Open Access Article
Open Access Article2D TiVCTx layered nanosheets grown on nickel foam as highly efficient electrocatalysts for the hydrogen evolution reaction
Yi Wen ,
Junsheng Yang*,
Haoran Zou,
Yiquan Fan,
Jie Li,
Yijian Kuang,
Wenkang Liu,
Kaisong Zhang and
Lieqiang Xiong
,
Junsheng Yang*,
Haoran Zou,
Yiquan Fan,
Jie Li,
Yijian Kuang,
Wenkang Liu,
Kaisong Zhang and
Lieqiang Xiong
School of Mechanical Engineering, Wuhan Polytechnic University, Wuhan, 430023, Hubei, China. E-mail: yangjunsheng2008@163.com; Fax: +86-027-85617871
First published on 18th August 2022
Abstract
Exploring highly efficient and durable catalysts for the hydrogen evolution reaction (HER) is crucial for the hydrogen economy and environmental protection issues. Numerous studies have now found that transition metal carbide MXenes are ideal candidates as catalysts for the hydrogen evolution reaction. However, MXenes are inclined to easily undergo lamellar structure agglomeration and stacking, which impedes their further applications. Besides, most of the extant research has focused on single transition metal carbides, and the investigation of double transition metal carbide MXenes is rather rare. In this research work, a three-dimensional (3D) TiVCTx-based conductive electrode was constructed by depositing 2D TiVCTx nanosheets on 3D network structured nickel foam (NF) to synthesize a hybrid electrode material (abbreviated as TiVCTx@NF). TiVCTx@NF exhibits efficient electrochemical properties with a low overpotential of 151 mV at 10 mA cm−2 and a small Tafel slope of 116 mV dec−1. Benefitting from the open layer structure and strong interfacial coupling effect, compared to the pristine structure, the resulting TiVCTx@NF has greatly increased active sites for the hydrogen evolution reaction (HER) and encounters less resistance for charge transfer. In addition, TiVCTx@NF exhibits better stability in long-term acidic electrolytes. This work provides a tactic to prepare three-dimensional network electrode materials and broadens the application of single transition metal carbide MXenes as water splitting electrodes in the HER, which is beneficial to the application of noble metal-free electrocatalysts.
1. Introduction
In recent years, due to the combustion and emission of a variety of chemical fuels, energy shortage and environmental pollution have become global problems. The development of various new and renewable energy sources is an important issue at present. As a secondary energy source, hydrogen energy is clean, efficient, and can be easily stored and transported, and is thus regarded as an ideal energy carrier.1,2 Water splitting is one of the most promising technologies for industrial hydrogen production.3 The best electrocatalysts for the HER are platinum electrodes or noble metals; however, due to the rising price of precious metals, the improvement of the catalytic activity and cost-effectiveness of existing metal hydrogen evolution electrodes has become a primary issue for manufacturers.4,5MXenes are two-dimensional transition metal compounds, which can be expressed as Mn+1XnTx (where M represents a transition metal element, X stands for C or N, and T represents –OH, –F and other functional groups). Many studies6–9 have shown that MXenes are excellent materials for water electrolysis due to their high specific surface area, good electrical conductivity and stability, which has aroused the extensive interest of scholars. Tang et al.10 proposed that due to the features of the MXene Ti3C2 such as its large surface area, excellent conductivity and adjustable terminations, it can be applied in the hydrogen evolution reaction (HER). Huang et al.11 prepared MoS2/Ti3C2 composite heterostructures by the hydrothermal method. When used as a HER electrocatalyst, MoS2/Ti3C2 exhibits excellent electrochemical activity with a low overpotential of ∼280 mV at 10 mA cm−2. Compared to MoS2 nanosheets, the catalytic current density induced by MoS2/Ti3C2 at an overpotential of ∼400 mV is more than 6.2 times that of the MoS2 nanosheets. Le et al.12 used ammonia heat treatment to enhance the hydrogen evolution catalysis of Ti3C2Tx MXenes by modification with a nitrogen heteroatom. As shown in the experimental results, compared to the Ti3C2Tx MXene, the nitrogen-doped Ti3C2Tx annealed at 600 °C exhibited superior HER electrocatalytic performance with an overpotential as low as 198 mV at 10 mA cm−2 and a much smaller Tafel slope of 92 mV dec−1. Liu et al.13 mixed H8MoN2S4 and Ti3C2Tx to prepare MoS2/Ti3C2Tx layered nanomaterials. The active sites of the two-dimensional nanomaterials are greatly increased, the exchange current density is more than 25 times that of MoS2, and the electrochemical performance is significantly improved.
With the deepening of research, scholars have tried to introduce transition metal elements into MXenes to obtain different electrochemical properties. According to a previous study,14 compared to Ti3C2, the TiVCTx MXene has some unique electrochemical properties due to the addition of the transition metal element V. Yazdanparast et al.15 successfully obtained MXene thin films of TiVCTx by etching and layering. The obtained TiVCTx nanosheets possess a large specific surface area and multiple active sites, and the functional group structure on the TiVCTx MXene has excellent stability. Li et al.16 investigated the electrochemical properties of the TiVCTx MXene, and the results showed that the TiVCTx MXene exhibited metallic properties with high electronic conductivity, and its comprehensive electrochemical performance is better than that of the Ti2C MXene. Li et al.17 prepared TiVCTx MXenes and investigated their electrochemical properties, and discovered that the performance of the TiVCTx MXenes did not yield the desired results. They then used o-phenylenediamine (o-PD) as a metamer for oxidant-free polymerization and mixed it with the prepared TiVCTx to obtain three-dimensionally stable TiVCTx/poly-o-PD hybrids. Electrochemical studies have shown that the TiVCTx/poly-o-PD hybrid possessed a high specific surface area, excellent cycling stability, and improved comprehensive electrochemical performance. Nevertheless, there are few reports on the hydrogen evolution performance of the TiVCTx MXene in the electrolysis of water.
Ni is an abundant transition metal element with the features of low cost and high catalytic activity in hydrogen production by water electrolysis.18 Previous studies have shown that the hybridization of nickel foam (NF) is beneficial to the improvement of the electrochemical performance of nanomaterials.19–22 Herein, a new strategy is proposed, and the novel hybrid TiVCTx@nickel foam (NF) is obtained by compounding nickel foam with layered MXene nanosheets. The surface element distribution, morphology and structure of TiVCTx@NF are systematically studied, and the electrochemical hydrogen evolution performance and stability of the hybrid nanomaterials are further investigated.
2. Experimental
2.1 Preparation of TiVAlC powders
In a preliminary work, we have successfully prepared TiVAlC powders using TiH2 (99.6% pure), V (99.6% pure), Al (99.5% pure) and C (99.8% pure) powders with an atomic ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by the activation reaction sintering method at a sintering temperature of 1500 °C, and the specific details about the preparation of the MAX TiVAlC are shown in Zou's study.23 Then, the fabricated TiVAlC powders were broken by a crushing machine (GJ500-1, Hangzhou, China) and sieved through a mesh 325 sieve.
1 by the activation reaction sintering method at a sintering temperature of 1500 °C, and the specific details about the preparation of the MAX TiVAlC are shown in Zou's study.23 Then, the fabricated TiVAlC powders were broken by a crushing machine (GJ500-1, Hangzhou, China) and sieved through a mesh 325 sieve.
2.2 Preparation of TiVCTx nanosheets
Layered TiVCTx was synthesized by liquid etching of the as-prepared TiVAlC powders. The etching was carried out by immersing the TiVAlC powders in a 40% HF solution with a solid-to-liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and stirred at 500 rpm for 48 h at 45 °C. Then, the suspension was centrifuged 7–8 times (3 min at a time) at 3500 rpm to separate the multilayered MXene. The centrifuged suspension supernatant was washed and filtered with distilled water until the pH of the supernatant was equal to ∼7. After that, the multilayered TiVCTx MXene was obtained. The obtained multilayered TiVCTx was stirred with 25% TMAOH solution for 24 h at room temperature. Then, the mixture was centrifuged at 8500 rpm for 5 min, and the supernatant was collected. The TiVCTx nanosheets were obtained by washing the supernatant with distilled water until the pH of the supernatant was neutral and filtered. The final TiVCTx nanosheet powders were dried at 50 °C for 12 h in a vacuum oven (DZF-6020, Shanghai, China).
20 and stirred at 500 rpm for 48 h at 45 °C. Then, the suspension was centrifuged 7–8 times (3 min at a time) at 3500 rpm to separate the multilayered MXene. The centrifuged suspension supernatant was washed and filtered with distilled water until the pH of the supernatant was equal to ∼7. After that, the multilayered TiVCTx MXene was obtained. The obtained multilayered TiVCTx was stirred with 25% TMAOH solution for 24 h at room temperature. Then, the mixture was centrifuged at 8500 rpm for 5 min, and the supernatant was collected. The TiVCTx nanosheets were obtained by washing the supernatant with distilled water until the pH of the supernatant was neutral and filtered. The final TiVCTx nanosheet powders were dried at 50 °C for 12 h in a vacuum oven (DZF-6020, Shanghai, China).
2.3 Preparation of TiVCTx nanosheets@NF
After sonication for 1 h, 5 mg of the TiVCTx nanosheets and 20 μL Nafion solution (5 wt%) were dispersed in a 980 μL deionized water/ethanol mixed solution with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then, 5 μL of the mixed solution was evenly added to commercial NF (1 cm × 1 cm). After that, the NF loaded with the catalyst was put into a vacuum sintering furnace and sintered in vacuum at 300 °C for 2 h, and TiVCTx nanosheets@NF was obtained.
1. Then, 5 μL of the mixed solution was evenly added to commercial NF (1 cm × 1 cm). After that, the NF loaded with the catalyst was put into a vacuum sintering furnace and sintered in vacuum at 300 °C for 2 h, and TiVCTx nanosheets@NF was obtained.
2.4 Characterization
The sample powders were analyzed by X-ray diffraction (XRD, D/MAX-rA) using an X-ray diffractometer equipped with Cu Kα radiation (λ = 1.541 Å). The morphologies of the samples were characterized by scanning electron microscopy (SEM, JSM-5600LV) and transmission electron microscopy (TEM, F200X). The elemental composition of the samples was characterized by scanning electron microscopy equipped with energy dispersive X-ray spectrometry (EDS). The sample surface was characterized by X-ray photoelectron spectroscopy using Mg Kα as the excitation source.2.5 Electrocatalytic performance evaluation
All electrochemical measurements were carried out using a typical three-electrode configuration with an electrochemical workstation (CHI660E, Shanghai, China). TiVCTx nanosheets@NF was used as the working electrode (the loading weight is 0.025 mg cm−2), and an Ag/AgCl electrode and glassy carbon electrode were used as the reference electrode and counter electrode, respectively. The HER testing was performed in 0.5 mol L−1 H2SO4.For the HER, the potential calibration of the reversible hydrogen electrode (RHE) was calibrated with respect to the following equation:24
| E(RHE) = E(Ag/AgCl) + 0.0591 pH + 0.196 | (1) |
Cyclic voltammetry (CV) was used to measure the double-layer capacitance (Cdl), which was recorded from −6 to 94 mV vs. RHE at scan rates from 20 to 120 mV s−1 at an interval of 20 mV s−1. Linear sweep voltammetry (LSV) was performed at a scan rate of 10 mV s−1 with 90% IR compensation by sweeping the potential from 0 to −0.6 V vs. RHE. Electrochemical impedance spectroscopy (EIS) was performed in a frequency range of 100 kHz to 0.01 Hz at an overpotential of open-circuit potential (OCP). The long-term stability test was performed by successive CV at a scan rate of 100 mV s−1 for 1000 cycles. Lastly, chronoamperometric measurements at the requisite overpotential to afford 10 mA cm−2 were used to evaluate the long-term durability.
3. Results and discussion
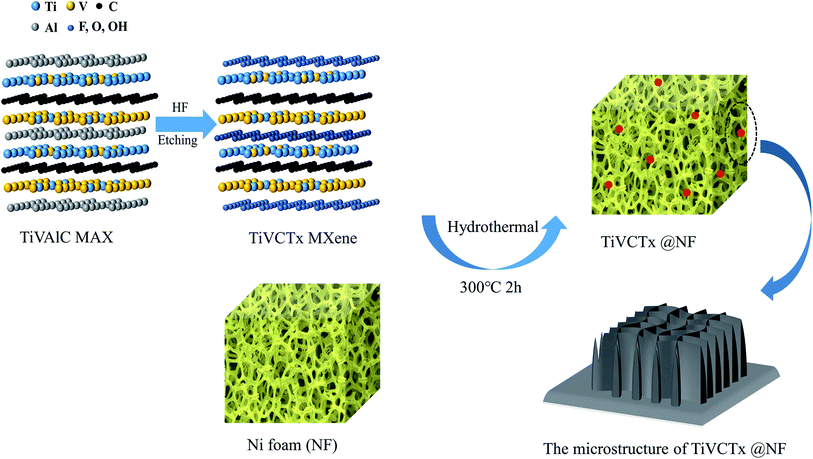
In this study, multi-layered TiVCTx MXenes with different abundant functional groups were successfully prepared through the etching method of removing Al atoms from TiVAlC powder using HF. Ultrathin nanostructured TiVCTx was obtained by exfoliation. Next, the TiVCTx nanosheet powders were loaded on commercial nickel foam (NF), and then placed in a vacuum furnace for annealing at 300 °C for 2 h to obtain TiVCTx@NF hybrids (Fig. 1). Fig. 2(a) shows an SEM image of the etched products. It can be clearly seen that a lamellar morphology appears, and these nanosheets are produced by HF etching away the Al in TiVAlC. In order to acquire the micromorphological and structural information of the TiVCTx MXene in more detail, Fig. 2(b) shows a TEM image of multilayer TiVCTx and Fig. 2(c) shows a TEM image of few-layer TiVCTx. The multilayered TiVCTx nanosheets exhibit clearly visible ripples, indicating the ultrathin feature of the TiVCTx nanosheets. The multilayered TiVCTx nanosheets present an unordered vertical arrangement. By calculation, the average nanolayer spacing is 2.2 nm (Fig. 2(d and e)).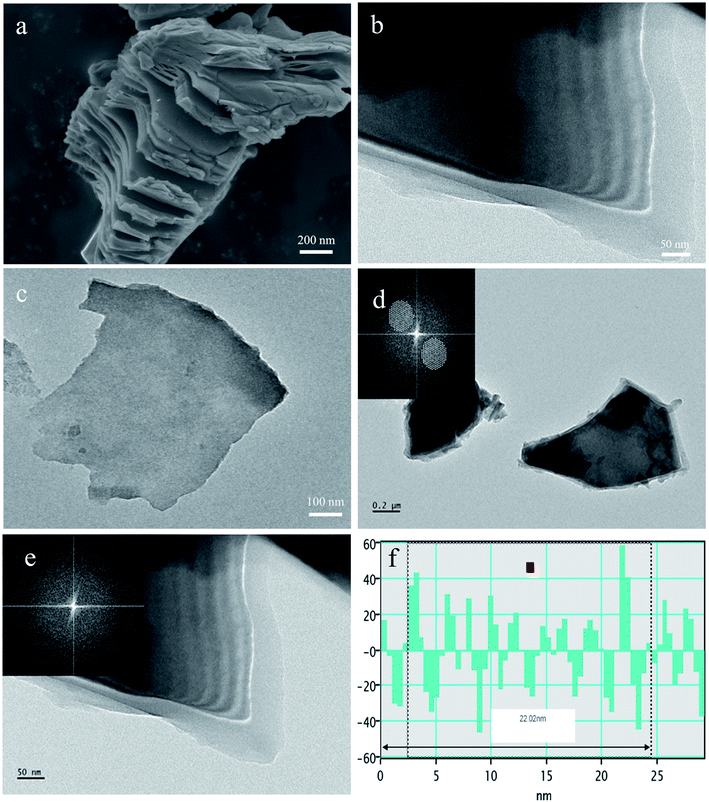 | ||
| Fig. 2 (a) SEM images of layered TiVCTx. (b and c) TEM images of TiVCTx. (d–f) The interlayer analysis of TiVCTx. | ||
Fig. 3(a) shows the XRD patterns of TiVAlC and the TiVCTx MXene after the HF solution etching. It can be seen that the XRD patterns before and after TiVAlC etching are obviously different, indicating the successful synthesis of the TiVCTx MXene. After the etching of TiVAlC, the diffraction peak intensity at 40.38° is significantly weakened.
Additionally, in the spectrum of the TiVCTx MXene, a diffraction peak appears at a small angle of 6.85°, indicating the introduction of other molecules (according to a study by Li et al.17). Fig. 3(b) shows the surface element distribution of the TiVCTx MXene. It can be seen that the Ti, V, C, F, and O elements are well and uniformly dispersed on the nanosheet structure. The presence of the element O is due to the intercalation of oxygen-containing groups between the nanosheets.
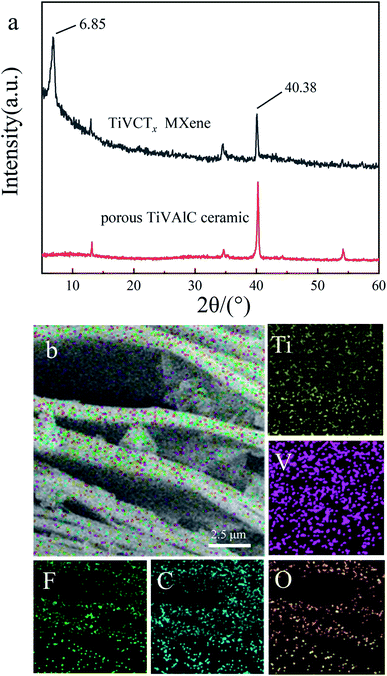 | ||
| Fig. 3 (a) XRD patterns of the TiVCTx MXene and TiVAlC powder. (b) Elemental mapping images of layered TiVCTx nanosheets. | ||
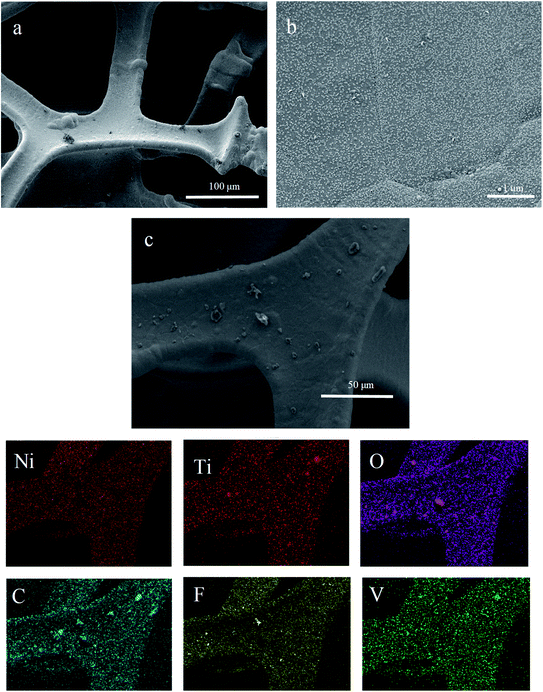
Fig. 4 shows the micromorphology and the results of the elemental distribution of the TiVCTx@NF hybrid materials. Fig. 4(a) exhibits the microstructure of the porous nickel foam framework. In Fig. 4(b), it can be clearly seen that plenty of white nanoparticles are attached to the surface of the nickel foam after hydrothermal treatment. In Fig. 4(c), it can be obviously seen that TiVCTx@NF is composed of Ni, Ti, O, C, F and V, and the six elements are uniformly distributed, indicating the successful synthesis of TiVCTx@NF.
As shown in Fig. 5, XPS was used to analyze the element valence composition of TiVCTx, and PeakFit was used to perform peak processing on the XPS data, and the corresponding characteristic peaks were fixed according to the binding energy. As shown in Fig. 5(b), the spectrum of Ti 2p contains two singlets and one doublet; the peaks at 454.8 eV and 455.36 eV correspond to Ti–C; the peaks at 456.3 eV, 459.2 eV and 464.70 eV correspond to Ti–O; and the peak at 461 eV corresponds to Ti–F. As shown in Fig. 5(c), the binding energy peaks identified for TiVCTx at 281.6, 282.1, 285.0, 285.3, and 286.3 eV correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O, graphitic C–C, C–V, and C–Ti. As shown in Fig. 5(d), the spectrum of F 1s contains two singlets and one doublet. The spectrum is easily fitted by one doublet peak of F–C, which contains two peaks at binding energies of 687.5 eV and 688.5 eV. The peak at 685.4 eV is related to the characteristic F–V, and the peak at 686.4 eV corresponds to C–Ti–Fx. The results suggest the embedding of –F in the MXene layered structure. As shown in Fig. 5(e), the spectra of V 2p and O 1s contain V–C, VxOy, V–F, O–Ti, O
O, C–O, graphitic C–C, C–V, and C–Ti. As shown in Fig. 5(d), the spectrum of F 1s contains two singlets and one doublet. The spectrum is easily fitted by one doublet peak of F–C, which contains two peaks at binding energies of 687.5 eV and 688.5 eV. The peak at 685.4 eV is related to the characteristic F–V, and the peak at 686.4 eV corresponds to C–Ti–Fx. The results suggest the embedding of –F in the MXene layered structure. As shown in Fig. 5(e), the spectra of V 2p and O 1s contain V–C, VxOy, V–F, O–Ti, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) V and –OH peaks, indicating the successful etching of Al and the combination of O. In general, it can be clearly seen from the above results that the Al atomic layer is completely etched, and new functional groups (–F, –OH, –O) are introduced. The addition of these functional groups (–F, –OH, –O) may increase the HER catalytic activity of the MXene nanosheets.25 With the increased adsorption energy of protons, the charge transfer resistance decreases. Due to the surface functional groups, the TiVCTx nanosheets are negatively charged. Combining the analysis results, the following equations are obtained:
V and –OH peaks, indicating the successful etching of Al and the combination of O. In general, it can be clearly seen from the above results that the Al atomic layer is completely etched, and new functional groups (–F, –OH, –O) are introduced. The addition of these functional groups (–F, –OH, –O) may increase the HER catalytic activity of the MXene nanosheets.25 With the increased adsorption energy of protons, the charge transfer resistance decreases. Due to the surface functional groups, the TiVCTx nanosheets are negatively charged. Combining the analysis results, the following equations are obtained:
| 2TiVAlC + 6HF → 2TiVC + 2AlF3 + 3H2↑ | (2) |
| TiVC + 2HF → TiVCF2 + H2↑ | (3) |
| TiVC + 2H2O → TiVCO2 + 2H2↑ | (4) |
| TiVC + 2H2O → TiVC(OH)2 + H2↑ | (5) |
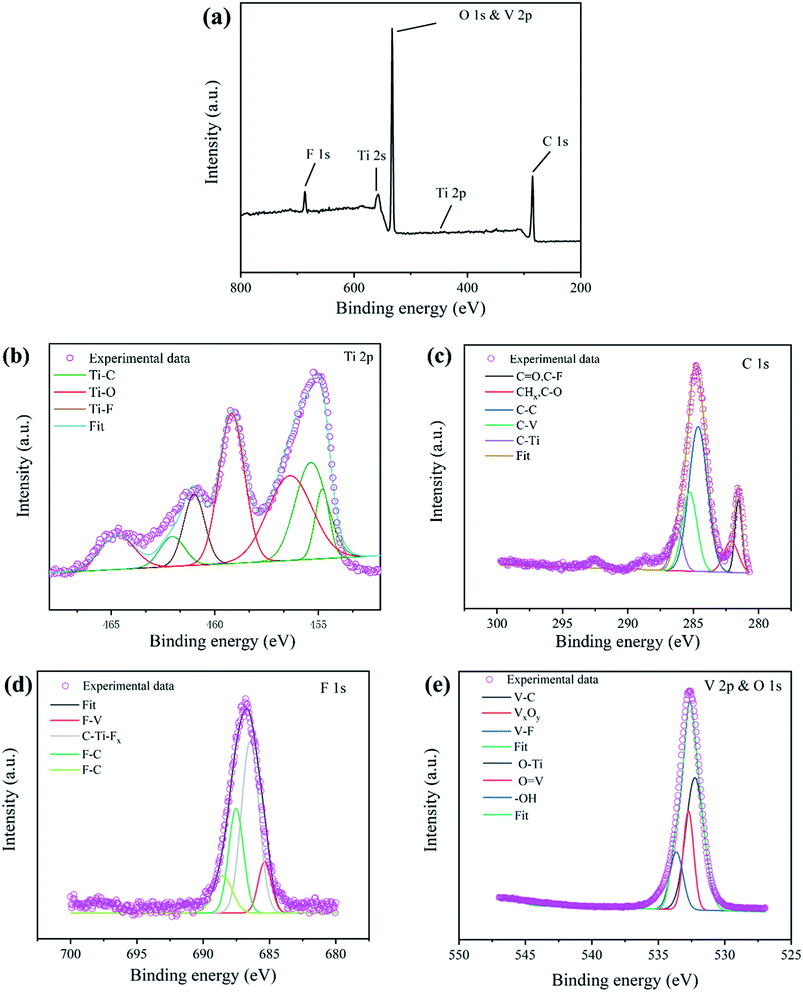 | ||
| Fig. 5 XPS spectra of the TiVCTx MXene: (a) the full spectrum, (b) Ti 2p, (c) C 1s, (d) F 1s, (e) V 2p and O 1s. | ||
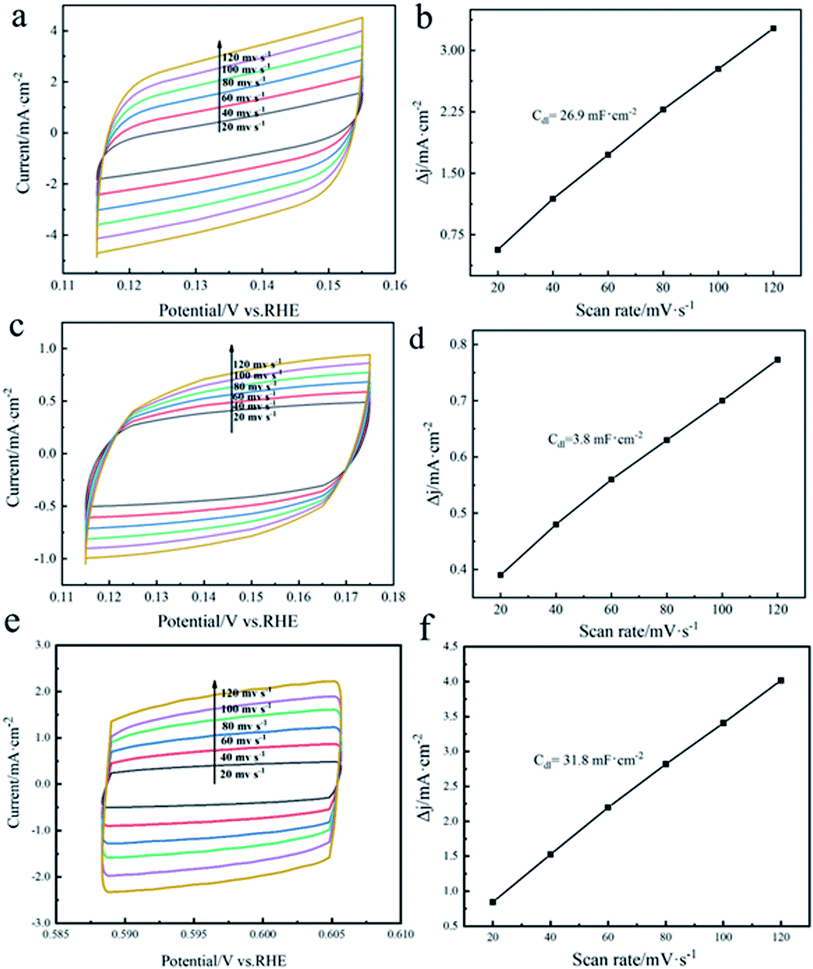
Electrochemical double-layer capacitance (Cdl) is an electrochemical parameter that can evaluate the difference in the electrochemically active surface area of different samples and can be determined by cyclic voltammetry (CV).26 The number of active sites is one of the important factors determining the HER activity, and the electrochemically active surface area depends on the number of active sites. Fig. 6(a), (c) and (e) present the cyclic voltammetry curves of TiVCTx@NF, TiVCTx and commercial Pt/C, respectively, measured at scan rates of 20–120 mV s−1 with an interval of 20 mV s−1. Fig. 6(b), (d) and (f) present the curves of capacitive current versus scan rate for TiVCTx@NF, TiVCTx and commercial Pt/C at 0.15 V, respectively. It can be seen from the above results that the Cdl of TiVCTx@NF is 26.9 mF cm−2, which is much larger than that of TiVCTx (3.8 mF cm−2), indicating that through the combination with nickel foam, the specific surface area is increased, the active sites are increased, and the hydrogen evolution catalytic performance is enhanced. Besides, the Cdl of TiVCTx@NF is close to the Cdl of the Pt/C electrode (31.8 mF cm−2).
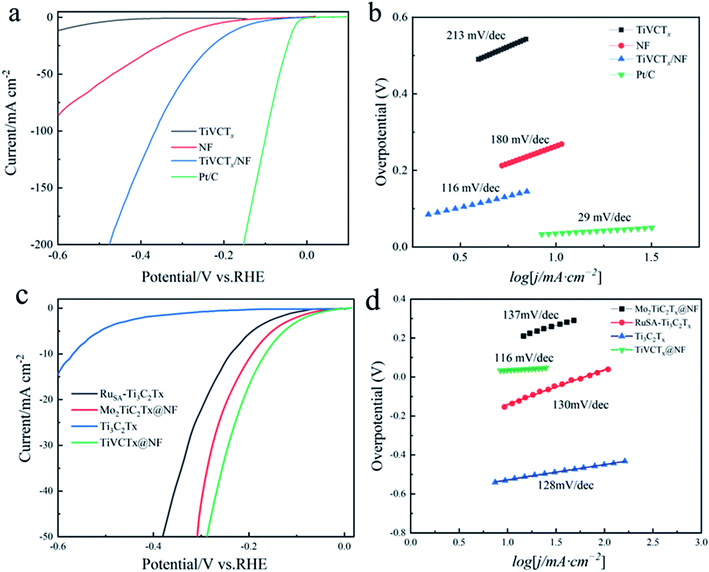
In order to explain the good electrochemical behavior of the TiVCTx nanosheets, the relevant tests on their hydrogen evolution performance were carried out. Using a standard three-electrode electrochemical system, the electrochemical activity of TiVCTx and its hybrid products for the HER was tested in a 0.5 mol L−1 H2SO4 electrolyte. Besides, the commercial platinum–carbon catalyst (10 wt% Pt/C) with extremely high hydrogen evolution activity and pure nickel foam (NF) were used as references in this test. Fig. 7(a) shows the HER polarization curves of TiVCTx, NF, TiVCTx@NF and commercial platinum–carbon in 0.5 mol L−1 H2SO4. It can be seen from the figure that the hydrogen evolution overpotential of the TiVCTx nanosheets obtained after exfoliation at 10 mA cm−2 is 583 mV, while the hydrogen evolution overpotential of TiVCTx@NF after modification is 151 mV, which is close to that of the platinum–carbon electrode (33 mV). The hydrogen evolution overpotential of NF at 10 mA cm−2 is 261 mV, indicating that the hydrogen evolution catalytic activity of NF is stronger than that of TiVCTx, and the hydrogen evolution catalytic activity is further improved after the combination of TiVCTx and NF. TiVCTx@NF can be selected as a candidate for excellent hydrogen evolution catalyst materials.
The Tafel slope reveals the rate-determining steps of the hydrogen evolution catalytic reaction. Generally, when the Tafel slope reaches 30 mV, 40 mV or 120 mV, the rate-determining step of the hydrogen evolution catalytic reaction is identified as a Tafel, Heyrovsky or Volmer step, respectively. To understand the kinetics of the hydrogen evolution catalytic reaction of the hybrid TiVCTx@NF in the HER, the Tafel slope was used to reveal its reaction rate-determining steps.27 Fig. 7(b) shows the Tafel slope curves of the four samples in 0.5 mol L−1 H2SO4. It can be seen that the Tafel slopes of TiVCTx, NF, TiVCTx@NF and commercial platinum–carbon are 213 mV dec−1, 180 mV dec−1, 116 mV dec−1 and 29 mV dec−1, respectively. The electrochemical test results show that the water splitting of the hybrid TiVCTx@NF in the hydrogen evolution catalytic process is based on the Volmer–Heyrovsky reaction mechanism,28 which involves the rapid hydrogen ion adsorption Volmer reaction (H3O+ + e− → Hads + H2O) and the slow hydrogen desorption Heyrovsky reaction (Hads + H3O+ + e− → H2 + H2O). Previous studies have shown that the active site of the HER is generally the –OH group, and the two basic steps of water splitting are as follows: (1) H3O+ ions are first adsorbed on the –OH group and then combined with electrons to generate a hydrogen atom. (2) The hydrogen atom generated in step 1 combines with an H3O+ ion and an electron to generate H2. The hydrogen desorption process in step 2 is the rate-determining step of the hydrogen evolution catalytic reaction. In addition, other scholars have reported the excellent electrochemical performance of MXene electrodes, as shown in Fig. 7(c) and (d); with the same electrolyte conditions, the TiVCTx@NF electrodes are superior to MXene electrodes (Ti3C2Tx,29 RuSA-Ti3C2Tx (ref. 30) and Mo2TiC2Tx@NF31).
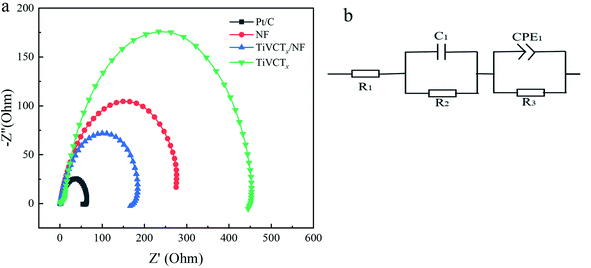
Furthermore, electrochemical impedance spectroscopy (EIS) was used to reveal the electron transfer kinetics of TiVCTx@NF during the hydrogen evolution reaction. Fig. 8(a) presents a Nyquist plot of the four samples. In the Nyquist diagram, the impedance value represents the resistance encountered in the transfer of charges in the HER, and the smaller the arc radius, the smaller the impedance value of the test sample, indicating that the hydrogen evolution catalytic reaction is more likely to occur. The size of the semicircle is related to the electrode surface properties and determines the size of the charge transfer resistance (Rct). It is obvious that the impedance value of the hybrid TiVCTx@NF is significantly lower than that of the TiVCTx MXene. The electrochemical impedance spectrum is fitted using the Z-view software; the fitted resistance circuit diagram is shown in Fig. 8(b) and the related resistance parameter fitting results are presented in Table 1. R1 represents the equivalent series resistance (ESR), which consists of three parts: the ionic resistance of the electrolyte, the resistance inside the active material and the current collector, and the interfacial contact resistance between the electrode and the electrolyte. R2 represents the diffusion resistance, which refers to the resistance caused by the transport of ions in a thin layer, which is related to the current changes, concentration changes, and electrode materials. R3 represents the charge transfer resistance (Rct). As shown in Table 1, the fitted resistance of TiVCTx@NF is smaller than that of the other two electrode materials, and the R3 of TiVCTx@NF is only 186.5 Ω. This indicates that the TiVCTx@NF electrode system has a smaller internal resistance and larger electroactive surface, and it is easier for ions to penetrate between the sheets of the electrode material. These results indicate that TiVCTx@NF has better electrochemical performance.
| Working electrode | R1 | R2 | R3 | C1 | CPE1-T | CPE2-T |
|---|---|---|---|---|---|---|
| TiVCTx | 15.62 | 115.1 | 2334 | 1.02 × 10−5 | 6.39 × 10−6 | 0.8533 |
| NF | 15.34 | 9.447 | 442.8 | 2.89 × 10−5 | 5.17 × 10−6 | 0.8693 |
| TiVCTx@NF | 1.33 | 3.695 | 186.5 | 0.0079 | 0.0021 | 0.8247 |
To evaluate the comprehensive performance of a hydrogen evolution catalyst, it is necessary not only to consider the catalytic activity of the hydrogen evolution reaction but also to test its stability. In order to examine the catalytic stability of the TiVCTx@NF composites in 0.5 mol L−1 H2SO4, long-term CV cycling tests for 1000 cycles were carried out. As shown in Fig. 9(a), the time-dependent electrical density curve remains almost unchanged for the HER after 1000 test cycles. As shown in Fig. 9(b), the catalytic activity for hydrogen evolution of TiVCTx@NF remains almost unchanged at 0.15 V for 12 h. From the above results, it can be seen that the hybrid TiVCTx@NF exhibits excellent HER catalytic activity and good stability during long-term electrolysis, indicating that TiVCTx@NF is a highly stable electrocatalyst for HER.
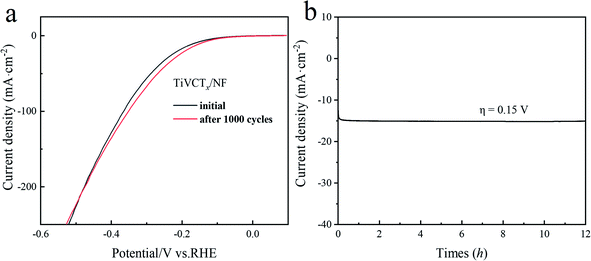 | ||
| Fig. 9 (a) Polarization curves of TiVCTx@NF initially and after 1000 CV cycles. (b) Current density versus time for TiVCTx@NF at a 150 mV potential. | ||
4. Conclusion
In summary, TiVCTx@NF was successfully prepared using the annealing method. Selecting a typical three-electrode system, the comprehensive electrochemical performance of TiVCTx@NF was tested. The experimental results show that the composite TiVCTx@NF exhibits excellent electrochemical properties with a low overpotential of 151 mV at 10 mA cm−2 and a small Tafel slope of 116 mV dec−1. In a 0.5 M H2SO4 solution, TiVCTx@NF exhibits excellent HER catalytic activity after 1000 test cycles and long-term stability over 12 h. Compared to the TiVCTx MXene, TiVCTx@NF has more active sites and less resistance encountered in the transfer of charges in the HER. Based on these excellent electrochemical properties, TiVCTx@NF is expected to be applied as an electrocatalytically active catalyst for water splitting.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work is financially supported by the Natural Science Foundation of China (51704221) and Outstanding Youth Science Foundation of Wuhan Polytechnic University (2018J05).References
- S. M. Fernández-Valverde, in Hydrogen: The Ecological Fuel for Mexican Future, Springer, 2007 Search PubMed.
- S. H. Jensen, P. H. Larsen and M. Mogensen, Hydrogen and synthetic fuel production from renewable energy sources, Int. J. Hydrogen Energy, 2007, 32, 3253–3257 CrossRef CAS.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Solar water splitting cells, Chem. Rev., 2010, 110, 6446–6473 CrossRef CAS PubMed.
- Y. Wei, R. A. Soomro, X. Xie and B. Xu, Design of efficient electrocatalysts for hydrogen evolution reaction based on 2D MXenes, J. Energy Chem., 2021, 55, 244–255 CrossRef.
- Z. Li, Z. Qi, S. Wang, T. Ma, L. Zhou, Z. Wu, X. Luan, F. Lin, M. Chen and J. T. Miller, In situ formed Pt3Ti nanoparticles on a two-dimensional transition metal carbide (MXene) used as efficient catalysts for hydrogen evolution reactions, Nano Lett., 2019, 19, 5102–5108 CrossRef CAS PubMed.
- H. Wang, Y. Wu, J. Zhang, G. Li, H. Huang, X. Zhang and Q. Jiang, Enhancement of the electrical properties of MXene Ti3C2 nanosheets by post-treatments of alkalization and calcination, Mater. Lett., 2015, 160, 537–540 CrossRef CAS.
- Z. Guo, L. Gao, Z. Xu, S. Teo, C. Zhang, Y. Kamata, S. Hayase and T. Ma, High electrical conductivity 2D MXene serves as additive of perovskite for efficient solar cells, Small, 2018, 14, 1802738 CrossRef PubMed.
- Y. Zhang, X. Zha, K. Luo, Y. Qin, X. Bai, J. Xu, C. Lin, Q. Huang and S. Du, Theoretical study on the electrical and mechanical properties of MXene multilayer structures through strain regulation, Chem. Phys. Lett., 2020, 760, 137997 CrossRef CAS.
- Z. W. Seh, K. D. Fredrickson, B. Anasori, J. Kibsgaard, A. L. Strickler, M. R. Lukatskaya, Y. Gogotsi, T. F. Jaramillo and A. Vojvodic, Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution, ACS Energy Lett., 2016, 1, 589–594 CrossRef CAS.
- R. Tang, S. Xiong, D. Gong, Y. Deng, Y. Wang, L. Su, C. Ding, L. Yang and C. Liao, Ti3C2 2D MXene: recent progress and perspectives in photocatalysis, ACS Appl. Mater. Interfaces, 2020, 12, 56663–56680 CrossRef CAS PubMed.
- L. Huang, L. Ai, M. Wang, J. Jiang and S. Wang, Hierarchical MoS2 nanosheets integrated Ti3C2 MXenes for electrocatalytic hydrogen evolution, Int. J. Hydrogen Energy, 2019, 44, 965–976 CrossRef CAS.
- T. A. Le, Q. V. Bui, N. Q. Tran, Y. Cho, Y. Hong, Y. Kawazoe and H. Lee, Synergistic effects of nitrogen doping on MXene for enhancement of hydrogen evolution reaction, ACS Sustainable Chem. Eng., 2019, 7, 16879–16888 CrossRef CAS.
- J. Liu, Y. Liu, D. Xu, Y. Zhu, W. Peng, Y. Li, F. Zhang and X. Fan, Hierarchical “nanoroll” like MoS2/Ti3C2Tx hybrid with high electrocatalytic hydrogen evolution activity, Appl. Catal., B, 2019, 241, 89–94 CrossRef CAS.
- Z. He, T. Rong, Y. Li, J. Ma, Q. Li, F. Wu, Y. Wang and F. Wang, Two-Dimensional TiVC Solid-Solution MXene as Surface-Enhanced Raman Scattering Substrate, ACS Nano, 2022, 16, 4072–4083 CrossRef CAS PubMed.
- S. Yazdanparast, S. Soltanmohammad, A. Fash-White, G. J. Tucker and G. L. Brennecka, Synthesis and surface chemistry of 2D TiVC solid-solution MXenes, ACS Appl. Mater. Interfaces, 2020, 12, 20129–20137 CrossRef CAS PubMed.
- Y. Li, L. Li, R. Huang, Y. Zhang and Y. Wen, Computational screening of pristine and functionalized ordered TiVC MXenes as highly efficient anode materials for lithium-ion batteries, Nanoscale, 2021, 13, 2995–3001 RSC.
- Y. Li, J. Zhang, Y. Cheng, K. Feng, J. Li, L. Yang and S. Yin, Stable TiVCTx/poly-o-phenylenediamine composites with three-dimensional tremella-like architecture for supercapacitor and Li-ion battery applications, Chem. Eng. J., 2022, 134578 CrossRef CAS.
- J. R. McKone, B. F. Sadtler, C. A. Werlang, N. S. Lewis and H. B. Gray, Ni-Mo nanopowders for efficient electrochemical hydrogen evolution, ACS Catal., 2013, 3, 166–169 CrossRef CAS.
- G. Wang, J. Huang, S. Chen, Y. Gao and D. Cao, Preparation and supercapacitance of CuO nanosheet arrays grown on nickel foam, J. Power Sources, 2011, 196, 5756–5760 CrossRef CAS.
- W. Kong, C. Lu, W. Zhang, J. Pu and Z. Wang, Homogeneous core-shell NiCo2S4 nanostructures supported on nickel foam for supercapacitors, J. Mater. Chem. A, 2015, 3, 12452–12460 RSC.
- T. Hussain, L. Truong, S. Hussain, S. A. Pital, S. Chun and M. Kaseem, Novel core-shell structured electrocatalyst with 1D-NiTe2 over 3D metal structure for efficient hydrogen evolution reactions, J. Alloys Compd., 2022, 165797 CrossRef CAS.
- T. Hussain, M. Hussain, S. Hussain and M. Kaseem, Microwave-assisted synthesis of NiTe2 photocatalyst as a facile and scalable approach for energy-efficient photocatalysis and detoxification of harmful organic dyes, Sep. Purif. Technol., 2022, 282, 120025 CrossRef CAS.
- H. Zou, X. Li, C. Zhang, Y. Wen, Y. Fan, Y. Liu, L. Xiong, X. Zheng and J. Yang, Reactive synthesis for porous TiVAlC ceramics by TiH2, V, Al and graphite powders, Ceram. Int., 2021, 47, 28288–28295 CrossRef CAS.
- S. Li, P. Tuo, J. Xie, X. Zhang, J. Xu, J. Bao, B. Pan and Y. Xie, Ultrathin MXene nanosheets with rich fluorine termination groups realizing efficient electrocatalytic hydrogen evolution, Nano Energy, 2018, 47, 512–518 CrossRef CAS.
- X. Wang, J. Wang, J. Qin, X. Xie, R. Yang and M. Cao, Surface charge engineering for covalently assembling three-dimensional MXene network for all-climate sodium ion batteries, ACS Appl. Mater. Interfaces, 2020, 12, 39181–39194 CrossRef CAS PubMed.
- P. Sharma and T. S. Bhatti, A review on electrochemical double-layer capacitors, Energy Convers. Manage., 2010, 51, 2901–2912 CrossRef CAS.
- S. A. Vilekar, I. Fishtik and R. Datta, Kinetics of the hydrogen electrode reaction, J. Electrochem. Soc., 2010, 157, B1040 CrossRef CAS.
- A. Lasia, Mechanism and kinetics of the hydrogen evolution reaction, Int. J. Hydrogen Energy, 2019, 44, 19484–19518 CrossRef CAS.
- A. D. Handoko, K. D. Fredrickson, B. Anasori, K. W. Convey, L. R. Johnson, Y. Gogotsi, A. Vojvodic and Z. W. Seh, Tuning the basal plane functionalization of two-dimensional metal carbides (MXenes) to control hydrogen evolution activity, ACS Appl. Energy Mater., 2017, 1, 173–180 CrossRef.
- V. Ramalingam, P. Varadhan, H. C. Fu, H. Kim, D. Zhang, S. Chen, L. Song, D. Ma, Y. Wang and H. N. Alshareef, Heteroatom-mediated interactions between ruthenium single atoms and an MXene support for efficient hydrogen evolution, Adv. Mater., 2019, 31, 1903841 CrossRef CAS PubMed.
- J. Wang, P. He, Y. Shen, L. Dai, Z. Li, Y. Wu and C. An, FeNi nanoparticles on Mo2TiC2Tx MXene@nickel foam as robust electrocatalysts for overall water splitting, Nano Res., 2021, 14, 3474–3481 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |