Open Access Article
Open Access ArticlePreparation and performance of chlorfenapyr microcapsules with a degradable polylactide-based polyurethane wall material
Linfang Zhu a,
Guangqi Jiang
a,
Guangqi Jiang *ab,
Jun Cena and
Linhuai Lia
*ab,
Jun Cena and
Linhuai Lia
aCollege of Chemistry and Chemical Engineering, Guizhou University, Guiyang, China
bState Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, Guiyang, China. E-mail: gqjiang@163.com
First published on 7th June 2022
Abstract
To improve the utilization rate of chlorfenapyr and make the wall material of chlorfenapyr microcapsules easily degradable, polylactide diol, toluene diisocyanate and 1,4-butanediol were used to prepare a chlorfenapyr microcapsule suspension by interfacial polymerization. The product was characterized by the methods of optical microscopy, scanning electron microscopy and Fourier-transform infrared spectroscopy. The results indicated that the microcapsule particles were spherical, with an encapsulation efficiency of 84.20%. The diluted product had good wetting and spreading abilities on cabbage leaves. Compared with other commercial formulations, the slow-release effect of the microcapsule suspension was more obvious and the release mechanisms conform to Fickian diffusion, with the release rate controllable by adjusting the external pH conditions. Furthermore, the wall material of the microcapsules showed good degradation performance in a phosphate-buffered solution. Microencapsulation by this method significantly increased the validity period of chlorfenapyr and the wall material was also degraded easily.
1 Introduction
Pesticide-based slow and controlled release systems allow the active ingredient of the pesticide to be released continuously and stably, so that the lowest effective level of pesticide can be maintained for a long time.1 Microencapsulation is a common method used to achieve slow and controlled release performance for many kinds of pesticides, and the slow and controlled release performance is mainly determined by the properties of the active ingredients of the pesticide and the nature of the wall materials of the microcapsules.2 Microencapsulation of pesticides can reduce the loss of pesticides by volatilization and degradation when exposed to external environments, thereby enhancing the utilization rate and reducing the toxicity of the pesticides.3 Recently, H. W. An et al. fabricated a universal drug delivery platform based on an intracellular self-assembly strategy to achieve effective drug delivery.4 H. Sawalha et al. demonstrated that the properties of the capsules could be optimized through the polymer solidification process, and the choice of appropriate non-solvents and oils can have an important role in the preparation of such capsules.5Polyurethane (PU) is widely used in the fields of medicine,6–8 pesticides9 and materials10 due to its low cost and good thermal stability. PU is also one of the commonly used materials in the preparation of microcapsules.11,12 However, PU is difficult to degrade and can pollute soil environments. To develop green and environmentally-friendly pesticide formulations, degradable materials have been used as wall materials for microcapsules and this has become a focus for agricultural chemists.13,14 Nowadays, it is easily to obtain degradable PU by adding biodegradable substances in its preparation process.15,16 Among these substances, polylactic acid (PLA) is low cost and has good degradability and biocompatibility. The final products of PLA degradations are CO2 and H2O, which are friendly to humans and the environment, resulting in PLA often being used as a carrier material for drugs and cells in encapsulation fields.17–20
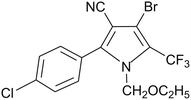
Chlorfenapyr is a widely used insecticide and acaricide in vegetables, fruits, and other crops, but damage to crops occasionally occurs in some areas and chlorfenapyr is highly toxic to aquatic organisms, thereby limiting its applications in agriculture.21–23 Commonly, commercial formulations of chlorfenapyr are microemulsions (ME), emulsifiable concentrates (EC) and other traditional formulations. Its microcapsule suspension with a general PU wall material has rarely appeared in the market and to the best of our knowledge, chlorfenapyr microcapsule suspension (MS) with a degradable PU wall material have not been reported so far. The structure of chlorfenapyr is shown in Fig. 1.
In this study, chlorfenapyr microcapsules were prepared by interfacial polymerization with optimized preparation conditions. The slow-release performance differences between the microcapsule formulation and other commercial formulations were investigated and the release mechanisms were studied. In addition, the wetting and spreading abilities of the diluted microcapsule suspension on the surface of cabbage leaves and the degradation performance of the microcapsule wall material in a phosphate-buffered solution (PBS) solution was also studied. The chlorfenapyr microcapsules showed excellent slow-release, wetting and degradation performance.
2 Materials and methods
2.1 Materials
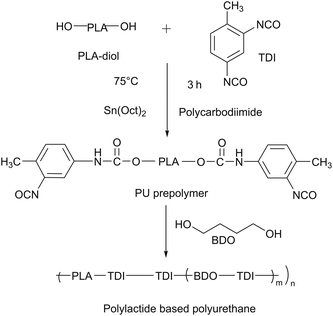
Chlorfenapyr was provided by Capot Chemical Co., Ltd (Zhejiang, China) and used as received. Polylactide diol (PLA-500, 500 g mol−1) was purchased from Hubei Yisheng New Material Co., Ltd (Hubei, China). Toluene diisocyanate (TDI, 98%, 80/20) was acquired from Meicheng International Trade Co., Ltd (Jiangsu, China). The 1,4-butanediol (BDO) is an analytical reagent and was purchased from the Tianjin Damao Chemical Reagent Factory (Tianjin, China). Stannous octoate (Sn(Oct)2, 95%) was provided by Shanghai Tengzhun Biotechnology Co., Ltd (Shanghai, China). Polycarbodiimide was purchased from Dongguan Shunjie Plastic Technology Co., Ltd (Guangdong, China). Agricultural emulsifier 600# was bought from Chengdu Jinshan Chemical Reagent Co., Ltd (Sichuan, China). Sodium dodecyl benzene sulfonate (SDBS) was supplied by the Tianjin Beichen Fangzheng Reagent Factory (Tianjin, China). Span 80 and Tween 20 were purchased from the Jiangsu Hai'an Petrochemical Factory (Jiangsu, China). Cyclohexanone, methanol and n-hexane, as analytical reagents, were bought from the Aladdin Industrial Corporation (Shanghai, China). 10% chlorfenapyr microemulsion was purchased from Guangdong Zhenge Biological Technology Co., Ltd (Guangdong, China). 10% chlorfenapyr suspension concentrate (SC) was bought from BASF SE Co., Ltd (Ludwigshafen, Germany).2.2 Preparation of chlorfenapyr microcapsules
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm to form a uniform O/W emulsion. The emulsion was placed into a 250 mL three-necked flask and 2.95 g of BDO solution were slowly added dropwise at a stirring speed of 600 rpm, before being placed in a 50 °C water bath for constant temperature solidification for 4 h. Finally, the mixture was naturally cooled and a chlorfenapyr microcapsule suspension could be obtained.
000 rpm to form a uniform O/W emulsion. The emulsion was placed into a 250 mL three-necked flask and 2.95 g of BDO solution were slowly added dropwise at a stirring speed of 600 rpm, before being placed in a 50 °C water bath for constant temperature solidification for 4 h. Finally, the mixture was naturally cooled and a chlorfenapyr microcapsule suspension could be obtained.2.3 Characterization
The chlorfenapyr concentration in the solution was determined using a LC-20A high-performance liquid chromatograph (HPLC, Shimadzu, Japan) with a UV detector, three parallel experiments were conducted for each of the microcapsule samples. The test conditions were methanol/water (92/8, v/v) as a mobile phase with a flow rate of 1 mL min−1. The sample injection volume was 5 μL and the detection wavelength was 261 nm.27 The active ingredients were separated using a Zorbax Eclipse XDB C18 column (4.6 mm × 250 mm, 5 μm, Daicel Investment Co., Ltd, China). The above solutions were filtered with a 0.22 μm nylon 66 filter membrane.
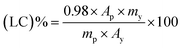 | (1) |
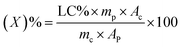 | (2) |
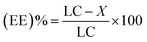 | (3) |
The surface tension values of the prepolymer, microcapsule and diluted samples were measured by an OCA 25 contact angle measuring instrument (Dataphys Instruments Co., Ltd, China) and we maintained the measured surface tension value of distilled water at 72.0 ± 0.2 mN m−1 at the beginning. Each sample was tested in triplicate. The environmental temperature during the measurement was 25 ± 2 °C.
3 Results and discussion
3.1 Screening of preparation conditions
During the preparation of microcapsule suspension, factors such as the emulsifier, core/shell ratio and the ratio of PLA-500 to TDI affected the morphology, encapsulation efficiency and stability of the microcapsule.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. When the core/shell ratio was 1
4. When the core/shell ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the formed capsule wall was thinner and the encapsulating effect of chlorfenapyr was 78.48%. When the core/shell ratio was 1
1, the formed capsule wall was thinner and the encapsulating effect of chlorfenapyr was 78.48%. When the core/shell ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the encapsulation efficiency of the microcapsules increased to 91.83%. However, as the core/shell ratio continued to decrease, the viscosity of the system increased, resulting in a decrease in the encapsulation efficiency. When the core/wall ratio was 1
2, the encapsulation efficiency of the microcapsules increased to 91.83%. However, as the core/shell ratio continued to decrease, the viscosity of the system increased, resulting in a decrease in the encapsulation efficiency. When the core/wall ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the fluidity of the system was so low that the reaction cannot proceed.
4, the fluidity of the system was so low that the reaction cannot proceed.
| Sample | Core/shell | Polycarbodiimide (%) | PLA-500/TDI | Encapsulation efficiency (%) |
|---|---|---|---|---|
| M1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
78.48 |
| M2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
91.83 |
| M3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
76.60 |
| M4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
— |
| M5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
84.20 |
| M6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
82.25 |
| M7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
84.25 |
According to the literature, polylactide-based PU materials have good degradation properties.36,37 The polycarbodiimide can react with the carboxylic acid produced by the hydrolysis of the polyurethane material, which can terminate its self-initiated cracking process, and is often used as a stabilizer for polyurethane materials.36,38 Our experimental results showed that the encapsulation efficiency of the microcapsule suspension without a stabilizer was reduced to ∼40% within 3 days, while the encapsulation efficiency of the microcapsule suspension was still be maintained above 80% after 60 days when 0.5% of stabilizer was added.
As shown in Table 1, the PLA-500/TDI ratio was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 1
2 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to prepare the PU prepolymer. The encapsulation efficiency of the microcapsules reached ∼84% when the PLA-500/TDI ratio was 1
3 to prepare the PU prepolymer. The encapsulation efficiency of the microcapsules reached ∼84% when the PLA-500/TDI ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 1
2 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, but in the case of 1
3, but in the case of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, there was serious agglomeration phenomenon in the suspension.
2, there was serious agglomeration phenomenon in the suspension.
Finnally, agricultural emulsifier 600#, a core/shell ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, stabilizer at 0.5% and a PLA-500/TDI ratio of 1
2, stabilizer at 0.5% and a PLA-500/TDI ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 were selected to prepare the 10% chlorfenapyr microcapsule suspension (sample M5).
3 were selected to prepare the 10% chlorfenapyr microcapsule suspension (sample M5).
3.2 Morphology analysis and element analysis
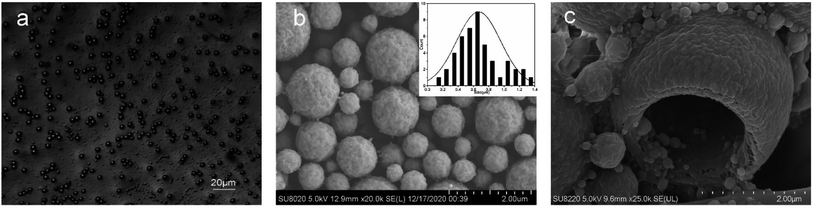
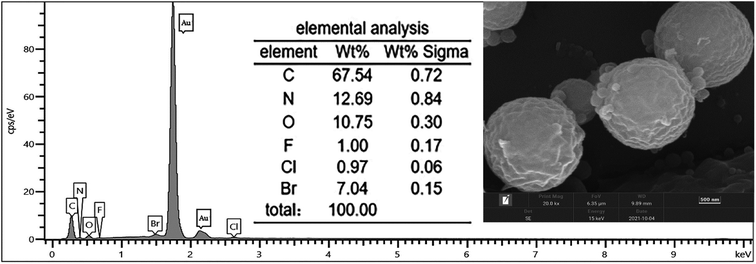
The OM image of the chlorfenapyr microcapsules is shown in Fig. 3(a). It can be seen that the obtained microcapsule had good dispersibility. The SEM images of the microcapsules are shown in Fig. 3(b) and (c). It is clear that the microcapsule particles were spherical and the wall material was relatively dense. Based on SEM image of Fig. 3(b), the particle size distribution of the prepared microcapsules is between 0.19 and 1.32 μm, and the average particle size is 0.667 μm. In Fig. 3(c), some incomplete capsule particles were found, which clearly demonstrated the core/shell structure of the microcapsules.39,40 In order to further verify whether chlorfenapyr is encapsulated in microcapsules, an element mapping analysis was performed using EDS. As shown in Fig. 4, the analysis date showed traces of fluorine, chlorine and bromine, element distribution provided evidence for the successful loading of chlorfenapyr.3.3 Particle size distribution
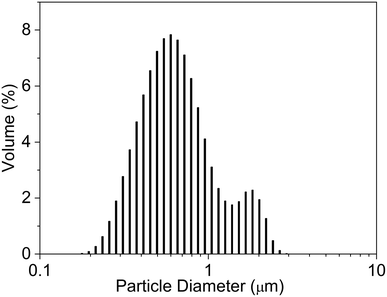
During the microcapsule formation, the shear forces, interfacial tension and viscous stress have a significant influence on the particle size distribution of microcapsules.31 The particle size distribution of the microcapsule sample M5 is given in Fig. 5. The average particle size of the microcapsules was 0.8 μm, the average/median ratio was 1.218 and the particle size distribution range was between 0.147 and 2.92 μm. There are two particle size centers for sample M5.3.4 FTIR spectral analysis
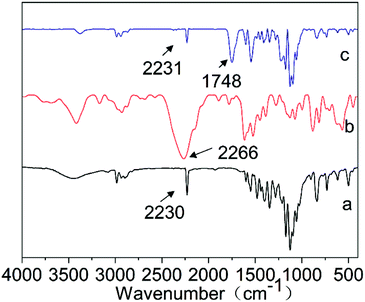
Fig. 6 shows the FTIR spectra of chlorfenapyr, TDI and the microcapsule sample M5. As shown in curve a, the peak at 2230 cm−1 was attributed to the characteristic stretching vibration peak of –CN in chlorfenapyr.41 In curve b, the characteristic absorption peak of –NCO of TDI was at 2266 cm−1.42,43 In curve c, the characteristic peak of –NCO disappeared completely, indicating that the TDI reacted completely in the system and polyurethane was formed.44,45 The appearance of the characteristic absorption peak at 1748 cm−1 proved that PLA was successfully introduced into the microcapsule wall material.2 The curve c had the characteristic peaks of chlorfenapyr, which proved that chlorfenapyr was successfully encapsulated in the microcapsules.3.5 Contact angle and surface tension analysis
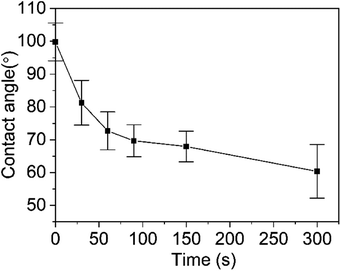
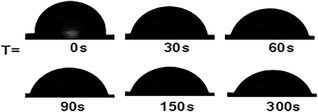
The wetting and spreading abilities of the pesticide formulations on the surface of the crop is often reflected by the contact angle and 90° is used as the limit of whether the droplets can be wetted on the surface of the leaves.46,47 Fig. 7 shows the changes of the contact angle of the diluted microcapsule sample M5 on the surface of the cabbage leaf with time. When the droplet was deposited on the leaf surface, the contact angle was 93.1°. Within 30 s, the contact angle of the droplet dropped rapidly to 79.8°. At 300 s, the contact angle decreased to 68.9°. The dynamic change of the droplet shapes during the wetting process is shown in Fig. 8, where it can be seen that the shape of the droplet changed significantly over time. Furthermore, the surface tension values of the prepolymer, microcapsule and diluted samples were measured. The prepolymer is 3.29 mN m−1, the chlorfenapyr microcapsule suspension is 1.98 mN m−1 and the sample diluted 500-fold with deionized water is 6.13 mN m−1.3.6 Slow-release performance
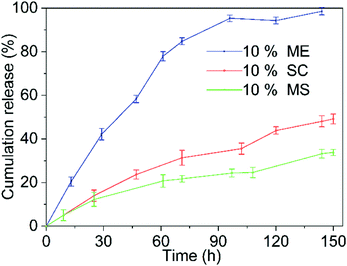
The slow-release performance of the microcapsule was determined by factors such as the active ingredients encapsulated in the microcapsules and the nature of the wall material.35,48 Fig. 9 shows the release curves of the 10% chlorfenapyr microemulsion, 10% chlorfenapyr suspension concentrate and 10% chlorfenapyr microcapsule suspension (microcapsule sample M5), respectively. Among them, the cumulative amount of chlorfenapyr released from the 10% chlorfenapyr microemulsion was 98.57% when the release time was 144 h. The cumulative amount of chlorfenapyr released from the 10% chlorfenapyr suspension concentrate was 49.16% when the time was 150 h. In comparison, the cumulative amount of chlorfenapyr released from the microcapsule sample M5 was much slower, with only 33.80% at 150 h. It can be seen that microencapsulation effectively extended the release time of chlorfenapyr.Fig. 10 shows the slow-release curves for the 10% chlorfenapyr microcapsule suspension in release media with different pH values. When the other conditions were the same, in release media of pH = 9, the release rate of chlorfenapyr was the fastest because alkaline conditions are most conducive to the hydrolysis of polylactide based polyurethane.49 Because the hydrolysis of polylactide-based polyurethane under acidic conditions was also faster than in neutral media, the cumulative release rate of chlorfenapyr at pH = 5 was slightly higher than at pH = 7, which was consistent with previous results.26,50
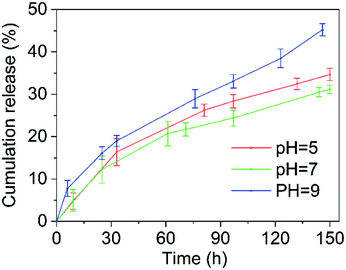 | ||
| Fig. 10 The release curves for the 10% chlorfenapyr MS in a methanol/water (3/2, v/v) solution with different pH values. | ||
3.7 Release kinetics of microcapsules
The establishment of the kinetic model can effectively evaluate the release mechanism of the active ingredients in the microcapsules. Commonly used mathematical models for microcapsule formulations are the zero-order, first-order, Higuchi and Korsmeyer–Peppas equations, where Mt and M∞ are the cumulative release amounts of the drugs at the time of t and ∞, respectively.48 The kinetic plots of chlorfenapyr microcapsule are shown in Fig. 11. Four different kinetic models were used to fit the slow-release curve of the chlorfenapyr microcapsule at pH = 7.51 The fitting results are shown in Table 2. Among these models, the Higuchi model had the best results. The R2 value reached 0.9808, which inferred that the release of chlorfenapyr in the microcapsule belonged to Fickian diffusion.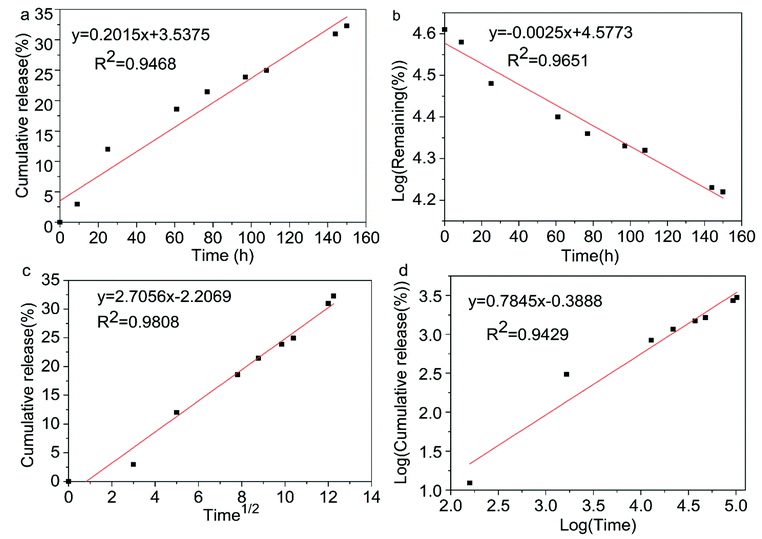 | ||
| Fig. 11 Kinetic plots of chlorfenapyr microcapsule: (a) zero-order equations; (b) first-order equations; (c) Higuchi equations; (d) Korsmeyer–Peppas equations. | ||
| Kinetic model | Fitted equation | R2 |
|---|---|---|
| Zero-order | Mt/M∞ = 0.2015t | 0.9468 |
| First-order | Ln(1 − Mt/M∞) = 0.0025t | 0.9651 |
| Higuchi | Mt/M∞ = 2.7056t1/2 | 0.9808 |
| Korsmeyer–Peppas | Mt/M∞ = 0.6779t0.7845 | 0.9429 |
3.8 Degradability of wall material of microcapsules
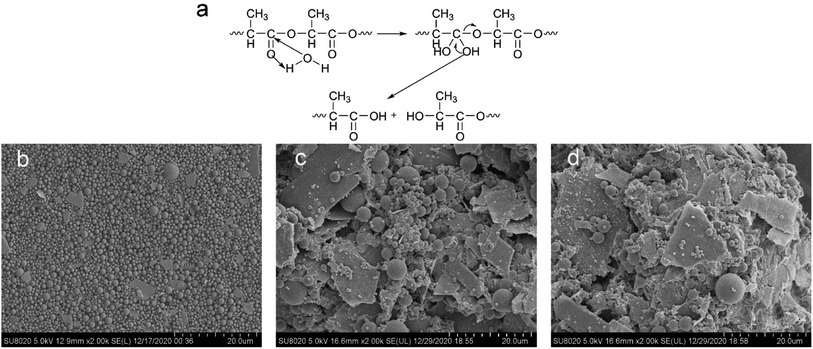
It has been reported in the literature that the ester group was first hydrolyzed in the hydrolysis process of the polylactide based polyurethane material. The carboxylic acid in the product can play a catalytic role and accelerate the degradation of the material.52 In Fig. 12(a), we provided the hydrolysis mechanism of the ester group on the polylactic acid molecular chain of the polyurethane wall material. Fig. 12(b) shows the SEM image of the original M5 sample. Fig. 12(c) shows the SEM image of the M5 sample after being incubated in PBS and hydrolyzed for 150 h. Fig. 12(d) shows the morphology of microcapsule sample M5 after 12 days incubation in PBS solution. In the case, most of the capsule particles were degraded into fragments, but there were still a few spherical particles. Meanwhile, the viscosity of the system reduced from 562.0 mPa s to 302.3 mPa s, which indirectly revealed the chemical degradation of the wall materials. Comparing Fig. 12(d) with Fig. 12(c), we found that after the microcapsules were incubated in PBS solution for 12 days, the samples showed more fragments.4 Conclusions
Chlorfenapyr microcapsules with degradable polylactide based polyurethane wall material was prepared by interfacial polymerization. This kind of microcapsule possessed high encapsulation efficiency and narrow particle size distribution, and its release mechanisms conformed to Fickian diffusion. Compared with other commercial formulations, chlorfenapyr microcapsules effectively prolonged the release time of chlorfenapyr and the release rate was controlled by adjusting the external pH conditions. The diluted microcapsule formulations had good wetting and spreading abilities on the surface of cabbage leaves. Furthermore, the polylactide-based polyurethane wall material of chlorfenapyr microcapsules showed excellent degradation performance in a PBS solution.Conflicts of interest
The authors report there are no competing interests to declare.Funding
This work was supported by the opening foundation of the Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, Grant No. 2016GDGP0102.Acknowledgements
We thank International Science Editing (https://www.internationalscienceediting.com) for editing this manuscript.References
- B. Liu, Y. Wang, F. Yang, X. Wang, H. Shen, H. Cui and D. Wu, Colloids Surf., B, 2016, 144, 38–45 CrossRef CAS PubMed.
- T. Zheng, K. Chen, W. Chen, B. Wu, Y. Sheng and Y. Xiao, J. Microencapsulation, 2019, 36(1), 62–71 CrossRef CAS PubMed.
- S. Kumar, G. Bhanjana, A. Sharma, M. C. Sidhu and N. Dilbaghi, Carbohydr. Polym., 2014, 101, 1061–1067 CrossRef CAS PubMed.
- H. W. An, M. Mamuti, X. Wang, H. Yao, M. D. Wang, L. Zhao and L. L. Li, Exploration, 2021, 1, 20210153 CrossRef.
- H. Sawalha, K. Schroën and R. Boom, Chem. Eng. J., 2011, 169, 1–10 CrossRef CAS.
- V. Kanyanta and A. Ivankovic, J. Mech. Behav. Biomed. Mater., 2010, 3(1), 51–62 CrossRef PubMed.
- P. Gentile, D. Bellucci, A. Sola, C. Mattu, V. Cannillo and G. Ciardelli, J. Mech. Behav. Biomed. Mater., 2015, 44, 53–60 CrossRef CAS.
- X. Liu, S. She, W. Tong and C. Gao, RSC Adv., 2015, 5(8), 5775–5780 RSC.
- E. Rosenbauer, M. Wagner, A. Musyanovych and K. Landfester, Macromolecules, 2010, 43(11), 5083–5093 CrossRef CAS.
- P. F. De Castro and D. G. Shchukin, Chem.–Eur. J., 2015, 21(31), 11174–11179 CrossRef PubMed.
- N. Tsuda, T. Ohtsubo and M. Fuji, Adv. Powder Technol., 2012, 23(6), 724–730 CrossRef CAS.
- Y. Xu, L. Wang, Y. Tong, S. Xiang, X. Guo, J. Li, H. Gao and X. Wu, J. Appl. Polym. Sci., 2016, 133(35), 43844 CrossRef.
- Y. Wang, C. Li, T. Wang, X. Li and X. Li, Langmuir, 2020, 36(41), 12336–12345 CrossRef CAS PubMed.
- J. Yang, Z. Zhou, Y. Liang, J. Tang, Y. Gao, J. Niu, H. Dong, R. Tang, G. Tang and Y. Cao, ACS Sustainable Chem. Eng., 2020, 8(35), 13440–13448 CrossRef CAS.
- Y. Xiao, B. Wu, X. Fu, R. Wang and J. Lei, Preparation of biodegradable microcapsules through an organic solvent-free interfacial polymerization method, Polym. Adv. Technol., 2019, 30(2), 483–488 CrossRef CAS.
- X. Zhao, T. Shou, R. Liang, S. Hu, P. Yu and L. Zhang, Ind. Crops Prod., 2020, 154, 112619 CrossRef CAS.
- X. Shi, J. Jiang, L. Sun and Z. Gan, Colloids Surf., B, 2011, 85(1), 73–80 CrossRef CAS PubMed.
- B. Liu, X. Zhou, F. Yang, H. Shen, S. Wang, B. Zhang, G. Zhi and D. Wu, Polym. Chem., 2014, 5(5), 1693–1701 RSC.
- D. Huang, D. Li, T. Wang, H. Shen, P. Zhao, B. Liu, Y. You, Y. Ma, F. Yang, D. Wu and S. Wang, Biomaterials, 2015, 52, 417–425 CrossRef CAS PubMed.
- Y. Duan, B. Zhang, L. Chu, H. H. Tong, W. Liu and G. Zhai, Colloids Surf., B, 2016, 141, 345–354 CrossRef CAS PubMed.
- S. Han, S. Yeom, S. Lee, S. C. Park, H. B. Kim, Y. M. Cho and S. W. Park, Hong Kong J. Emerg. Med., 2019, 26(6), 375–378 CrossRef.
- A. A. Romeh and R. A. Ibrahim Saber, J. Environ. Manage., 2020, 260, 110104 CrossRef CAS PubMed.
- A. Younas, H. N. Rashid, D. Hussain, S. T. R. Naqvi, M. Ali Khan, B. Fatima and S. Majeed, J. Environ. Manage., 2021, 284, 112017 CrossRef CAS PubMed.
- M. Kaur, K. M. Gupta, A. E. Poursaid, P. Karra, A. Mahalingam, H. A. Aliyar and P. F. Kiser, Drug Delivery Transl. Res., 2011, 1(3), 223–237 CrossRef CAS PubMed.
- F. Yu, Y. Wang, Y. Zhao, J. Chou and X. Li, Materials, 2021, 14(13), 3753 CrossRef CAS.
- R. Wang and Y. Xiao, J. Appl. Polym. Sci., 2019, 137(16), 48594 CrossRef.
- Y. Cao, J. Chen, Y. Wang, J. Liang, L. Chen and Y. Lu, Sci. Total Environ., 2005, 350(1–3), 38–46 CrossRef CAS PubMed.
- C. Cao, Y. Song, Z. Zhou, L. Cao, F. Li and Q. Huang, Soft Matter, 2018, 14(39), 8030–8035 RSC.
- B. Liu, Y. Fan, H. Li, W. Zhao, S. Luo, H. Wang, B. Guan, Q. Li, J. Yue, Z. Dong, Y. Wang and L. Jiang, Adv. Funct. Mater., 2021, 31, 2006606 CrossRef CAS.
- T. Wu, X. Fang, Y. Yang, W. Meng, P. Yao, Q. Liu, B. Zhang, F. Liu, A. Zou and J. Cheng, J. Agric. Food Chem., 2020, 68, 12549–12557 CrossRef CAS PubMed.
- M. Li, W. Xu, D. Hu and B. Song, Colloids Surf., A, 2018, 553, 578–585 CrossRef CAS.
- T. Wu, X. Fang, Y. Yang, W. Meng, P. Yao, Q. Liu, B. Zhang, F. Liu, A. Zou and J. Cheng, J. Agric. Food Chem., 2020, 68(45), 12549–12557 CrossRef CAS PubMed.
- S. Likhitkar and A. K. Bajpai, Carbohydr. Polym., 2012, 87(1), 300–308 CrossRef CAS PubMed.
- S. Xue, D. Pei, W. Jiang, Y. Mu and X. Wan, Polymer, 2016, 99, 340–348 CrossRef CAS.
- J. Luo, X. Huang, T. F. Jing, D. X. Zhang, B. Li and F. Liu, ACS Sustainable Chem. Eng., 2018, 6(12), 17194–17203 CrossRef CAS.
- J. G. Kennemur and B. M. Novak, Polymer, 2011, 52(8), 1693–1710 CrossRef CAS.
- S. Chen, C. Ma and G. Zhang, Prog. Org. Coat., 2017, 104, 58–63 CrossRef CAS.
- Z. Lu, W. Guan and L. Tang, Prog. Org. Coat., 2019, 132, 328–335 CrossRef CAS.
- J. Zhou, W. Xu, Y. Wang and B. Shi, RSC Adv., 2017, 7(34), 21196–21204 RSC.
- F. Zhang, T. Zhao, D. Ruiz-Molina, Y. Liu, C. Roscini, J. Leng and S. K. Smoukov, ACS Appl. Mater. Interfaces, 2020, 12(41), 47059–47064 CrossRef CAS PubMed.
- N. Abbas, M. Hayat, H. Fatima, S. Manzoor, S. Nawaz, F. Mabood, G. Yasmean, A. Majeed and S. Manzoor, Sep. Sci. Technol., 2021, 56(3), 518–526 CrossRef CAS.
- Z. Zhang, H. Song, X. Men and Z. Luo, Wear, 2008, 264(7–8), 599–605 CrossRef CAS.
- J. Kim, T. S. Pathak, J.-H. Yun, K.-P. Kim, T.-J. Park, Y. Kim and K.-J. Paeng, J. Polym. Environ., 2013, 21(1), 224–232 CrossRef CAS.
- P. K. S. Pillai, S. Li, L. Bouzidi and S. S. Narine, Ind. Crops Prod., 2016, 83, 568–576 CrossRef CAS.
- S. Zhan, S. Chen, L. Chen and W. Hou, Powder Technol., 2016, 292, 217–222 CrossRef CAS.
- V. Starov, N. Ivanova and R. G. Rubio, Adv. Colloid Interface Sci., 2010, 161(1–2), 153–162 CrossRef CAS PubMed.
- N. A. Ivanova and V. M. Starov, Curr. Opin. Colloid Interface Sci., 2011, 16(4), 285–291 CrossRef CAS.
- C. G. England, M. C. Miller, A. Kuttan, J. O. Trent and H. B. Frieboes, Eur. J. Pharm. Biopharm., 2015, 92, 120–129 CrossRef CAS PubMed.
- D. K. Chattopadhyay and K. V. S. N. Raju, Structural engineering of polyurethane coatings for high performance applications, Prog. Polym. Sci., 2007, 32(3), 352–418 CrossRef CAS.
- S. Motokucho, Y. Nakayama, H. Morikawa and H. Nakatani, J. Appl. Polym. Sci., 2018, 135(8), 45897 CrossRef.
- Y. Xu, R. Zhao, H. Chen, X. Guo, Y. Huang, H. Gao and X. Wu, J. Polym. Res., 2021, 28(1), 1–11 CrossRef.
- D. Macocinschi, D. Filip, M. F. Zaltariov and C. D. Varganici, Polym. Degrad. Stab., 2015, 121, 238–246 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |