandey, A. Soni, U. V. Waghmare and K. Biswas, J. Am. Chem. Soc., 2020, 142, 15595–15603 CrossRef CAS PubMed.
- M. Roknuzzaman, K. Ostrikov, H. Wang, A. Du and T. Tesfamichael, Sci. Rep., 2017, 7, 14025 CrossRef PubMed.
- Z. Ma, F. Li, G. Qi, L. Wang, C. Liu, K. Wang, G. Xiao and B. Zou, Nanoscale, 2019, 11, 820–825 RSC.
- S. Li, J. Luo, J. Liu and J. Tang, J. Phys. Chem. Lett., 2019, 10, 1999–2007 CrossRef CAS PubMed.
- C. C. Stoumpos, D. H. Cao, D. J. Clark, J. Young, J. M. Rondinelli, J. I. Jang, J. T. Hupp and M. G. Kanatzidis, Chem. Mater., 2016, 28, 2852–2867 CrossRef CAS.
- E. R. Dohner, A. Jaffe, L. R. Bradshaw and H. I. Karunadasa, J. Am. Chem. Soc., 2014, 136, 13154–13157 CrossRef CAS PubMed.
- B. Yang, J. Chen, F. Hong, X. Mao, K. Zheng, S. Yang, Y. Li, T. Pullerits, W. Deng and K. Han, Angew. Chem., Int. Ed., 2017, 56, 12471 CrossRef CAS PubMed.
- B. Yang, J. Chen, S. Yang, F. Hong, L. Sun, P. Han, T. Pullerits, W. Deng and K. Han, Angew. Chem., Int. Ed., 2018, 57, 5359–5363 CrossRef CAS PubMed.
- L. Zhou, J.-F. Liao, Z.-G. Huang, J.-H. Wei, X.-D. Wang, W.-G. Li, H.-Y. Chen, D.-B. Kuang and C.-Y. Su, Angew. Chem., Int. Ed., 2019, 58, 5277–5281 CrossRef CAS PubMed.
- R. T. Williams and K. S. Song, J. Phys. Chem. Solids, 1990, 51, 679–716 CrossRef CAS.
- B. M. Benin, D. N. Dirin, V. Morad, M. Wörle, S. Yakunin, G. Rainò, O. Nazarenko, M. Fischer, I. Infante and M. V. Kovalenko, Angew. Chem., Int. Ed., 2018, 57, 11329–11333 CrossRef CAS PubMed.
- K. M. McCall, C. C. Stoumpos, S. S. Kostina, M. G. Kanatzidis and B. W. Wessels, Chem. Mater., 2017, 29, 4129–4145 CrossRef CAS.
- L. Lian, M. Zheng, P. Zhang, Z. Zheng, K. Du, W. Lei, J. Gao, G. Niu, D. Zhang, T. Zhai, S. Jin, J. Tang, X. Zhang and J. Zhang, Chem. Mater., 2020, 32, 3462–3468 CrossRef CAS.
- B. Ke, R. Zeng, Z. Zhao, Q. Wei, X. Xue, K. Bai, C. Cai, W. Zhou, Z. Xia and B. Zou, J. Phys. Chem. Lett., 2020, 11, 340–348 CrossRef CAS PubMed.
- J. Luo, X. Wang, S. Li, J. Liu, Y. Guo, G. Niu, L. Yao, Y. Fu, L. Gao, Q. Dong, C. Zhao, M. Leng, F. Ma, W. Liang, L. Wang, S. Jin, J. Han, L. Zhang, J. Etheridge, J. Wang, Y. Yan, E. H. Sargent and J. Tang, Nature, 2018, 563, 541–545 CrossRef CAS PubMed.
- L. Zhou, L. Zhang, H. Li, W. Shen, M. Li and R. He, Adv. Funct. Mater., 2021, 31, 2108561 CrossRef CAS.
- Y. P. Varshni, Physica, 1967, 34, 149–154 CrossRef CAS.
- J. M. Frost, K. T. Butler, F. Brivio, C. H. Hendon, M. van Schilfgaarde and A. Walsh, Nano Lett., 2014, 14, 2584–2590 CrossRef CAS PubMed.
- H. Yang, Y. Zhang, J. Pan, J. Yin, O. M. Bakr and O. F. Mohammed, Chem. Mater., 2017, 29, 8978–8982 CrossRef CAS.
- Y. Jing, Y. Liu, M. Li and Z. Xia, Adv. Opt. Mater., 2021, 9, 2002213 CrossRef CAS.
- I. B. Koutselas, L. Ducasse and G. C. Papavassiliou, J. Phys.: Condens. Matter, 1996, 8, 1217–1227 CrossRef CAS.
- Y. Toyozawa, Prog. Theor. Phys., 1962, 27, 89–104 CrossRef CAS.
- J. A. Steele, P. Puech, M. Keshavarz, R. Yang, S. Banerjee, E. Debroye, C. W. Kim, H. Yuan, N. H. Heo, J. Vanacken, A. Walsh, J. Hofkens and M. B. J. Roeffaers, ACS Nano, 2018, 12, 8081–8090 CrossRef CAS PubMed.
- R. Zeng, L. Zhang, Y. Xue, B. Ke, Z. Zhao, D. Huang, Q. Wei, W. Zhou and B. Zou, J. Phys. Chem. Lett., 2020, 11, 2053–2061 CrossRef CAS PubMed.
- H. Peng, S. Yao, Y. Guo, R. Zhi, X. Wang, F. Ge, Y. Tian, J. Wang and B. Zou, J. Phys. Chem. Lett., 2020, 11, 4703–4710 CrossRef CAS PubMed.
- S. Rudin, T. L. Reinecke and B. Segall, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 42, 11218–11231 CrossRef CAS PubMed.
- A. D. Wright, C. Verdi, R. L. Milot, G. E. Eperon, M. A. Perez-Osorio, H. J. Snaith, F. Giustino, M. B. Johnston and L. M. Herz, Nat. Commun., 2016, 7, 11755 CrossRef PubMed.
- L. Zhou, J.-F. Liao, Y. Qin, X.-D. Wang, J.-H. Wei, M. Li, D.-B. Kuang and R. He, Adv. Funct. Mater., 2021, 31, 2102654 CrossRef CAS.
- B. Luo, D. Liang, S. Sun, Y. Xiao, X. Lian, X. Li, M.-D. Li, X.-C. Huang and J. Z. Zhang, J. Phys. Chem. Lett., 2020, 11, 199–205 CrossRef CAS PubMed.
- K. M. McCall, C. C. Stoumpos, O. Y. Kontsevoi, G. C. B. Alexander, B. W. Wessels and M. G. Kanatzidis, Chem. Mater., 2019, 31, 2644–2650 CrossRef CAS.
- K. Thirumal, W. K. Chong, W. Xie, R. Ganguly, S. K. Muduli, M. Sherburne, M. Asta, S. Mhaisalkar, T. C. Sum, H. S. Soo and N. Mathews, Chem. Mater., 2017, 29, 3947–3953 CrossRef CAS.
- P. Shen, X. Ma, F. Pan, Y.-n. Wang, B. Liu and H. Ye, J. Phys. Chem. C, 2021, 125, 6767–6772 CrossRef CAS.
- P. Pal, S. Saha, A. Banik, A. Sarkar and K. Biswas, Chem.–Eur. J., 2018, 24, 1811–1815 CrossRef CAS PubMed.
- H. Yang, W. Shi, T. Cai, K. Hills-Kimball, Z. Liu, L. Dube and O. Chen, Nanoscale, 2020, 12, 23191–23199 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: methods including synthesis details and characterization techniques, schematic of synthesis, additional PXRD, FESEM, TEM, TGA, and FTIR spectra, optical measurements, comparison tables for reported PLQY of 2D lead halide perovskites, electron-longitudinal optical phonon coupling constant and fitted lifetime values of bulk and NPLs of RbPb2Br5. See https://doi.org/10.1039/d2sc02992h |
| This journal is © The Royal Society of Chemistry 2022 |