Open Access Article
Open Access ArticleSelective synthesis of 3-formylbenzofuran and 3-acylbenzofuran using a chalcone rearrangement strategy†
Akira Nakamura ,
Akira Imamiya,
Yuichiro Ikegami,
Fei Rao,
Harumi Yuguchi,
Yasuyoshi Miki and
Tomohiro Maegawa
,
Akira Imamiya,
Yuichiro Ikegami,
Fei Rao,
Harumi Yuguchi,
Yasuyoshi Miki and
Tomohiro Maegawa *
*
School of Pharmaceutical Sciences, Kindai University, 3-4-1 Kowakae, Higashi-osaka, Osaka 577-8502, Japan. E-mail: maegawa@phar.kindai.ac.jp
First published on 25th October 2022
Abstract
We developed a method for highly selective synthesis of two benzofuran isomers, by rearranging and subsequently transforming 2-hydroxychalcones. Depending on the reaction conditions, synthesis of 3-formylbenzofurans, unconventional products, and 3-acylbenzofurans was achieved through cyclized 2,3-dihydrobenzofurans obtained from the rearranged products. The facile synthesis of 3-formylbenzofurans facilitated synthesis of the natural product, puerariafuran, from the corresponding chalcone.
Introduction
Benzofuran and its derivatives are present as scaffolds in many natural products and biologically active compounds.1 These compounds have attracted much attention in the pharmaceutical and pesticide industries because of their promising antibacterial, antimicrobial, antitumor, and antidiabetic activities.2 Hence, the synthesis of benzofurans has aroused considerable interest and various methodologies have been reported and applied for the synthesis of natural products.3Our current research focuses on the development of methods for synthesizing various heterocycles used for the rearrangement of chalcones.4 Combination of chalcones and rearrangement reactions has been reported, but has limited utility for the construction of isoflavones.5 We also reported the synthesis of pterocarpin via isoflavones, through hypervalent iodine-mediated oxidative rearrangement of 2,2′-hydroxychalcone derivatives. In this study, we found that 3-acylbenzofuran was formed from 2-hydroxychalcone derivatives.4b The synthesis of 3-acylbenzofurans is an unexplored topic; little is known about routes to effective synthesis. For example, Friedel–Crafts acylation of benzofurans results in low C2/C3 regioselectivity,6 and most existing methods can synthesize 3-acylbenzofurans with substituents at the C2 position.7
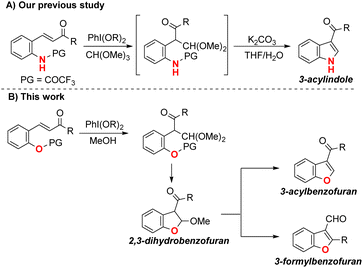
We recently developed a method for concise one-pot synthesis of 3-acylindoles. This method first rearranges the N-COCF3-protected 2-aminochalcone with phenyliodine diacetate (PhI(OAc)2); 3-acylindoles are then produced under basic conditions via deprotection and a cyclization reaction (Scheme 1A).4c To apply the chalcone-rearrangement strategy for the synthesis of 3-acylbenzofurans, we investigated the reaction of the protected 2-hydroxychalcone. Unexpectedly, our approach produced not only 3-acylbenzofuran, but also 3-formylbenzofuran (with high selectivity) from 2,3-dihydrobenzofuran (Scheme 1B).
Results and discussion
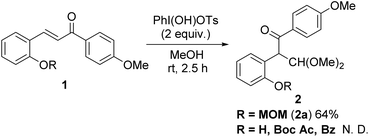
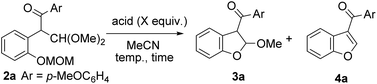
We started by testing the rearrangement conditions using 2-hydroxychalcone 1 with a hypervalent iodine reagent (Scheme 2). No rearrangement product 2 was obtained without the protecting group (R = H). Attempts were made to synthesize chalcones protected with the trifluoroacetyl group used in our previous indole synthesis, but the chalcones were unstable and could not be isolated. Then, the reaction was evaluated with chalcones bearing various protecting groups, such as t-butyloxycarbonyl (Boc), acetyl (Ac) and benzoyl (Bz), but no rearranged products were obtained. The desired product was obtained with methoxymethyl (MOM) protection. The reaction of MOM-protected hydroxychalcone with two equivalents of hydroxy(tosyloxy)iodobenzene (PhI(OH)OTs) gave the corresponding rearranged product 2a in 64% yield.We next examined deprotection followed by simultaneous cyclization to the corresponding 3-acylbenzofuran under acidic conditions (Table 1). With excess AcOH, no reaction was observed, even with reflux (entry 1). The desired benzofuran 4a was not obtained using trifluoroacetic acid (TFA), whereas 2,3-dihydrobenzofuran 3a, the precursor of 4a, was isolated in 56% yield (entry 2). The use of p-toluenesulfonic acid (p-TsOH) increased the yield of 3a slightly to 62% (entry 3), but 3a was not obtained, and nor was 4a with heating (entry 4). The use of the solvents CH2Cl2, THF, and MeOH did not yield the desired 4a. We finally optimized the conditions to obtain 3a in 80% yield using 0.1 equivalent of p-TsOH (entry 5).
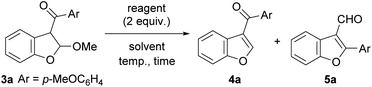
Subsequently, the transformation of 2,3-dihydrobenzofuran 3a into 4a was studied under basic and acidic conditions (Table 2). When the reaction was performed using K2CO3, aromatization proceeded at room temperature affording 3-acylbenzofuran 4a in 97% yield (entry 1). Pyridine was ineffective and only trace amounts of 4a were obtained upon reflux (entry 2). However, 4a was obtained in 98% yield in AcOH (entry 3). Other acids (pyridinium p-toluenesulfonate (PPTS) and TFA) in toluene also gave 4a in high yields under reflux conditions (entries 4 and 5). Surprisingly, unexpected formation of 3-formylbenzofuran 5a was observed with p-TsOH when 1,1,1,3,3,3-hexafluoro-2-propanol was used as a solvent. 3-Formylbenzofurans are found in the skeletons of natural products,8 and few synthetic methods are known.9
| Entry | Reagent | Solvent | Temp. [°C] | Time [h] | Yieldb [%] | |
|---|---|---|---|---|---|---|
| 4a | 5a | |||||
| a The reactions were performed with 2a (0.2 mmol) in 2 ml of solvent.b Isolated yield. | ||||||
| 1 | K2CO3 | THF | r.t. | 4 | 97 | — |
| 2 | Pyridine | THF | 70 | 12 | Trace | — |
| 3 | — | AcOH | 110 | 3 | 98 | — |
| 4 | PPTS | PhCH3 | 110 | 7 | 95 | Trace |
| 5 | TFA | PhCH3 | 110 | 4 | 95 | Trace |
| 6 | p-TsOH | (CF3)2CHOH | r.t. | 0.5 | — | 98 |
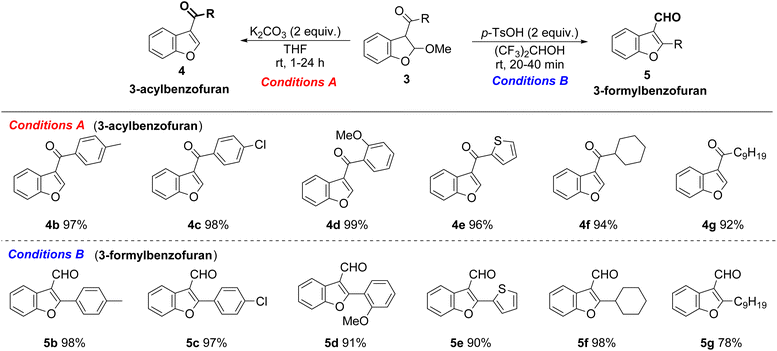
On optimizing the conditions for the selective synthesis of benzofuran isomers, the transformation was extended to various 2,3-dihydrobenzofurans 3 with aryl or alkyl groups on the ketone moiety (Table 3). A series of electron-donating groups (e.g., p-methyl and o-methoxy) and electron-withdrawing substituents (e.g., p-chloro) on the phenyl ring reacted smoothly to give the respective 3-acylbenzofurans (4b–4d) and 3-formylbenzofuran (5b–5d) in excellent yields. The reaction with 2,3-dihydrobenzofuran with a thiophenyl group afforded 4e and 5e in yields of 96% and 90%, respectively. During the transformation of 3f and 3g with alkyl groups, 3-acylbenzofurans (4f and 4g) and 3-formylbenzofuran 5f were obtained in high yields, whereas the yield of 3-formylbenzofuran 5g was decreased slightly.
| a Reaction conditions: conditions A, K2CO3 (2 equiv.), THF (0.1 M). Conditions B, p-TsOH (2 equiv.), (CF3)2CHOH (0.1 M). |
|---|
 |
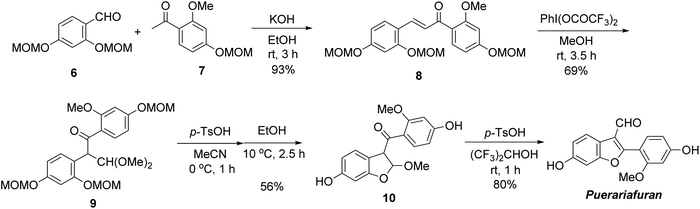
Next, we applied this novel 3-formylbenzofuran synthesis method to natural products (Scheme 3). Puerariafuran was chosen as the synthetic target, as it exhibits biological activities such as the inhibition of advanced glycation end products (AGEs).8g Recently, Lin et al. synthesized puerariafuran,10 but the number of steps and total yield could be improved. First, chalcone 8 was prepared by condensing MOM-protected aldehyde 6 and acetophenone 7 in 93% yield. Oxidative rearrangement with hypervalent iodine reagent was achieved by [bis(trifluoroacetoxy)iodo]benzene (PhI(OCOCF3)2), affording 9 in 69% yield. To synthesize 2,3-dihydrobenzofuran 10, we attempted to perform simultaneous deprotection and cyclization reactions under acidic conditions. Partial decomposition was observed as the reaction progressed, but adding an excess of EtOH suppressed the decomposition and dihydrobenzofuran 10 was obtained in 56% yield.11 Final conversion to the 3-formylbenzofuran skeleton was achieved on treatment with p-TsOH in (CF3)2CHOH, giving puerariafuran in 80% yield. Our protocol for puerariafuran synthesis has seven steps and an overall yield of 18% from commercial aldehyde and acetophenone; it is more efficient than the previous synthesis method (11 steps and 5.3% total yield).
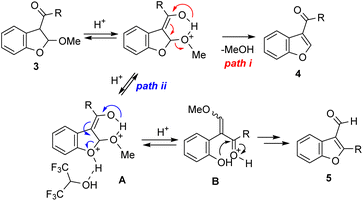
The reaction mechanism for obtaining two types of benzofuran from 2,3-dihydrobenzofurans 3 under acidic conditions was thought to be as follows (Scheme 4). Using relatively weak acids, 3-acylbenzofurans 4 were obtained via aromatization in association with methanol elimination (path i). The mechanism of 3-formylbenzofuran 5 formation is via a diprotonated intermediate A (path ii),12 which is stabilized with (CF3)2CHOH.13,14 Subsequent THF ring opening of intermediate A gives B, and the ring-closure at the ketone moiety then lead to 5 after aromatization and hydrolysis. According to Zanatta,15 another possible pathway for the formation of 5 is isomerization from 4. However, the reaction of 4 with p-TsOH and some MeOH in (CF3)2CHOH resulted in no reaction.16
Conclusions
In summary, we developed a new method for highly selective synthesis of two benzofuran isomers based on rearrangement of the MOM-protected 2-hydroxychalcone. The key intermediates, 2,3-dihydrobenzofurans, could be selectively transformed into different benzofuran isomers using different reaction conditions. 3-Acylbenzofurans were obtained under basic or weakly acidic conditions in THF. Using (CF3)2CHOH as a solvent with p-TsOH generated 3-formylbenzofurans selectively. A variety of 2,3-dihydrobenzofurans were selectively converted into benzofuran isomers in high yields, and the efficient total synthesis of puerariafuran proves the practicality of this method. Currently, we are developing this methodology for application to other heterocycles.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by JSPS KAKENHI Grant No. 19K16329 and 18K05132, and also supported by 2021 Kindai University Research Enchancement Grant (KD2106). We also thank the Kindai University Joint Research Center for use of their facilities.Notes and references
- (a) A. Radadiya and A. Shah, Bioactive Benzofuran Derivatives: An Insight on Lead Developments, Radioligands and Advances of the Last Decade, Eur. J. Med. Chem., 2015, 97, 356 CrossRef CAS; (b) Y. Miao, Y. Hu, J. Yang, T. Liu, J. Sun and X. Wang, Natural Source, Bioactivity and Synthesis of Benzofuran Derivatives, RSC Adv., 2019, 9, 27510 RSC.
- (a) H. K. Shamsuzzaman, Bioactive Benzofuran Derivatives: A Review, Eur. J. Med. Chem., 2015, 97, 483 CrossRef; (b) R. J. Nevagi, S. N. Dighe and S. N. Dighe, Biological and Medicinal Significance of Benzofuran, Eur. J. Med. Chem., 2015, 97, 561 CrossRef CAS; (c) Z. Xu, S. Zhao, Z. Lv, L. Feng, Y. Wang, F. Zhang, L. Bai and J. Deng, Benzofuran Derivatives and their Anti-tubercular, Anti-bacterial Activities, Eur. J. Med. Chem., 2019, 162, 266 CrossRef CAS.
- (a) M. M. Heravi, V. Zadsirjan, H. Hamidi and P. H. T. Amiri, Total Synthesis of Natural Products Containing Benzofuran Rings, RSC Adv., 2017, 7, 24470 RSC; (b) L. Chiummiento, R. D'Orsi, M. Funicello and P. Lupattelli, Last Decade of Unconventional Methodologies for the Synthesis of Substituted Benzofurans, Molecules, 2020, 25, 2327 CrossRef CAS.
- (a) Y. Miki, S. Kobayashi, N. Ogawa and H. Hachiken, Reaction of Chalcone with Penyliodine(III) Bis(trifluoroacetate) (PIFA): Synthesis of (±)-Homopterocarpin, Synlett, 1994, 1001 CrossRef CAS; (b) Y. Miki, R. Fujita and K. Matsushita, Oxidative Rearrangement of Pentaalkoxychalcones with Phenyliodine(III) Bis(trifluoro-acetate) (PIFA): Synthesis of (±)-10-Bromopterocarpin and (±)-Pterocarpin, J. Chem. Soc., Perkin Trans. 1, 1998, 2533 RSC; (c) A. Nakamura, S. Tanaka, A. Imamiya, R. Takane, C. Ohta, K. Fujimura, T. Maegawa and Y. Miki, Synthesis of 3-Acylindoles by Oxidative Rearrangement of 2-Aminochalcones Using a Hypervalent Iodine Reagent and Cyclization Sequence, Org. Biomol. Chem., 2017, 15, 6702 RSC; (d) A. Nakamura, R. Takane, J. Tanaka, J. Morimoto and T. Maegawa, Construction of Azaisoflavone Derivatives by Hypervalent Iodine Reagent-Mediated Oxidative Rearrangement of 2′-Nitrochalcone, Heterocycles, 2018, 97, 785 CrossRef CAS.
- (a) L. Farkas, A. Gottsegen, M. Nogradi and S. Antus, Synthesis of Sophorol, Violanone, Lonchocarpan, Claussequinone, Philenopteran, Leiocalycin, and Some Other Natural Isoflavonoids by the Oxidative Rearrangement of Chalcones with Thallium(III) Nitrate, J. Chem. Soc., Perkin Trans. 1, 1974, 305 RSC; (b) R. V. Suresh, C. S. Rukmani Iyer and P. R. Iyer, Thallium(III) Nitrate Mediated Synthesis of Erythrinin-A, Dihydropyrano- and Pyranoisoflavones, Heterocycles, 1985, 23, 859 CrossRef CAS; (c) R. V. Suresh, C. S. Rukmani Iyer and P. R. Iyer, A Naturally Occurring Pyranoisoflavone, Tetrahedron, 1985, 41, 2479 CrossRef CAS; (d) M. Tsukayama, Y. Kawamura, H. Tamaki and T. Horie, Synthesis of Parvisoflavones A and B, Chem. Pharm. Bull., 1991, 39, 1704 CrossRef CAS; (e) Y. Kawamura, M. Maruyama, T. Tokuoka and M. Tsukayama, Synthesis of Isoflavones from 2′-Hydroxychalcones Using Poly[4-(diacetoxy)iodo]styrene or Related Hypervalent Iodine Reagent, Synthesis, 2002, 17, 2490 CrossRef; (f) M. M. Hossain, Y. Kawamura, K. Yamashita and M. Tsukayama, Microwave-Assisted Regioselective Synthesis of Natural 6-Prenylpolyhydroxyisoflavones and their Hydrates with Hypervalent Iodine Reagents, Tetrahedron, 2006, 62, 8625 CrossRef CAS; (g) R. S. Khupse and P. W. Erhardt, Practical Synthesis of Lespedezol A1, J. Nat. Prod., 2008, 71, 275 CrossRef CAS; (h) S. Tamura, K. Yoshihira, M. Tokumaru, X. Zisheng and N. Murakami, Inhibitors for Expression of IgE Eeceptor on Human Mast Cell from Puerariae Flos, Bioorg. Med. Chem. Lett., 2010, 20, 3872 CrossRef CAS PubMed; (i) S. Sato and H. Ishikawa, Total Synthesis of Two Isoflavone Bis-C-glycosides: Genistein and Orobol 6,8-di-C-β-D-Glucopyranosides, Synthesis, 2010, 18, 3126 CrossRef; (j) K. H. Almabruk, J. H. Chang and T. Mahmud, Total Synthesis of (±)-Isoperbergins and Correction of the Chemical Structure of Perbergin, J. Nat. Prod., 2016, 79, 2391 CrossRef CAS PubMed.
- (a) V. Morizur, D. Hector, S. Olivero, J. R. Desmurs and E. Dunach, Metal Sulfonate Polymers as Catalysts for the Heterogeneous Acylation of Aromatic Derivatives, Eur. J. Org. Chem., 2016, 3126 CrossRef CAS; (b) Only one example for selective C3-acylation in ionic solvent; P. H. Tran, H. T. Nguyen, P. E. Hansen and T. N. Le, An Efficient and Green Method for Regio- and Chemo-Selective Friedel-Crafts Acylations Using a Deep Eutectic Solvent ([CholineCl][ZnCl2]3), RSC Adv., 2016, 6, 37031 RSC; (c) I. Komoto, J. Matsuo and S. Kobayashi, Catalytic Friedel-Crafts Acylation of Heteroaromatics, Top. Catal., 2002, 19, 43 CrossRef CAS.
- For selected examples, see (a) H. Yuan, K.-J. Bi, B. Li, R.-C. Yue, J. Ye, Y.-H. Shen, L. Shan, H.-Z. Jin, Q.-Y. Sun and W.-D. Zhang, Construction of 2-Substituted-3-functionalized Benzofurans via Intramolecular Heck Coupling: Application to Enantioselective Total Synthesis of Daphnodorin B, Org. Lett., 2013, 15, 4742 CrossRef CAS; (b) H. Hammoud, Q. Zhao and L. Desaubry, Synthesis of Hydroxybenzofurans by Condensation of Quinones with Benzoylacetone: Revised Structure of the Adducts, Tetrahedron Lett., 2016, 57, 4044 CrossRef CAS; (c) M. Begala, P. Caboni, M. J. Matos and G. L. Delogu, Unexpected One-Step Synthesis of 3-Benzoyl-2-phenylbenzofurans under Wittig Conditions, Tetrahedron Lett., 2018, 59, 1711 CrossRef CAS; (d) W. Huang, J. Xu, C. L., Z. Chen and Y. Gu, Lewis Acid-Catalyzed Synthesis of Benzofurans and 4,5,6,7-Tetrahydrobenzofurans from Acrolein Dimer and 1,3-Dicarbonyl Compounds, J. Org. Chem., 2019, 84, 2941 CrossRef CAS; (e) P. Natho, L. A. T. Allen, A. J. P. White and P. J. Parsons, Transition-Metal-Free Access to Heteroaromatic-Fused 4-Tetralones by the Oxidative Ring Expansion of the Cyclobutanol Moiety, J. Org. Chem., 2019, 84, 9611 CrossRef CAS PubMed; (f) L. Wang, Y. Zhang, M. Zhang, P. Bao, X. Lv, H.-G. L., X. Zhao, J.-S. Li, Z. Luo and W. Wei, Metal-Free I2O5-Mediated Oxidative Synthesis of Sulfonylated Benzofurans through Cyclization Reaction of 1,6-Enynes and Arylsulfonylhydrazides, Tetrahedron Lett., 2019, 60, 1845 CrossRef CAS.
- (a) M. A. Ferreira, M. Moir and R. H. Thomson, New pterocarpenes from Brya Ebenus, J. Chem. Soc., Perkin Trans. 1, 1974, 2429 RSC; (b) M. Halabalaki, N. Aligiannis, Z. Papoutsi, S. Mitakou, P. Moutsatsou, C. Sekeris and A. L. Skaltsounis, Three New Arylobenzofurans from Onobrychis ebenoides and Evaluation of Their Binding Affinity for the Estrogen Receptor, J. Nat. Prod., 2000, 63, 1672 CrossRef CAS; (c) J. Takashima, S. Asano and A. Ohsaki, Planta Med., 2002, 68, 621 CrossRef CAS PubMed; (d) H. Tanaka, T. Oh-Uchi, H. Etoh, M. Sako, M. Sato, T. Fukai and Y. Tateishi, An Arylbenzofuran and Four Isoflavonoids from the Roots of Erythrina Poeppigiana, Phytochemistry, 2003, 63, 597 CrossRef CAS PubMed; (e) H. Tanaka, M. Hirata, H. Etoh, M. Sako, M. Sato, J. Murata, H. Murata, D. Darnaedi and T. Fukai, Six New Constituents from the Roots of Erythrina Variegata, Chem. Biodiversity, 2004, 1, 1101 CrossRef CAS; (f) D. S. Jang, J. M. Kim, Y. M. Lee, Y. S. Kim, J.-H. Kim and J. S. Kim, Puerariafuran, a New Inhibitor of Advanced Glycation End Products (AGEs) Isolated from the Roots of Pueraria Lobate, Chem. Pharm. Bull., 2006, 54, 1315 CrossRef CAS; (g) M. Halabalaki, X. Alexi, N. Aligiannis, M. N. Alexis and A. L. Skaltsounis, Ebenfurans IV–VIII from Onobrychis Ebenoides: Evidence that C-Prenylation is the Key Determinant of the Cytotoxicity of 3-Formyl-2-arylbenzofurans, J. Nat. Prod., 2008, 71, 1934 CrossRef CAS; (h) P. H. Nguyen, T. N. A. Nguyen, T. T. Dao, H. W. Kang, D. T. Ndinteh, J. T. Mbafor and W. K. Oh, AMP-Activated Protein Kinase (AMPK) Activation by Benzofurans and Coumestans Isolated from Erythrina Abyssinica, J. Nat. Prod., 2010, 73, 598 CrossRef CAS PubMed; (i) S. D. Chen, H. Gao, Q. C. Zhu, Y. Q. Wang, T. Li, Z. Q. Mu, H. L. Wu, T. Peng, X. S. Yao and A.-E. Houttuynoids, Anti-Herpes Simplex Virus Active Flavonoids with Novel Skeletons from Houttuynia cordata, Org. Lett., 2012, 14, 1772 CrossRef CAS; (j) G. Y. Luo, M. Zhou, Y. Liu, Q. Ye, J. Gu, T. F. Huang, G. L. Zhang and Y. G. Luo, 3-Formyl-2-Arylbenzofurans from the Aerial Parts of Itea Ilicifolia, Phytochem. Lett., 2014, 10, 19 CrossRef CAS.
- (a) Z. Yang, H. B. Liu, C. M. Lee, H. M. Chang and H. N. C. Wong, Compounds from Danshen. Part 7. Regioselective Introduction of Carbon-3 Substituents to 5-Alkyl-7-methoxy-2-phenylbenzo[b]furans: Synthesis of a Novel Adenosine A1 Receptor Ligand and its Derivatives, J. Org. Chem., 1992, 57, 7248 CrossRef CAS; (b) R. Gastpar, M. Goldbrunner, D. Marko and E. von Angerer, Methoxy-Substituted 3-Formyl-2-phenylindoles Inhibit Tubulin Polymerization, J. Med. Chem., 1998, 41, 4965 CrossRef CAS.
- Y. Tang, C. Jiang, X. Zhang, C. Liu, J. Lin, Y. Wang, C. Du, X. Peng, W. Li, Y. Liu and M. Cheng, Collective Syntheses of 2-(3-Methylbenzofuran-2-yl)phenol-Derived Natural Products by a Cascade [3,3]-Sigmatropic Rearrangement/Aromatization Strategy, J. Org. Chem., 2017, 82, 11102 CrossRef CAS PubMed.
- Although 2,2-dimethyl-1,3-propandiol was used in MOM deprotection, EtOH also gave favorable results; J. H. Koh and M. R. Gagne, PdII- and PtII-Mediated Polycyclization Reactions of 1,5- and 1,6-Dienes: Evidence in Support of Carbocation Intermediates, Angew. Chem., Int. Ed., 2004, 43, 3459 CrossRef CAS PubMed.
- See reported reactions to go through diprotonated or dicationic intermediates under acidic conditions. (a) T. Suzuki, T. Ohwada and K. Shudo, Superacid-Catalyzed Electrocyclization of 1-Phenyl-2-propen-1-ones to 1-Indanones. Kinetic and Theoretical Studies of Electrocyclization of Oxonium-Carbenium Dications, J. Am. Chem. Soc., 1997, 119, 6774 CrossRef CAS; (b) D. A. Klumpp, Y. Zhang, M. J. O'Connor, P. M. Esteves and L. S. de Almeida, Aza-Nazarov Reaction and the Role of Superelectrophiles, Org. Lett., 2007, 9, 3085 CrossRef CAS; (c) N. Ghavtadze, R. Fröhlich, K. Bergander and E.-U. Würthwein, Superelectrophilic Intermediates in the Synthesis of Novel Indenole Derivatives via 1,5-Cyclization Reactions of 5-Aryl-1-azapenta-1,4-dien-3-ones, Synthesis, 2008, 21, 3397 Search PubMed; (d) H. Kurouchi, K. Kawamoto, H. Sugimoto, S. Nakamura, Y. Otani and T. Ohwada, Activation of Electrophilicity of Stable Y-Delocalized Carbamate Cations in Intramolecular Aromatic Substitution Reaction: Evidence for Formation of Diprotonated Carbamates Leading to Generation of Isocyanates, J. Org. Chem., 2012, 77, 9313 CrossRef CAS; (e) Y.-K. Wu, T. Niu and F. G. West, Construction of α-Amido-indanones via Formal Allenamide Hydroacylation-Nazarov Cyclization, Chem. Commun., 2012, 48, 9186 RSC.
- For reviews on (CF3)2CHOH; (a) T. Sugiishi, M. Matsugi, H. Hamamoto and H. Amii, Enhancement of Stereoselectivities in Asymmetric Synthesis Using Fluorinated Solvents, Auxiliaries, and Catalysts, RSC Adv., 2015, 5, 17269 RSC; (b) J. Wencel-Delord and F. A. Colobert, A Remarkable Solvent Effect of Fluorinated Alcohols on Transition Metal Catalysed C–H Functionalizations, Org. Chem. Front., 2016, 3, 394 RSC; (c) C. Yu, J. Sanjosé-Orduna, F. Patureau and M. Pérez-Temprano, Emerging Unconventional Organic Solvents for C–H Bond and Related Functionalization Reactions, Chem. Soc. Rev., 2020, 49, 1643 RSC.
- (a) V. Pozhydaiev, M. Power, V. Gandon, J. Moran and D. Lebœuf, Exploiting Hexafluoroisopropanol (HFIP) in Lewis and Brønsted Acid-Catalyzed Reactions, Chem. Commun., 2020, 56, 11548 RSC; (b) M. A. Hussein, A. H. Dinh, V. T. Huynh and T. V. Nguyen, Synthesis of Tertiary Amines by Direct Brønsted Acid Catalyzed Reductive Amination, Chem. Commun., 2020, 56, 8691 RSC.
- M. A. Marangoni, P. A. Moraes, A. F. Camargo, H. G. Bonacorso, M. A. P. Martins and N. Zanatta, Synthesis of a Novel 1,4-Dicarbonyl Scaffold – Ethyl 3-Formyl-4,5-dihydro-furan-2-carboxylate- and Its Application to the Synthesis of Pyridazines, Synthesis, 2020, 52, 2528 CrossRef CAS.
- The related conversions are known to require heating under basic conditions; (a) Z. V. Chirkova, M. V. Kabanova, D. V. Luferenko, S. I. Filimonov and I. G. Abramov, Formation of 4-Hydroxy-5-[aryl(alkyl)-1H-pyrazol-4-yl]benzene-1,2-dicarbo-nitriles in Reactions of Benzofurans with Hydrazines, Russ. J. Org. Chem., 2015, 51, 644 CrossRef CAS; (b) K. Srinivas, R. Sharma and C. V. Ramana, Interrupting Base-Mediated Benzofuran Ring Transformation with Michael Acceptors, J. Org. Chem., 2017, 82, 9816 CrossRef CAS; (c) I. Khelifi, G. Zhao, N. Ghermani, O. Provot and M. Alami, Unexpected Oxidative Ring Opening of Electron-Rich 3-Aminobenzofurans into α-Ketoimines Derivatives, J. Org. Chem., 2019, 84, 1725 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra06080a |
| This journal is © The Royal Society of Chemistry 2022 |