Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
N-doped nonalternant aromatic belt via a six-fold annulative double N-arylation†
Hiroki
Sato
a,
Rie
Suizu
 bc,
Tomoki
Kato
b,
Akiko
Yagi
a,
Yasutomo
Segawa
bc,
Tomoki
Kato
b,
Akiko
Yagi
a,
Yasutomo
Segawa
 *bdef,
Kunio
Awaga
bg and
Kenichiro
Itami
*bdef,
Kunio
Awaga
bg and
Kenichiro
Itami
 *abfg
*abfg
aInstitute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Chikusa, Nagoya, 464-8602, Japan. E-mail: Itami@chem.nagoya-u.ac.jp
bGraduate School of Science, Nagoya University, Chikusa, Nagoya, 464-8602, Japan. E-mail: segawa@ims.ac.jp
cJapan Science and Technology Agency (JST), PRESTO, 4-1-8 Honcho, Kawaguchi, Saitama 332-0012, Japan
dInstitute for Molecular Science, Myodaiji, Okazaki, 444-8787, Japan
eDepartment of Structural Molecular Science, SOKENDAI (The Graduate University for Advanced Studies), Myodaiji, Okazaki, 444-8787, Japan
fJST-ERATO, Nagoya University, Itami Molecular Nanocarbon Project, Chikusa, Nagoya, 464-8602, Japan
gIntegrated Research Consortium on Chemical Sciences (IRCCS), Nagoya University, Japan
First published on 9th August 2022
Abstract
The design and synthesis of nitrogen (N)-doped molecular nanocarbons are of importance since N-doped nanocarbons have received significant attention in materials science. Herein, we report the synthesis and X-ray crystal structure of a nitrogen-inserted nonalternant aromatic belt. The palladium-catalyzed six-fold annulative double N-arylation provided an aromatic belt bearing six nitrogen atoms in one step from cyclo[6]paraphenylene-Z-ethenylene, the precursor of the (6,6)carbon nanobelt. The C3i-symmetric structure of the aromatic belt in the solid state was revealed using X-ray crystallography. The multistep (electro)chemical oxidation behavior of the belt, which was facilitated by the six p-methoxyaniline moieties, was studied, and a stable dication species was successfully identified by X-ray crystallography. The present study not only shows the unique structure and properties of the N-doped nonalternant aromatic belt but also expands the scope of accessibility of synthetically difficult belt molecules by the conventional intramolecular contraction pathway.
Introduction
The research field of nitrogen (N)-doped nanocarbon materials has been expanding over the last few decades because N doping creates interactive sites in pristine nanocarbons and modifies their electronic properties (Fig. 1a).1 Whereas current production of intrinsically N-doped nanocarbons by post-treatment or in situ synthesis methods provides random dopant patterns and structures,1a,b,e organic synthesis, in contrast, enables the formation of structurally uniform and atomically precise carbon nanostructures.2,3 Such nanocarbon structures, also called molecular nanocarbons, are of high importance for their use as discrete models, ligands, electronically tunable organic materials, and templates for the growth of precise large nanocarbons. For example, a number of N-doped nanographenes have been reported to date as discrete models for N-doped graphene sheets (Fig. 1b). Their unique properties and tunable electronic structures with structural uniformity have attracted considerable attention.2a,3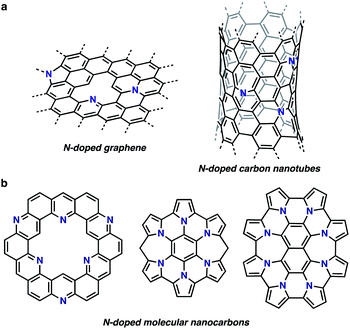 | ||
| Fig. 1 Structures of N-doped nanocarbons. (a) N-doped graphene and carbon nanotubes. (b) Examples of atomically precise N-doped molecular nanocarbons. | ||
The tubular belt structures formed by annulated conjugated aromatic rings pose formidable synthetic challenges because of their high strain.3 To date, ring-shaped,4 planar,5 bowl-shaped,6 and triangle- or square-shaped aromatic molecules have only been reported.7 An N-doped aromatic belt with nonconjugated sp3-linkers can also release the belt strain.8 A recent unsuccessful synthetic endeavor of a tetraazapentacene belt also attests to the aforementioned challenge associated with the synthesis of fully conjugated N-doped aromatic belts.9
Herein, we report the synthesis of an N-doped aromatic belt via annulative amination reactions of the Z-stilbene-based macrocyclic intermediate 1 (Fig. 2). The crystal structure and optical properties of 2 are presented. We also report structural determination of the corresponding dication salts of 2 obtained via chemical oxidation.
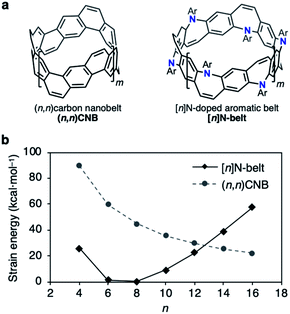 | ||
| Fig. 2 (a) Structures of (n,n)CNB and [n]N-belt where n = 2(m + 2). (b) Plot of the strain energies of (n,n)CNB and [n]N-belt (kcal mol−1) versus n calculated by the B3LYP/6-31G(d) level of theory; Ar = Ph (see the ESI for details†). | ||
Results and discussion
The difficulty of accessing belt molecules lies in the final ring-closing step in which a particular precursor forms the highly strained belt structures. Thus, available synthetic strategies are rather limited. In our previous work, fully fused carbon nanobelts (CNBs, Fig. 2a, left)10a,b were synthesized via intramolecular reductive or oxidative C–C bond-forming reactions in the final annulation step. On the other hand, the strategy of inserting belt components intermolecularly during annulation events (N-belt, Fig. 2a, right) has not yet been studied, although this approach has potential benefits regarding substrate flexibility and variety. Based on the recent progress in Buchwald–Hartwig amination reactions, we speculated an intermolecular synthetic strategy for N-doped belt synthesis via annulative C–N bond formation reactions of zigzag macrocycles.To estimate its synthetic difficulty, the strain energy of the N-belt was calculated. The calculation of the strain energy of [n]N-belt was performed by using density functional theory (DFT) B3LYP/6-31G(d) level of theory with hypothetical homodesmotic reactions (see Fig. S4 in the ESI for details†). Fig. 2b shows the strain energy of [n]N-belt and (n,n)CNB. It was clearly seen that the size-dependency of the strain energy of [n]N-belt was different from that of (n,n)CNB.10a Because of the nonplanar structure of 7-membered rings in [n]N-belt, [6]- and [8]N-belt have almost no strain (1.3 and 0.4 kcal mol−1, respectively), whereas small (n = 4) and large [n]N-belts (n = 10–16) have high strain energy. According to these analyses, [6]- and [8]N-belt would be suitable targets for the synthesis of N-doped belt molecules.
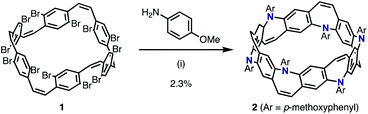
We envisioned that the Z-stilbene-based macrocycle 1 could afford the target [6]N-belt in one step by the 12-fold annulative Buchwald–Hartwig reactions11 (or the so-called annulative double N-arylation reactions12). Z-stilbene-based macrocyclic precursor 1 was prepared following our previous report.10a Next, palladium-catalyzed amination reactions between 1 and aniline were performed. In the first attempt, debrominated, hydroaminated, and over-/less-aminated ring products were detected by mass spectrometry but not the desired belt molecules. Several amines such as 4-(dimethylamino)aniline, ethyl 4-aminobenzoate, 4-aminopyridine, and benzylamine were screened, aiming at the reduction of these undesired products. Through this screening, it was found that p-methoxyaniline afforded the desired belt molecule 2 (the methoxy derivative of [6]N-belt), probably because the electron-donating methoxy groups promoted the reductive elimination. Among the various conditions tested, Pd(dba)2 (dba = dibenzylideneacetone) and PMe(tBu)2·HBF4 salt as the catalyst and ligand, respectively, furnished the desired compound most efficiently. In particular, the reaction between 1 and p-methoxyaniline using 0.60 equiv. Pd(dba)2, 1.2 equiv. PMe(tBu)2·HBF4, and 30 equiv. of NaOtBu in m-xylene at 140 °C for 8 h provided 2 in 2.3% isolated yield (Scheme 1). The structure of nanobelt 2 was fully characterized by 1H and 13C NMR spectroscopies, MALDI-TOF MS, and X-ray diffraction.
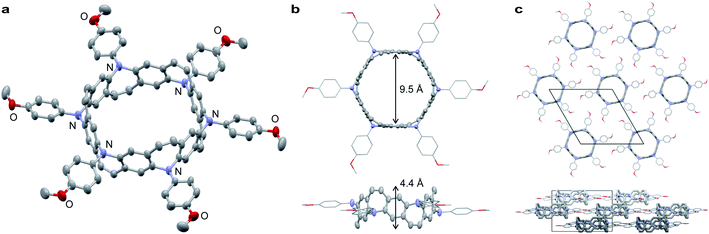
Belt 2 is a yellow powder that can be dissolved in halogenated solvents. A single crystal of 2 was obtained by the slow diffusion of n-pentane into a 1,2-dichloroethane solution. The crystal structure of 2 belongs to the trigonal space group R3. As shown in Fig. 3a and b, 2 exhibits a tubular belt structure with approximately 9.5 Å pore and 4.4 Å height. Six p-methoxyphenyl substituents project radially out from the center of the belt. The sum of the three C–N–C angles around N (ΣN) is 351.6° indicating that the nitrogen atoms are almost sp2-hybridized. A disordered n-pentane molecule was incorporated in the cavity of the belt. The packing structure of 2 revealed a columnar structure with side-by-side packing, and there were no notable π–π interactions between neighboring molecules (Fig. 3c).
To gain insight into the redox properties of 2, its chemical and electrochemical oxidation experiments were performed, and ultraviolet-visible-near infrared (UV-Vis-NIR) absorption measurements were conducted. Neutral 2 exhibited a single absorption peak at 334 nm and no obvious fluorescence upon irradiation of 334 nm light. With the addition of AgSbF6 as an oxidant, a new absorption peak appeared at around 520 nm (Fig. 4), and the peak intensity continuously increased for additions of up to 3.0 equiv. of AgSbF6. With more than 3.0 equiv. of the oxidant, insoluble solids started to precipitate, resulting in weaker absorption bands (Fig. S2†), probably due to intermolecular oxidative coupling reactions. No other peak, except the one at 520 nm, was observed during the incremental addition of the oxidant. Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements were carried out in dichloromethane (0.1 M nBu4NPF6) (Fig. S3†). CV analysis showed a reversible redox process, and DPV revealed multiple oxidation steps including EOx = 0.42 and 0.54 [EOxvs. Fc/Fc+].
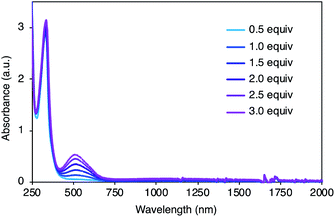 | ||
| Fig. 4 UV-Vis-NIR absorption spectra of 2 (5.0 × 10−5 M) with AgSbF6 (0.5–3.0 equiv.) in dichloromethane. | ||
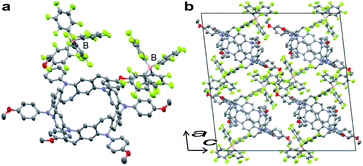
Stoichiometrically controlled chemical oxidation of 2 and structural characterization of oxidized species were attempted. Oxidants with different counter anions and varying oxidant stoichiometry were screened. It was found that the stabilities of the cations were highly dependent on the counter anions. The purple color of the solutions derived from the radical cations disappeared within a short period for BF4− or PF6− counter anions, whereas the solutions remained purple for a while in the case of SbF6− or SbCl6−. Treatment of 2 with 2 equiv. of AgB(C6F5)4 in dichloromethane and slow diffusion in n-hexane at −30 °C over a period of 1 week resulted in the formation of thick purple single crystals of the N-doped nanobelt dication salt 22+·2[B(C6F5)4]−. The structure of 22+·2[B(C6F5)4]− was determined by X-ray diffraction (Fig. 5). The space group was monoclinic I2/a. Unlike the neutral compound, where one-sixth of the molecule is an asymmetric unit, half of the molecule is crystallographically independent of the dication compound, and the belt structure contracts uniaxially. Because of the instability of 22+ in solution, spectral analysis could not be performed.
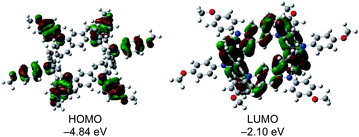
To further investigate the electronic nature of 2, DFT calculations were carried out at the B3LYP/6-31 G (d) level. The calculated optimal structure of 2 (Fig. S5†) matches well with the experimental results obtained by X-ray analysis. Notably, DFT studies revealed that the energies of all orbitals between HOMO and HOMO−5 lie within a narrow range of 0.10 eV (Fig. S6†). Furthermore, the electron density on the HOMO was localized on the p-methoxyphenylamine moieties, while that on the LUMO was localized on the central belt (Fig. 6). DFT calculations performed on the oxidized species revealed that the optimal structures of 2n+ in different oxidation states (n = 1–3) were similar (Fig. S7†) and spins were localized on the p-methoxyphenylamine moieties (Fig. S8†). Time-dependent DFT calculations of 2 and the cations also supported the experimental results (Table S2†). The absorption peak of 2 at 334 nm can be assigned to the HOMO−6 → LUMO+1 (360.6 nm, f = 0.9802) and HOMO−6 → LUMO+2 (360.6 nm, f = 0.9802) transitions at the belt center. The calculations for 2n+ show similar absorption bands in different oxidation states around 550 nm (Fig. S9†), which may correspond to the observed absorption band at 520 nm in the chemical oxidation experiment.
Conclusions
We successfully synthesized a novel class of N-doped aromatic belts via a six-fold annulative double N-arylation reaction. This new synthetic strategy can expand the scope of accessibility of synthetically difficult belt molecules using the conventional intramolecular contraction pathway. N-doped belt 2 was chemically oxidized, and the generated diradical species 22+·2[B(C6F5)4]− was successfully characterized. Further studies on the detailed electronic and magnetic properties of 2n+ are required for their future applications.Author contributions
K. I., Y. S., and H. S. conceived the concept and prepared the manuscript with feedback from the other authors. H.S. performed the synthetic experiments. Y. S. and R. S. performed the X-ray crystallographic measurements. H. S., R. S., T. K., and A. Y. performed the photophysical and electrochemical measurements. A. Y. and K. A. provided critical comments.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the ERATO (JPMJER1302 to K. I.) and FOREST program (JPMJFR211R to Y. S.) from JST, and JSPS KAKENHI (JP22H02068 to Y. S., 19H05463 to K. I.). H. S. is a recipient of the JSPS Research Fellowship for Young Scientists (PD). We thank K. Oyama, M. Kuwayama, H. Shudo, and Y. Otsubo for the support of experiments. Calculations were performed using the resources of the Research Center for Computational Science, Okazaki, Japan (22-IMS-C192). ITbM is supported by the World Premier International Research Center Initiative (WPI), Japan.Notes and references
- (a) H. Wang, T. Maiyalagan and X. Wang, ACS Catal., 2012, 2, 781–794 CrossRef CAS; (b) X. Wang, G. Sun, P. Routh, D.-H. Kim, W. Huang and P. Chen, Chem. Soc. Rev., 2014, 43, 7067–7098 RSC; (c) W. J. Lee, U. N. Maiti, J. M. Lee, J. Lim, T. H. Han and S. O. Kim, Chem. Commun., 2014, 50, 6818–6830 RSC; (d) Q. Wei, X. Tong, G. Zhang, J. Qiao, Q. Gong and S. Sun, Catalysts, 2015, 5, 1574–1602 CrossRef CAS; (e) Y. Deng, Y. Xie, K. Zou and X. Ji, J. Mater. Chem. A, 2016, 4, 1144–1173 RSC; (f) M. R. Benzigar, S. N. Talapaneni, S. Joseph, K. Ramadass, G. Singh, J. Scaranto, U. Ravon, K. Al-Bahily and A. Vinu, Chem. Soc. Rev., 2018, 47, 2680–2721 RSC; (g) J. Wu, Z. Pan, Y. Zhang, B. Wang and H. Peng, J. Mater. Chem. A, 2018, 6, 12932–12944 RSC; (h) I.-Y. Jeon, H.-J. Noh and J.-B. Baek, Chem.–Asian J., 2020, 15, 2282–2293 CrossRef CAS PubMed.
- (a) A. Narita, X.-Y. Wang, X. Feng and K. Müllen, Chem. Soc. Rev., 2015, 44, 6616–6643 RSC; (b) M. Stępień, M. Gońka, M. Żyła and N. Sprutta, Chem. Rev., 2017, 117, 3479–3716 CrossRef PubMed.
- (a) Y. Segawa, A. Yagi, K. Matsui and K. Itami, Angew. Chem., Int. Ed., 2016, 55, 5136–5158 CrossRef CAS PubMed; (b) M. A. Majewski and M. Stępień, Angew. Chem., Int. Ed., 2019, 58, 86–116 CrossRef CAS PubMed; (c) S. Mirzaei, E. Castro and R. Hernández Sánchez, Chem. – Eur. J., 2021, 27, 8642–8655 CrossRef CAS PubMed; (d) M. Hermann, D. Wassy and B. Esser, Angew. Chem., Int. Ed., 2021, 60, 15743–15766 CrossRef CAS PubMed.
- (a) J. Song, N. Aratani, H. Shinokubo and A. Osuka, J. Am. Chem. Soc., 2010, 132, 16356–16357 CrossRef CAS PubMed; (b) E. R. Darzi, E. S. Hirst, C. D. Weber, L. N. Zakharov, M. C. Lonergan and R. Jasti, ACS Cent. Sci., 2015, 1, 335–342 CrossRef CAS PubMed; (c) T. Kuwabara, J. Orii, Y. Segawa and K. Itami, Angew. Chem., Int. Ed., 2015, 54, 9646–9649 CrossRef CAS PubMed; (d) M. Ball, B. Fowler, P. Li, L. A. Joyce, F. Li, T. Liu, D. Paley, Y. Zhong, H. Li, S. Xiao, F. Ng, M. L. Steigerwald and C. Nuckolls, J. Am. Chem. Soc., 2015, 137, 9982–9987 CrossRef CAS PubMed; (e) J. M. Van Raden, E. R. Darzi, L. N. Zakharov and R. Jasti, Org. Biomol. Chem., 2016, 14, 5721–5727 RSC; (f) S. Xue, D. Kuzuhara, N. Aratani and H. Yamada, Org. Lett., 2019, 21, 2069–2072 CrossRef CAS PubMed; (g) F. Schwer, S. Zank, M. Freiberger, R. Kaur, S. Frühwald, C. C. Robertson, A. Görling, T. Drewello, D. M. Guldi and M. von Delius, Org. Mater., 2022, 4, 7–17 CrossRef CAS.
- A. Tatibouët, R. Hancock, M. Demeunynck and J. Lhomme, Angew. Chem., Int. Ed. Engl., 1997, 36, 1190–1191 CrossRef.
- (a) D. Myśliwiec and M. Stępień, Angew. Chem., Int. Ed., 2013, 52, 1713–1717 CrossRef PubMed; (b) M. Zhao, S. H. Pun, Q. Gong and Q. Miao, Angew. Chem., Int. Ed., 2021, 60, 24124–24130 CrossRef CAS PubMed.
- J. Zhu, Y. Han, Y. Ni, G. Li and J. Wu, J. Am. Chem. Soc., 2021, 143, 2716–2721 CrossRef CAS PubMed.
- F. Zhang, X.-S. Du, D.-W. Zhang, Y.-F. Wang, H.-Y. Lu and C.-F. Chen, Angew. Chem., Int. Ed., 2021, 60, 15291–15295 CrossRef CAS PubMed.
- J. Wang and Q. Miao, Org. Lett., 2019, 21, 10120–10124 CrossRef CAS PubMed.
- (a) G. Povie, Y. Segawa, T. Nishihara, Y. Miyauchi and K. Itami, Science, 2017, 356, 172–175 CrossRef CAS PubMed; (b) G. Povie, Y. Segawa, T. Nishihara, Y. Miyauchi and K. Itami, J. Am. Chem. Soc., 2018, 140, 10054–10059 CrossRef CAS PubMed; (c) K. Y. Cheung, S. Gui, C. Deng, H. Liang, Z. Xia, Z. Liu, L. Chi and Q. Miao, Chem, 2019, 5, 838–847 CrossRef CAS; (d) K. Y. Cheung, K. Watanabe, Y. Segawa and K. Itami, Nat. Chem., 2021, 13, 255–259 CrossRef CAS PubMed; (e) Z. Xia, S. H. Pun, H. Chen and Q. Miao, Angew. Chem., Int. Ed., 2021, 60, 10311–10318 CrossRef CAS PubMed; (f) H. M. Bergman, G. R. Kiel, R. C. Handford, Y. Liu and T. D. Tilley, J. Am. Chem. Soc., 2021, 143, 8619–8624 CrossRef CAS PubMed.
- P. Ruiz-Castillo and S. L. Buchwald, Chem. Rev., 2016, 116, 12564–12649 CrossRef CAS PubMed.
- (a) K. Nozaki, K. Takahashi, K. Nakano, T. Hiyama, H.-Z. Tang, M. Fujiki, S. Yamaguchi and K. Tamao, Angew. Chem., Int. Ed., 2003, 42, 2051–2053 CrossRef CAS PubMed; (b) H. Christensen, C. Schjøth-Eskesen, M. Jensen, S. Sinning and H. H. Jensen, Chem. – Eur. J., 2011, 17, 10618–10627 CrossRef CAS PubMed; (c) X. Zhang, Y. Yang and Y. Liang, Tetrahedron Lett., 2012, 53, 6406–6408 CrossRef CAS; (d) N. Božinović, I. Opsenica and B. A. Šolaja, Synlett, 2013, 24, 49–52 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2133103 and 2133104. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc02647c |
| This journal is © The Royal Society of Chemistry 2022 |