Open Access Article
Open Access ArticleCopper-catalyzed cross coupling reaction of sulfonyl hydrazides with 3-aminoindazoles†
Guipeng Feng *a,
Jie Mengb,
Shaohong Xua,
Yao Gaoa,
Yingying Zhua and
Ziyu Huanga
*a,
Jie Mengb,
Shaohong Xua,
Yao Gaoa,
Yingying Zhua and
Ziyu Huanga
aSchool of Pharmacy, Xinxiang University, Xinxiang 453003, P. R. China. E-mail: fengguipengheda@163.com
bSchool of Basic Medical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, 250012, P.R. China
First published on 25th October 2022
Abstract
A novel Cu-catalyzed radical–radical cross coupling reaction of 3-aminoindazoles with sulfonyl hydrazides has been disclosed, enabling the production of diverse 1,3-substituted aminoindazoles in good yields. This methodology is distinguished by readily available starting materials, wide substrate scope and operational simplicity. In addition, a gram-scale reaction has been well demonstrated.
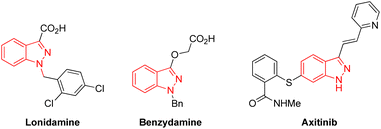
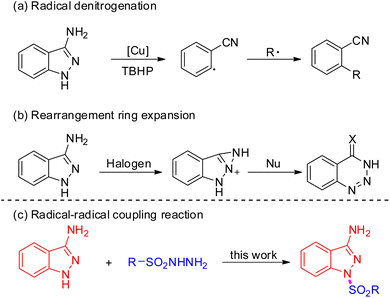
Indazoles have emerged as privileged core structural motifs in pharmaceutically relevant compounds, which exhibit potent antitumor, anti-HIV, antidepressant, anti-inflammatory, and contraceptive activity (Fig. 1).1 Among them, 3-aminoindazoles, possessing a free amine group and an indazole skeleton, have emerged as versatile reagents widely applied in the construction of valuable nitrogen-containing heterocyclic compounds.2 In general, the condensation annulation of 3-aminoindazoles with carbonyl compounds represented straightforward and efficient access to various polynitrogen heterocycles.3 Many delightful achievements have also been made in radical-initiated denitrogenative reactions in the past several years, which represents one of the most significant achievements in synthetic chemistry (Scheme 1a).4 For instance, Song's group developed a Cu-catalyzed denitrogenative ring-opening of 3-aminoindazoles to provide cyano substituted aryl radicals through oxidative cleavage the two C–N bonds.5 Later on, Liu and co-workers demonstrated a Cu-catalyzed oxidative dual arylation of acrylamides for the synthesis of cyanoarylated oxindoles.6 In addition, the rearrangement of 3-aminoindazoles with nucleophilic reagents provides a simple and reliable approach to afford diverse complex nitrogen-containing heterocycles (Scheme 1b).7 In 2018, the Song group firstly discovered a novel oxidative rearrangement of 3-aminoindazoles, enabling the synthesis of 1,2,3-benzotriazine-4(3H)-ones.7a In 2020, the group of Cui further reported an iodine-catalyzed oxidative rearrangement of 3-aminoindazoles with anilines for straightforward synthesis of 1,2,3-benzotriazole.7b Although a variety of condensation annulation, denitrogenative transannulation and rearrangement ring expansion have been well studied, the radical–radical cross coupling reaction of 3-aminoindazoles with sulfonyl hydrazide has never been documented so far.
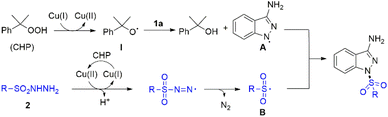
Due to the unique biological and chemical properties of sulfone compound, it allows the sulfone group to be installed in drugs or potentially active molecules.8 For instance, mesotrione could serve as an important herbicide; bicalutamide was proved to show anticancer property; eletriptan is an antimigraine agent.9 Therefore, the development of novel, versatile strategies to construct different useful skeletons bearing sulfonyl groups would be highly significant in organic synthesis. On the other hand, sulfonyl hydrazide moiety has gained significant attention, which could readily release hydrazide group to generate the sulfonyl radicals in situ under metal-free catalysts or transition metal catalysts conditions.10 However, methods for the construction of molecules bearing both a sulfonyl group and 3-aminoindazole motif have not been reported. Based on the above consideration, herein, we describe a novel Cu-catalyzed oxidative coupling strategy of 3-aminoindazoles with sulfonyl hydrazides for affording diverse functionalized 1,3-substituted aminoindazoles (Scheme 1c).
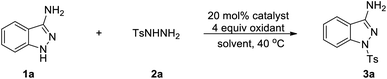
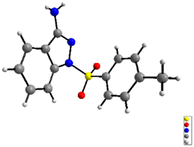
We initiated our investigation with 1H-indazol-3-amine (1a) and 4-methylbenzenesulfonohydrazide (2a) as the starting materials to determine the optimal conditions (Table 1). To our delight, the 1-tosyl-1H-indazol-3-amine 3a was obtained in 22% yield in the presence of Cu(OAc)2·H2O and TBHP in CH3CN at 40 °C under air for 18 h (Table 1, entry 1). The structure of 3a was confirmed by X-ray crystallographic analysis (Fig. 2). Encouraged by this result, we then turned our attention to optimize different solvents for this coupling reaction and the product yield in DMSO was as high as 29% (entries 2–5). Subsequently, a variety of copper salts were investigated and CuI gave the best result (entries 4–7). At this juncture, the effect of different oxidants was also investigated; conspicuously, CHP (cumene hydroperoxide) displayed the best performance (56% yield) compared with H2O2 or K2S2O8 (entries 14 and 15). When K2CO3 was added into the reaction system, the yield of the target product was increased to 65% (entry 16). When changing the molar ratio of substrates by using 1a (0.3 mmol) and 2a (0.2 mmol), the yield of the reaction was increased to 73% (entry 17). Moreover, when the reaction was performed in the absence of CuI or CHP under otherwise identical conditions, a stagnant reaction was observed, implying that metal catalyst and oxidant were all obligatory for this transformation (entries 18 and 19).
| Entry | Catalyst | Oxidant | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.36 mmol, 1.5 equiv.), oxidant (0.8 mmol, 4.0 equiv.), and solvent (2.0 mL) in a test tube at 40 °C for 18 h.b Isolated yields.c 2 equiv. K2CO3.d 1a (0.3 mmol, 1.5 equiv.), 2a (0.2 mmol). n.d. = not detected. TBHP = tert-butyl hydroperoxide (70% in water). CHP = cumene hydroperoxide. | ||||
| 1 | Cu(OAc)2·H2O | TBHP | CH3CN | 22 |
| 2 | Cu(OAc)2·H2O | TBHP | DCE | 24 |
| 3 | Cu(OAc)2·H2O | TBHP | THF | <5 |
| 4 | Cu(OAc)2·H2O | TBHP | EA | 18 |
| 5 | Cu(OAc)2·H2O | TBHP | DMSO | 29 |
| 6 | CuI | TBHP | DMSO | 40 |
| 7 | CuBr | TBHP | DMSO | 37 |
| 8 | CuBr2 | TBHP | DMSO | <5 |
| 9 | Cu(acac)2 | TBHP | DMSO | 27 |
| 10 | Cu(OTf)2 | TBHP | DMSO | <5 |
| 11 | CuSO4·5H2O | TBHP | DMSO | 12 |
| 12 | Cu(Tc) | TBHP | DMSO | 25 |
| 13 | CuI | CHP | DMSO | 56 |
| 14 | CuI | H2O2 | DMSO | 14 |
| 15 | CuI | K2S2O8 | DMSO | n.d. |
| 16c | CuI | CHP | DMSO | 65 |
| 17d | CuI | CHP | DMSO | 73 |
| 18 | CHP | DMSO | n.d. | |
| 19 | CuI | DMSO | n.d. | |
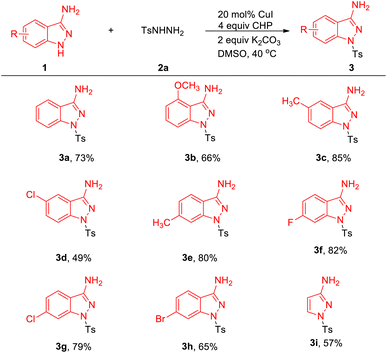
With these optimized reaction conditions in hand, we evaluated the scope of 1H-indazol-3-amine 1 (Table 2). Delightedly, electron-donating and electron-withdrawing substituents on aryl rings of 1H-indazol-3-amines were well tolerated under optimized reaction conditions and proceeded well with 2a, resulting in the products 3a–3h in 53–85% yields.11 Gratifyingly, halogen-containing motifs (3d, 3g, 3h) also exhibited excellent reactivity under the reaction conditions, highlighting the potential of this process in combination with further conventional cross-coupling transformations. It is noteworthy that 1H-pyrazol-3-amine was also compatible with this coupling reaction and corresponding product 3i was obtained in 57% yield.
| a Reaction conditions: 1 (0.3 mmol, 1.5 equiv.), 2a (0.2 mmol), CuI (0.04 mmol, 20 mol%), CHP (0.8 mmol, 4.0 equiv.), K2CO3 (0.4 mmol, 2.0 equiv.) and DMSO (2.0 mL) in a test tube at 40 °C for 18 h. |
|---|
 |
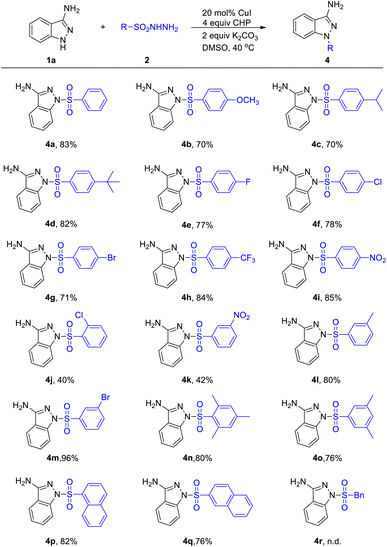
We next evaluated the scope of this coupling reaction with sulfonyl hydrazides as the substrates (Table 3). Pleasingly, various ortho-, meta-, and para-substituted arylsulfonyl hydrazides underwent smooth sulfonylations and provided the desired products (4a–4m) in good to excellent yields. Strong electron withdrawing group, such as NO2 and CF3, were proved to be suitable substituents to deliver products 4h, 4i and 4k in satisfactory yield. Moreover, increased steric congestion was inconspicuous and 2-Cl-phenylsulfonyl hydrazide could be converted into the corresponding product in 40% yields (4j). When introducing multiple substituents to the benzene ring, it has a slight effect on the yields (4n and 4o). To our delight, naphthalene-1-sulfonohydrazide and naphthalene-2-sulfonohydrazide were shown to be slightly less efficient yet nonetheless suitable substrates, affording the desired products 4p and 4q in 82% and 76% yield, respectively. It is regrettable that when phenylmethanesulfonohydrazide was used as a coupling component, it does not work (4r).
| a Reaction conditions: 1a (0.3 mmol, 1.5 equiv.), 2 (0.2 mmol), CuI (0.04 mmol, 20 mol%), CHP (0.8 mmol, 4.0 equiv.), K2CO3 (0.4 mmol, 2.0 equiv.) and DMSO (2.0 mL) in a test tube at 40 °C for 18 h. |
|---|
 |
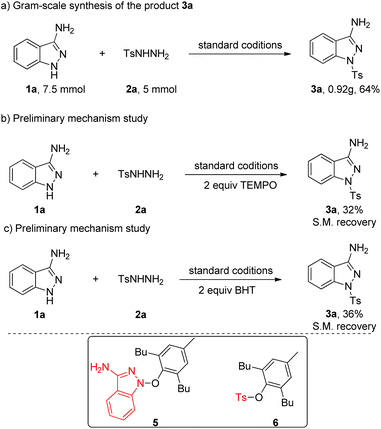
We also inspected the scalability of this copper-catalyzed coupling reaction and the current protocol could be readily executed on a gram scale by successfully reacting 5 mmol of 2a with 1a in one pot to obtain 3a in 69% yield (Scheme 2a). To shed light on the reaction mechanism for this transformation, control experiments were performed, as shown in Scheme 2b and c. As expected, the addition of well-known radical scavenger TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) or BHT (3,5-di-tert-butyl-4-hydroxytoluene) suppressed the reaction, the yield of the corresponding target product 3a has been greatly reduced and only remaining starting material is separated, which indicated that free radical intermediate may be involved in this transformation. Then we carefully analyzed the reaction solution in the presence of BHT, radical adducts 5 and 6 were detected by LC-MS, respectively, which confirms that this transformation undergoes a free radical process.
Based on the above experiments and previous studies from the literature,4c,12 we propose a plausible mechanism for this transformation, which is depicted in Scheme 3. Initially, Cu(I) species is oxidized by CHP through one-electron transfer to obtain the radical I and the Cu(II) species. The radical I abstracts one hydrogen atom from the 1H-indazol-3-amine 1a to generate the radical intermediate A and releases 2-phenylpropan-2-ol. Meanwhile, the sulfonyl radical B was generated was generated from 2 in the presence of copper catalyst and CHP via single electron transfer and deprotonation process. Then the coupling reaction of radical intermediate A and radical intermediate B gave final product.
Conclusions
In summary, we have implemented a novel Cu-catalyzed radical–radical cross coupling reaction of 3-aminoindazoles with sulfonyl hydrazides to furnish a range of 1,3-substituted aminoindazole derivatives under mild conditions. Our strategy features easily available starting materials, operational simplicity, broad substrate scope and good functionality tolerance. Moreover, a gram-scale reaction has been well conducted. Preliminary mechanistic studies suggest that this coupling reaction may undergo a free radical process. Further explorations on the synthetic utility of this transformation are currently underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from University-Industry Collaborative Education Program (202102144039, 202101226005).Notes and references
- (a) D. D. Gaikwad, A. D. Chapolikar, C. G. Devkate, K. D. Warad, A. P. Tayade, R. P. Pawar and A. J. Domb, Eur. J. Med. Chem., 2015, 90, 707–731 CrossRef CAS; (b) Y. Hu, D. Cole, R. A. Denny, D. R. Anderson, M. Ipek, Y. Ni, X. Wang, S. Thaisrivongs, T. Chamberlain, J. P. Hall, J. Liu, M. Luong, L.-L. Lin, J.-B. Telliez and A. Gopalsamy, Bioorg. Med. Chem. Lett., 2011, 21, 4758–4761 CrossRef CAS PubMed; (c) J. Schoene, T. Gazzi, P. Lindemann, M. Christmann, A. Volkamer and M. Nazaré, ChemMedChem, 2019, 14, 1514–1527 CrossRef CAS PubMed; (d) D. D. Gaikwad, A. D. Chapolikar, C. G. Devkate, K. D. Warad, A. P. Tayade, R. P. Pawar and A. J. Domb, Eur. J. Med. Chem., 2015, 90, 707–731 CrossRef CAS PubMed; (e) G. Chen, M. Hu and Y. Peng, J. Org. Chem., 2018, 83, 1591–1597 CrossRef CAS.
- (a) Y. Guo and Q. Gao, Org. Biomol. Chem., 2022, 20, 7138–7150 RSC; (b) W.-C. Yang, C.-Y. Chen, J.-F. Li and Z.-L. Wang, Chin. J. Catal., 2021, 42, 1865–1875 CrossRef CAS.
- (a) S. G. Balwe and Y. T. Jeong, Org. Chem. Front., 2018, 5, 1628–1632 RSC; (b) S. G. Balwe, S. S. Vagh and Y. T. Jeong, Tetrahedron Lett., 2020, 61, 152101 CrossRef CAS; (c) W. Kong, Y. Zhou and Q. Song, Adv. Synth. Catal., 2018, 360, 1943–1948 CrossRef CAS; (d) Y. Zhou, Y. Lou, Y. Wang and Q. Song, Org. Chem. Front., 2019, 6, 3355–3359 RSC; (e) X. Geng, Z. Xu, Y. Cai and L. Wang, Org. Lett., 2021, 23, 8343–8347 CrossRef CAS; (f) X. Liu, J. Zhou, J. Lin, Z. Zhang, S. Wu, Q. He and H. Cao, J. Org. Chem., 2021, 86, 9107–9116 CrossRef CAS; (g) J. Zhou, W. Li, H. Zheng, Y. Pei, X. Liu and H. Cao, Org. Lett., 2021, 23, 2754–2759 CrossRef.
- (a) Y. Zhou, Y. Wang, Y. Lou and Q. Song, Org. Lett., 2019, 21, 8869–8873 CrossRef CAS PubMed; (b) Y. Zhou, Y. Wang, Y. Lou and Q. Song, Chem. Commun., 2019, 55, 10265–10268 RSC; (c) Y. Zhou, L. Lin, Y. Wang, J. Zhu and Q. Song, Org. Lett., 2019, 21, 7630–7634 CrossRef CAS; (d) Y. Zhou, Y. Wang, Z. Song, T. Nakano and Q. Song, Org. Chem. Front., 2020, 7, 25–29 RSC; (e) J. Teng, S. Sun, J.-T. Yu and J. Cheng, J. Org. Chem., 2019, 84, 15669–15676 CrossRef CAS.
- Y. Zhou, S. Deng, S. Mai and Q. Song, Org. Lett., 2018, 20, 6161–6165 CrossRef CAS.
- Y. Guo, P.-F. Huang, B.-Q. Xiong, J.-H. Fan and Y. Liu, Org. Biomol. Chem., 2022, 20, 6844–6853 RSC.
- (a) Y. Zhou, Y. Wang, Y. Lou and Q. Song, Org. Lett., 2018, 20, 6494–6497 CrossRef CAS PubMed; (b) J. Ren, X. Yan, X. Cui, C. Pi, Y. Wu and X. Cui, Green Chem., 2020, 22, 265–269 RSC.
- (a) A. V. Ivachtchenko, E. S. Golovina, M. G. Kadieva, V. M. Kysil, O. D. Mitkin, S. E. Tkachenko and I. M. Okun, J. Med. Chem., 2011, 54, 8161–8173 CrossRef CAS; (b) X.-Y. Zhu, M. Li, Y.-P. Han, S. Chen, X.-S. Li and Y.-M. Liang, J. Org. Chem., 2017, 82, 8761–8768 CrossRef CAS.
- K. Hofman, N. W. Liu and G. Manolikakes, Chem.–Eur. J., 2018, 24, 11852–11863 CrossRef CAS.
- (a) J. Zhu, W.-C. Yang, X.-d. Wang and L. Wu, Adv. Synth. Catal., 2018, 360, 386–400 CrossRef CAS; (b) K. Hofman, N.-W. Liu and G. Manolikakes, Chem.–Eur. J., 2018, 24, 11852–11863 CrossRef CAS PubMed; (c) J. Liu and L. Zheng, Adv. Synth. Catal., 2019, 361, 1710–1732 CrossRef CAS; (d) O. M. Mulina, A. I. Ilovaisky, V. D. Parshin and A. O. Terent'ev, Adv. Synth. Catal., 2020, 362, 4579–4654 CrossRef CAS.
- CCDC 2203869 for 3a.†.
- (a) K. Sun, X.-L. Chen, S.-J. Li, D.-H. Wei, X.-C. Liu, Y.-L. Zhang, Y. Liu, L.-L. Fan, L.-B. Qu, B. Yu, K. Li, Y.-Q. Sun and Y.-F. Zhao, J. Org. Chem., 2018, 83, 14419–14430 CrossRef CAS; (b) Y. Liu, G. Zheng, Q. Zhang, Y. Li and Q. Zhang, J. Org. Chem., 2017, 82, 2269–2275 CrossRef CAS; (c) K. Sun, X.-L. Chen, S.-J. Li, D.-H. Wei, X.-C. Liu, Y.-L. Zhang, Y. Liu, L.-L. Fan, L.-B. Qu, B. Yu, K. Li, Y.-Q. Sun and Y.-F. Zhao, J. Org. Chem., 2018, 83, 14419–14430 CrossRef CAS; (d) F. Chen, Y. Shao, M. Li, C. Yang, S.-J. Su, H. Jiang, Z. Ke and W. Zeng, Nat. Commun., 2021, 12, 3304 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2203869. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ra05956h |
| This journal is © The Royal Society of Chemistry 2022 |