 Open Access Article
Open Access ArticleApplications of nanocomposites based on zeolitic imidazolate framework-8 in photodynamic and synergistic anti-tumor therapy
Wen Kang
 a,
Ying Tian
a,
Ying Tian
 b,
Ying Zhao
b,
Ying Zhao
 *a,
Xindao Yin
*a,
Xindao Yin
 *a and
Zhaogang Teng
*a and
Zhaogang Teng
 *c
*c
aDepartment of Radiology, Nanjing First Hospital, Nanjing Medical University, Nanjing 210006, P. R. China. E-mail: zhaoyingmed@163.com; y.163yy@163.com; tzg@fudan.edu.cn
bDepartment of Radiology, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing 210029, P. R. China
cKey Laboratory for Organic Electronics and Information Displays and Institute of Advanced Materials (IAM), Nanjing University of Posts and Telecommunications, Nanjing 210046, P. R. China
First published on 9th June 2022
Abstract
Due to the limitations resulting from hypoxia and the self-aggregation of photosensitizers, photodynamic therapy (PDT) has not been applied clinically to treat most types of solid tumors. Zeolitic imidazolate framework-8 (ZIF-8) is a common metal–organic framework that has ultra-high porosity, an adjustable structure, good biocompatibility, and pH-induced biodegradability. In this review, we summarize the applications of ZIF-8 and its derivatives in PDT. This review is divided into two parts. In the first part, we summarize progress in the application of ZIF-8 to enhance PDT and realize theranostics. We discuss the use of ZIF-8 to avoid the self-aggregation of photosensitizers, alleviate hypoxia, increase the PDT penetration depth, and combine PDT with multi-modal imaging. In the second part, we summarize how ZIF-8 can achieve synergistic PDT with other anti-tumor therapies, including chemotherapy, photothermal therapy, chemodynamic therapy, starvation therapy, protein therapy, gene therapy, and immunotherapy. Finally, we highlight the challenges that must be overcome for ZIF-8 to be widely applied in PDT. To the best of our knowledge, this is the first review of ZIF-8-based nanoplatforms for PDT.
Introduction
Cancer is an urgent problem that threatens human life and health,1 and how to cure cancer at the lowest possible cost is an important issue. Traditional cancer treatments such as surgery, chemotherapy, radiation therapy, and immunotherapy have several side effects.2–4 The application of nanomaterials in the biomedical field provides a new direction for cancer researchers. Nanomaterials can be applied to facilitate controlled drug transport, reduce side effects in normal tissues, and improve the curative effect of cancer treatment.5,6 At present, liposomes, polymeric micelles, and inorganic nanoparticles [NPs; e.g., gold NPs (AuNPs), magnetic NPs, upconversion NPs (UPNPs), and mesoporous silica NPs] are widely used in drug delivery systems.7,8 However, the hypersensitivity of liposomes, immunogenicity and complicated biological characteristics of polymeric micelles, and the toxicity of inorganic NPs restrict their clinical applications.9–12 In recent years, metal–organic frameworks (MOFs) have attracted considerable attention as a new type of nanomaterial. MOFs are a class of porous materials composed of metal ions or their clusters coordinated with organic ligands.13 Conventional MOFs have various advantages, including ultra-high porosity, high surface area, and adjustable structure,14–16 giving MOFs great potential in gas storage,17 chemical separation,18,19 catalysis,20,21 sensing,22 drug delivery,23 and other fields. Nanoscale MOFs (nMOFs) display high drug loading efficiency, high biodegradability, and low biological toxicity.24 Thus, nMOFs are regarded as ideal nanocarriers of biomacromolecules.25Zeolitic imidazolate framework-8 (ZIF-8) is a common MOF constructed from zinc ions and 2-methylimidazole (H-MeIM). ZIF-8 was first synthesized by Park and colleagues in 2006 via a solvothermal method.26 In this solvothermal synthesis, zinc nitrate tetrahydrate and H-MeIM were mixed and dissolved in dimethylformamide (DMF). After heating (5 °C min−1) the solution to 140 °C and keeping this state for 24 h, the solution was cooled (0.4 °C min−1) to room temperature. Then the mother liquor was removed, and chloroform was added. Colorless polyhedral crystals were collected from the upper layer, washed with DMF, and dried in air to obtain ZIF-8. In comparison to other MOFs, ZIF-8 has better biocompatibility because it is composed of constituents of the physiological system.27,28 Under physiological conditions (pH 7.4) and alkaline conditions, ZIF-8 shows excellent chemical and thermal stability. However, acidic conditions (pH 5.0–6.0) destroy the coordination between H-MeIM and zinc ions, leading to the decomposition of the ZIF-8 structure.26 This feature allows the construction of pH-controlled drug delivery systems based on ZIF-8.29 Since the first solvothermal synthesis of ZIF-8, additional solvothermal methods along with microwave-assisted, sono-chemical, mechanochemical, dry-gel, microfluidic, and high-throughput methods have been reported to synthesize ZIF-8 (Fig. 1).26,30–36 ZIF-8 NPs with different average particle sizes can be obtained using different synthetic methods. Among reported methods, the solvothermal synthesis of ZIF-8 in DMF results in the largest particle size (approximately 150–200 μm). ZIF-8 with relatively uniform particle sizes can be synthetized via sono-chemical routes.
 | ||
| Fig. 1 SEM images and particle sizes of ZIF-8 samples prepared using different synthetic methods. [This figure has been reproduced from ref. 36 with permission from Elsevier Inc., copyright 2015]. | ||
The delivery of functional molecules by ZIF-8 is typically based on pore encapsulation, the core–shell structure, surface attachment, ion doping, and in situ growth (Fig. 2).37–40 However, due to the small apertures (diameter of approximately 3.4 Å) of ZIF-8,41 some drugs can only be adsorbed on the surface of ZIF-8. To solve this problem, Tsung and colleagues developed a general one-pot synthetic route to encapsulate small molecules and improve the drug-loading capacity of ZIF-8; they incorporated fluorescein to imitate drugs that are larger than the ZIF-8 apertures. Zinc nitrate hexahydrate (150 mg) and H-MeIM (330 mg) were dissolved in 7.15 mL of methanol. After fluorescein-containing methanol solution was mixed with the zinc solution, H-MeIM solution was added into the zinc-based solution under magnetic stirring for 5 min. The change in color of the solution from bright green to milky white indicated the formation of ZIF-8 NPs. The obtained solution was centrifuged at 7000 rpm for 10 min and washed with methanol to obtain the fluorescein-loaded ZIF-8.28 This general process provides an effective method to embed different therapeutic molecules in ZIF-8 and expands the available ZIF-8-based strategies for tumor treatment.
 | ||
| Fig. 2 Schematic overview of common strategies to deliver functional molecules and the applications of ZIF-8 nanoplatforms in photodynamic therapy and synergistic therapy. | ||
Generally speaking, photodynamic therapy (PDT) is a kind of phototherapy in which singlet oxygen (1O2) and reactive oxygen species (ROS) are produced to kill tumor cells at the areas of photosensitizer (PS) accumulation under light irradiation at a specific wavelength [generally ultraviolet, visible, or near-infrared (NIR) light] and dose.42–44 PDT kills tumor cells directly or indirectly by inducing cell apoptosis, necrosis, and/or autophagy, triggering inflammation and immune response, and damaging the vasculature system.45–47 For PDT to be effectively applied in cancer treatment, a combination of light, a PS, and O2 is required. However, the hypoxia of tumor sites and the nonselective distribution of PSs limit the therapeutic efficacy of conventional PDT.46 Because ZIF-8 can be used to load PSs,48 it has great prospects in PDT for cancer. This review summarizes PDT-related nanocomposites based on ZIF-8 and its derivatives (Table 1) and discusses problems related to these nanoplatforms.
| Therapy modalities | Nanoplatforms | Payloads | Ref. |
|---|---|---|---|
| Monotherapy | |||
| PS delivery | ZnPc@ZIF-8 | ZnPc | 53 |
| ZnPc–COOH@ZIF-8 | ZnPc–COOH | 54 | |
| ZIF-8@Ce6-HA | Ce6 | 57 | |
| MPEG2000-ZIF/PC | PC | 64 | |
| PS@ZIF-8-PMMA-S-S-mPEG | D–A photosensitizers | 68 | |
| Alleviation of hypoxia | CAT-PS-ZIF@Mem | AlPcS4,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CAT CAT |
75 |
| UCNPs/MB@ZIF-8@catalase | UCNPs, MB,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CAT CAT |
78 | |
| Au@ZIF-8 | AuNPs, Ce6 | 84 | |
| Increase the penetration depth | UCNPs-g-C3N4–CDs@ZIF-8 | g-C3N4, CDs, UCNPs | 89 |
| Multi-modal imaging-guided PDT | BSA–MnO2/Ce6@ZIF-8 | Ce6, (BSA)-MnO2 | 94 |
| ZIF-8–IR820–MnPc–HA | IR820, MnPc | 99 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Synergistic PDT | |||
| PDT and chemotherapy | g-C3N4@ZIF-8 | g-C3N4, DOX | 102 |
| AuNCs@MOF-DOX | AuNCs, DOX | 103 | |
| F127–MnO2-ZIF@DOX/C3N4 | g-C3N4, MnO2, DOX | 105 | |
| ZDZP@PP | DOX, PpIX | 106 | |
| BCP/Cit-Fe(III)@ZIF-8 | DOX, BCP, Cit-Fe(III) | 108 | |
| FZIF-8/DOX-PD-FA - | DOX, Ce6 | 112 | |
| PDT and PTT | Fe3O4/ZIF-8-Au25 | Au25(SR)18−, Fe3O4 | 48 |
| PDAs-MB-CAT-ZIF-8 | MB, CAT, PDAs | 118 | |
| ZCNs* | 119 | ||
| PDT and CDT | O2–Cu/ZIF-8@Ce6/ZIF-8@F127 | O2, Cu2+, Ce6 | 71 |
| PDT and ST | UCNPs/TAPP@ZIF-8@Catalase/GOx | UCNPs, TAPP, CAT, GOx | 73 |
| PEG-COOH@Enzymes@TPP–DNB@ZIF-8 | TPP–DNB, CAT, GOx | 130 | |
| PDT and protein therapy | Ce6/Cyt c@ZIF-8/HA | Cyt c, Ce6 | 134 |
| PDT and gene therapy | DNAzyme@ZIF-8 | DNAzyme | 138 |
| PDT, PTT, and chemotherapy | FeNC@PAA* | 143 | |
| PDT, CDT, and ST | Ce6/GOx@ZIF-8/PDA@MnO2 | GOx, MnO2, Ce6 | 146 |
| PDT, CDT, and chemotherapy | MIL-88-ICG@ZIF-8-DOX | ICG, DOX | 147 |
| (DOX and ICG)@H-PMOF@mem* | ICG, DOX | 150 | |
| PDT, PTT, chemotherapy, and immunotherapy | CuZPMn@CpG | PpIX, CuS, DOX, CpG, MnO2 | 151 |
Individual PDT nanoplatforms
ZIF-8 has also been employed to encapsulate phycocyanin, an unstable new PS, via co-precipitation.63,64 Together, the ZIF-8 skeleton and MPEG2000–COOH modified on the surface of ZIF-8 greatly improved the stability and cell uptake of phycocyanin. At the same time, papaverine, an inhibitor of mitochondrial complex I,65 combined with MPEG2000-ZIF/phycocyanin to overcome hypoxia by restricting mitochondrial respiration at tumor sites and increasing ROS production for efficient PDT.
Another way to overcome PS inefficiency is to introduce donor (D) and acceptor (A) molecules to develop D–A PSs.66,67 For example, Wang et al. utilized ZIF-8 to construct a nanocarrier (PS@ZIF-8-PMMA-S–S-mPEG) to handle the problem that water molecules reduce the efficiency of D–A PSs (Fig. 3).68 The carrier realized the self-assembly of PS-loaded ZIF-8 in vivo. In this system, poly(methyl methacrylate) (PMMA) and disulfide-linked methoxy polyethylene glycol (S–S-mPEG) were connected with ZIF-8. The reduction of the disulfide bond and cleavage of mPEG led to the self-assembly of PS@ZIF-8-PMMA by PMMA fusion between adjacent nanoplatforms in tumor cells. These self-assemblies, which had sizes exceeding 200 nm, helped reduce drug extravasation and extend the retention time of organic PSs in tumors.69 ZIF-8 can separate PSs from water and carry O2 for PDT, thereby enhancing ROS generation in cancer PDT.
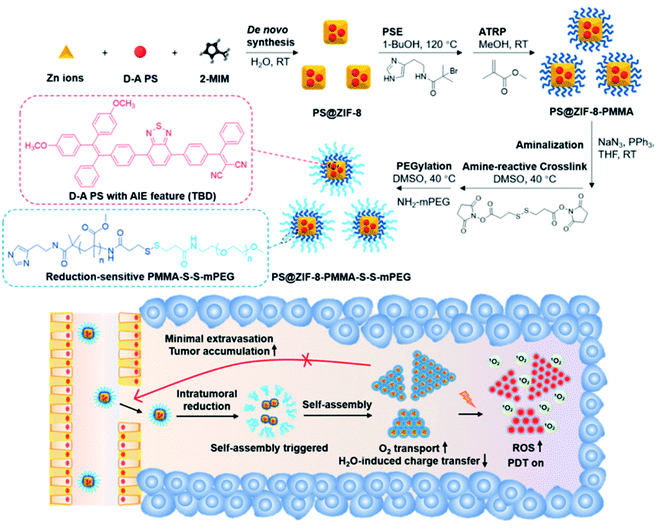 | ||
| Fig. 3 Schematic of the self-assembly of PS@ZIF-8-PMMA-S–S-mPEG. [This figure has been reproduced from ref. 68 with permission from American Chemical Society, copyright 2020]. | ||
ZIF-8 was also used as a vehicle for CAT-like materials in an O2 self-sufficient nanoplatform (Au@ZIF-8) in which AuNPs were connected to the surface of ZIF-8.84 In addition to emitting fluorescence, AuNPs can simulate CAT-like properties.85 Au@ZIF-8 can catalyze endogenous H2O2 to produce O2 and relieve tumor hypoxia, thereby enhancing the curative effect of PDT. ZIF-8 plays an essential role in stabilizing the AuNPs so that they are not removed from blood circulation.
 | ||
| Fig. 4 (A) Schematic of the anti-tumor mechanism of UCNPs-g-C3N4–CDs@ZIF-8. (B) UV-visible absorbance spectra of g-C3N4, CDs, and g-C3N4–CDs. (C) The emission spectrum upon 360 nm excitation and the absorption spectrum of CDs. (D) The emission spectrum of UCNPs under 980 nm laser excitation and the absorption spectrum of the g-C3N4–CDs PS. [This figure has been reproduced from ref. 89 with permission from American Chemical Society, copyright 2017]. | ||
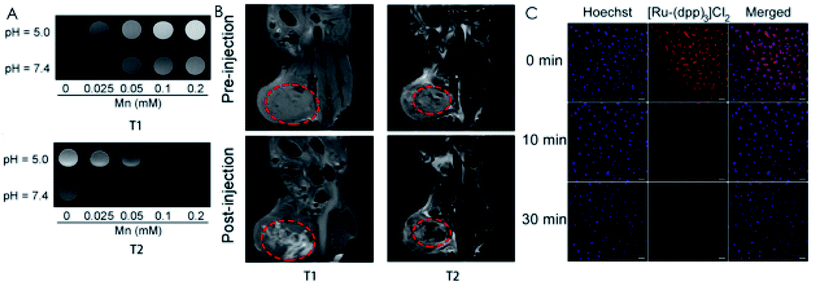 | ||
| Fig. 5 (A) T1-and T2-weighted MRI images of NPs at pH 5.0 and 7.4. (B) In vivo T1-and T2-weighted MRI images before and after injection with NPs. (C) CLSM images of HeLa cells incubated with BSA–MnO2/Ce6@ZIF-8 for different times after staining with Hoechst 33342 and [Ru-(dpp)3]Cl2. [This figure has been reproduced from ref. 94 with permission from American Chemical Society, copyright 2019]. | ||
Photoacoustic (PA) imaging has become a promising technique for deep tumors due to the great acoustic depth of penetration and optical resolution.97,98 Qu et al. designed ZIF-8–IR820–MnPc–HA (ZIMH) NPs that combined PA imaging with NIR-II fluorescence imaging to improve the visualization and localization of tumors (Fig. 6).99 To form the ZIMH NPs, IR820 and MnPc were encapsulated uniformly in the porous structure of ZIF-8 to reduce quenching and produce 1O2 under 808 nm irradiation. This multi-modal imaging-guided PDT provides a new strategy for the early and accurate diagnosis and treatment of tumors.
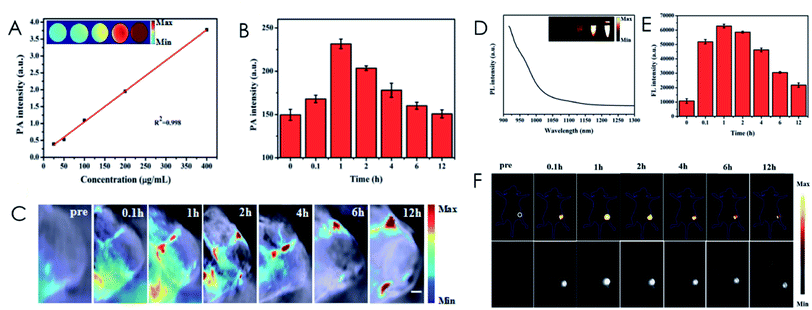 | ||
| Fig. 6 The linear relationships between the PA (A) and fluorescence (D) intensity and the ZIMH concentration. PA (B) and fluorescence (E) intensity of the tumor sites of mice in vivo at different times after the injection of ZIMH. PA (C) and fluorescence (F) images of the tumors at different time points after ZIMH injection. [This figure has been reproduced from ref. 99 with permission from Royal Society of Chemistry, copyright 2021]. | ||
Synergistic PDT nanoplatforms
Amphiphilic block copolymer was combined with ZIF-8 as a drug carrier to construct a pH/H2O2 dual-controlled associative PDT and chemotherapy nanoplatform (termed BCP/Cit-Fe(III)@ZIF-8).107,108 To form this nanoplatform, DOX and poly(L-lactic acid)-block-poly(sodium 4-styrenesulfonate) (BCP) were self-assembled in aqueous solution followed by the attachment of ferric citrate [Cit-Fe(III)] through electrostatic interaction. Finally, ZIF-8 was grown on the surfaces of the NPs. Under co-stimulation by H2O2 and visible light and the catalysis of Cit-Fe(III), sufficient ROS were released (Fig. 7).109–111 At the same time, the ROS were oxidized and decomposed sulfonate-containing polymeric vesicles, causing the encapsulated DOX to spread into ZIF-8. Under low pH, ZIF-8 disintegrated to achieve controlled drug release and an excellent anti-cancer effect.
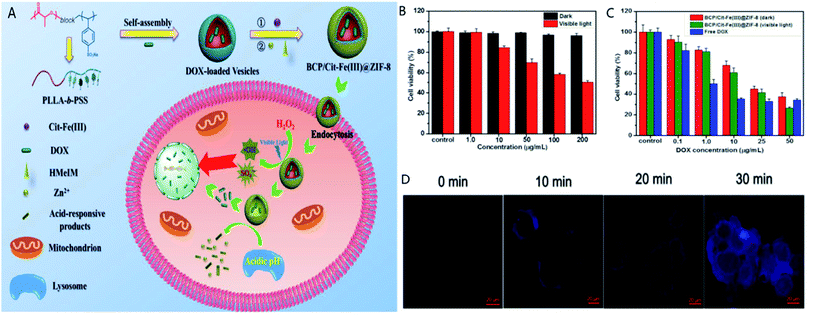 | ||
| Fig. 7 (A) Anti-tumor mechanism of BCP/Cit-Fe(III)@ZIF-8 in vivo. (B) Cell viability in the presence of different concentrations of the nanoplatform in the dark and under visible light. (C) Cell viability in the presence of various DOX concentrations after different treatments. (D) Intracellular hydroxyl radicals detected by incubating MCF-7 cells with coumarin under visible light. [This figure has been reproduced from ref. 108 with permission from American Chemical Society, copyright 2019]. | ||
In another photo-chemotherapy nanosystem, Qin and his colleagues loaded Gd-doped silicon NPs (Si–Gd NPs) into the ZIF-8 matrix to endow it with MRI properties.112 In this system, the combination of MRI/fluorescence imaging modalities and folic acid polyethylene glycol-maleimide (MaL-PEG-FA) as a cancer target molecule allows for a precise treatment effect.113
In addition, ZIF-8 has been directly pyrolyzed to obtain ZIF-8-derived carbon nanoparticles that served as both a PTA and PS rather than using two different drugs.119
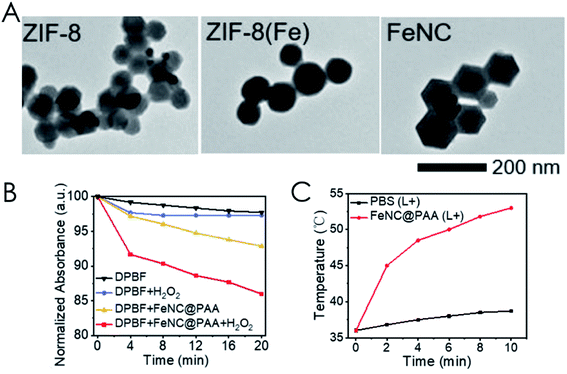 | ||
| Fig. 9 (A) TEM images of ZIF-8 (Zn), ZIF-8 (Fe/Zn), and FeNC NPs. (B) Decay curves of DPBF-normalized absorption at 410 nm under different conditions and 808 nm laser irradiation (0.5 W cm−2). (C) Temperature change of the tumor site during irradiation (0.3 W cm−2, 10 min). [This figure has been reproduced from ref. 143 with permission from American Chemical Society, copyright 2021]. | ||
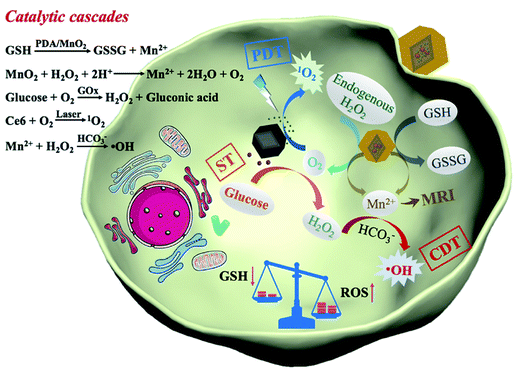 | ||
| Fig. 10 Schematic of the reaction mechanism of CGZPM in vivo. [This figure has been reproduced from ref. 146 with permission from Elsevier Inc., copyright 2021]. | ||
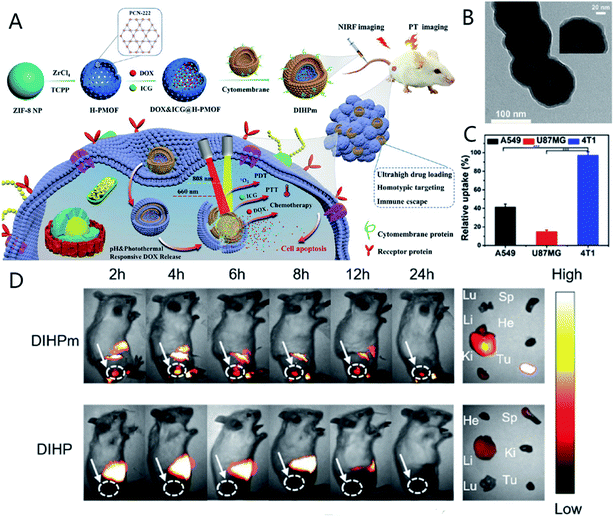 | ||
| Fig. 11 (A) Schematic of the synthesis and reaction mechanism of DIHPm. (B) TEM images of DIHPm. (C) Relative cellular uptake of DIHPm against various cell lines (A549, U87MG, and 4T1). (D) Fluorescence images of 4T1 tumor-bearing mice at different timepoints after the intravenous injection of DIHPm or DIHP. [This figure has been adapted from ref. 150 with permission from American Chemical Society, copyright 2021]. | ||
 | ||
| Fig. 12 Schematic diagram of the synthesis and applications of CuZPMn@CpG nanocomposite. [This figure has been reproduced from ref. 151 with permission from Royal Society of Chemistry, copyright 2018]. | ||
Conclusion and outlook
Multifunctional nanoplatforms based on ZIF-8 and its derivatives have shown outstanding application prospects in PDT. The porosity and easy modification of the ZIF-8 skeleton largely overcome the low photosensitizer efficiency and hypoxia in conventional PDT. The reaction of ZIF-8 in acidic conditions also contributes to the precise release of drugs in tumor sites. ZIF-8 can also be used as an intermediary to realize synergistic PDT and other anti-tumor therapies by delivering different small-molecule therapeutic agents. However, ZIF-8 still has some weaknesses.(1) As a carrier in PDT, ZIF-8 still needs to face challenges of low accumulation at tumor sites and unclear metabolic mechanisms in vivo. Without any surface modification, ZIF-8 has a short blood circulation time and low accumulation in tumor cells.155 Thus, modifiers must be added to ZIF-8 to overcome these limitations. In addition, most studies on ZIF-8-based nanomaterials have not explored the metabolic mechanisms and long-term side effects, and the effects of these nanomaterials on the immune system remain unknown.
(2) Although zinc, a component of ZIF-8, is one of the vital transition metal in organisms and essential for life, the accumulation of zinc in cells can damage the cells.156 Chen et al. thought that smaller ZIF-8 nanoplatforms would lead to more zinc accumulation. Although more zinc can provide more ROS, excess zinc can result in cytotoxicity.157 Further studies are needed to design ZIF-8 nanoplatforms with suitable sizes to decrease toxicity to normal tissues.
(3) The design of synergistic therapeutic nanoplatforms based on ZIF-8 remains complicated. Although there is a universal one-pot route to encapsulate small molecules, a simple and versatile way to integrate multiple therapeutic drugs into nanosystems has not been identified.
(4) Most ZIF-8 nanoplatforms for PDT are designed to alleviate hypoxia and deliver PSs. Thus, low tissue penetration depth in PDT remains a problem. Future work could focus on designing better ZIF-8-based PSs to extend the excitation wavelength and increase the penetration depth in PDT.
(5) PDT based on ZIF-8 has been primarily studied at the cell level and/or animal level, and human clinical data are lacking. Whether these nanoplatforms can pass rigorous clinical evaluation is still uncertain. We anticipate that researchers can overcome the remaining problems to build ZIF-8-based nanoplatforms for application in clinical treatment in the near future.
Author contributions
Wen Kang: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing – original draft, writing – review & editing.Ying Tian: conceptualization, methodology, software, validation, data curation, writing – original draft, writing – review & editing, project administration, funding acquisition.
Ying Zhao: software, validation, formal analysis, resources, data curation, writing – review & editing, visualization, project administration, funding acquisition.
Xindao Yin: conceptualization, software, investigation, resources, data curation, writing – review & editing, visualization, supervision, project administration, funding acquisition.
Zhaogang Teng: conceptualization, methodology, validation, investigation, writing – review & editing, supervision, project administration.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.Acknowledgements
This work was supported by National Natural Science Foundation of China (grant numbers 81901806 and 81971681).References
- R. L. Siegel, K. D. Miller and A. Jemal, Ca-Cancer J. Clin., 2020, 70, 7–30 CrossRef PubMed.
- M. Jablonická, L. Žideková and B. Mladosievičová, Vnitr. Lek., 2021, 67, 26–31 CrossRef.
- M. Dominguez and R. Malani, Curr. Pain Headache Rep., 2021, 25, 33 CrossRef PubMed.
- P. Iglesias, J. C. Sánchez and J. J. Díez, Pituitary, 2021, 24, 630–643 CrossRef CAS PubMed.
- J. Yu, Y. Ju, L. Zhao, X. Chu, W. Yang, Y. Tian, F. Sheng, J. Lin, F. Liu, Y. Dong and Y. Hou, ACS Nano, 2016, 10, 159–169 CrossRef CAS PubMed.
- O. C. Farokhzad and R. Langer, ACS Nano, 2009, 3, 16–20 CrossRef CAS PubMed.
- J. Shi, P. W. Kantoff, R. Wooster and O. C. Farokhzad, Nat. Rev. Cancer, 2017, 17, 20–37 CrossRef CAS PubMed.
- F. Wang, C. Li, J. Cheng and Z. Yuan, Int. J. Environ. Res. Public Health, 2016, 13, 1182 CrossRef PubMed.
- J. L. Paris, A. Baeza and M. Vallet-Regí, Expert Opin. Drug Delivery, 2019, 16, 1095–1112 CrossRef CAS PubMed.
- M. Yokoyama, J. Drug Targeting, 2014, 22, 576–583 CrossRef CAS PubMed.
- L. Larue, B. Myrzakhmetov, A. Ben-Mihoub, A. Moussaron, N. Thomas, P. Arnoux, F. Baros, R. Vanderesse, S. Acherar and C. Frochot, Pharmaceuticals, 2019, 12, 163 CrossRef CAS PubMed.
- N. Thotakura, P. Parashar and K. Raza, Expert Opin. Drug Metab. Toxicol., 2021, 17, 323–332 CrossRef CAS PubMed.
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- J. L. C. Rowsell and O. M. Yaghi, Microporous Mesoporous Mater., 2004, 73, 3–14 CrossRef CAS.
- K. K. Tanabe, Z. Wang and S. M. Cohen, J. Am. Chem. Soc., 2008, 130, 8508–8517 CrossRef CAS PubMed.
- C. Wang, D. Liu and W. Lin, J. Am. Chem. Soc., 2013, 135, 13222–13234 CrossRef CAS PubMed.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS PubMed.
- J. Li, P. M. Bhatt, J. Li, M. Eddaoudi and Y. Liu, Adv. Mater., 2020, 32, 2002563 CrossRef CAS PubMed.
- W.-Q. Tang, J.-Y. Xu and Z.-Y. Gu, Chem.–Asian J., 2019, 14, 3462–3473 CrossRef CAS PubMed.
- H. Wan, Y. Wang, J. Chen, H. M. Meng and Z. Li, Mikrochim. Acta, 2021, 188, 130 CrossRef CAS PubMed.
- Y.-B. Huang, J. Liang, X.-S. Wang and R. Cao, Chem. Soc. Rev., 2016, 46, 126–157 RSC.
- L. Du, W. Chen, P. Zhu, Y. Tian, Y. Chen and C. Wu, Biotechnol. J., 2021, 16, 1900424 CrossRef CAS PubMed.
- J. W. M. Osterrieth and D. Fairen-Jimenez, Biotechnol. J., 2021, 16, 2000005 CrossRef CAS PubMed.
- D. Gao, Y. Gao, J. Shen and Q. Wang, Photodiagn. Photodyn. Ther., 2020, 32, 102026 CrossRef CAS PubMed.
- M.-X. Wu and Y.-W. Yang, Adv. Mater., 2017, 29, 1606134 CrossRef PubMed.
- K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- C. Andreini, L. Banci, I. Bertini and A. Rosato, J. Proteome Res., 2006, 5, 3173–3178 CrossRef CAS PubMed.
- J. Zhuang, C.-H. Kuo, L.-Y. Chou, D.-Y. Liu, E. Weerapana and C.-K. Tsung, ACS Nano, 2014, 8, 2812–2819 CrossRef CAS PubMed.
- J. Yan, C. Liu, Q. Wu, J. Zhou, X. Xu, L. Zhang, D. Wang, F. Yang and H. Zhang, Anal. Chem., 2020, 92, 11453–11461 CrossRef CAS PubMed.
- H.-Y. Cho, J. Kim, S.-N. Kim and W.-S. Ahn, Microporous Mesoporous Mater., 2013, 169, 180–184 CrossRef CAS.
- Q. Shi, Z. Chen, Z. Song, J. Li and J. Dong, Angew. Chem., Int. Ed. Engl., 2011, 50, 672–675 CrossRef CAS PubMed.
- F. Hillman, J. Brito and H.-K. Jeong, ACS Appl. Mater. Interfaces, 2018, 10, 5586–5593 CrossRef CAS PubMed.
- R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O'Keeffe and O. M. Yaghi, Science, 2008, 319, 939–943 CrossRef CAS PubMed.
- P. J. Beldon, L. Fábián, R. S. Stein, A. Thirumurugan, A. K. Cheetham and T. Friščić, Angew. Chem., Int. Ed. Engl., 2010, 49, 9640–9643 CrossRef CAS PubMed.
- M. Faustini, J. Kim, G.-Y. Jeong, J. Y. Kim, H. R. Moon, W.-S. Ahn and D.-P. Kim, J. Am. Chem. Soc., 2013, 135, 14619–14626 CrossRef CAS PubMed.
- Y.-R. Lee, M.-S. Jang, H.-Y. Cho, H.-J. Kwon, S. Kim and W.-S. Ahn, Chem. Eng. J., 2015, 271, 276–280 CrossRef CAS.
- A. Dhakshinamoorthy, A. M. Asiri and H. Garcia, Dalton Trans., 2020, 49, 11059–11072 RSC.
- C. Chu, M. Su, J. Zhu, D. Li, H. Cheng, X. Chen and G. Liu, Theranostics, 2019, 9, 3134–3149 CrossRef CAS PubMed.
- Y. Wang, X. Dai, Y. Zhan, X. Ding, M. Wang and X. Wang, Int. J. Biol. Macromol., 2019, 137, 77–86 CrossRef CAS PubMed.
- C. Hu, Y. Yu, S. Chao, H. Zhu, Y. Pei, L. Chen and Z. Pei, Molecules, 2021, 26, 3878 CrossRef CAS PubMed.
- O. Karagiaridi, M. B. Lalonde, W. Bury, A. A. Sarjeant, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 18790–18796 CrossRef CAS PubMed.
- M. C. DeRosa and R. J. Crutchley, Coord. Chem. Rev., 2002, 233–234, 351–371 CrossRef CAS.
- M. L. Agarwal, M. E. Clay, E. J. Harvey, H. H. Evans, A. R. Antunez and N. L. Oleinick, Cancer Res., 1991, 51, 5993–5996 CAS.
- P. Vijayaraghavan, C.-H. Liu, R. Vankayala, C.-S. Chiang and K. C. Hwang, Adv. Mater., 2014, 26, 6689–6695 CrossRef CAS PubMed.
- D. Kessel and N. L. Oleinick, Photochem. Photobiol., 2018, 94, 213–218 CrossRef CAS PubMed.
- D. Van Straten, V. Mashayekhi, H. S. De Bruijn, S. Oliveira and D. J. Robinson, Cancers, 2017, 9, 19 CrossRef PubMed.
- W. M. Star, H. P. A. Marijnissen, A. E. van den Berg-Blok, J. A. C. Versteeg, K. A. P. Franken and H. S. Reinhold, Cancer Res., 1986, 46, 2532–2540 CAS.
- D. Yang, G. Yang, S. Gai, F. He, G. An, Y. Dai, R. Lv and P. Yang, Nanoscale, 2015, 7, 19568–19578 RSC.
- S. Kwiatkowski, B. Knap, D. Przystupski, J. Saczko, E. Kędzierska, K. Knap-Czop, J. Kotlińska, O. Michel, K. Kotowski and J. Kulbacka, Biomed. Pharmacother., 2018, 106, 1098–1107 CrossRef CAS PubMed.
- I. S. Mfouo-Tynga, L. D. Dias, N. M. Inada and C. Kurachi, Photodiagn. Photodyn. Ther., 2021, 34, 102091 CrossRef CAS PubMed.
- R. Baskaran, J. Lee and S.-G. Yang, Biomater. Res., 2018, 22, 25 CrossRef PubMed.
- S. Wang, X. Wang, L. Yu and M. Sun, Photodiagn. Photodyn. Ther., 2021, 34, 102254 CrossRef CAS PubMed.
- D. Xu, Y. You, F. Zeng, Y. Wang, C. Liang, H. Feng and X. Ma, ACS Appl. Mater. Interfaces, 2018, 10, 15517–15523 CrossRef CAS PubMed.
- M.-R. Song, D.-Y. Li, F.-Y. Nian, J.-P. Xue and J.-J. Chen, J. Mater. Sci., 2018, 53, 2351–2361 CrossRef CAS.
- A. Narumi, R. Rachi, H. Yamazaki, S. Kawaguchi, M. Kikuchi, H. Konno, T. Osaki, Y. Okamoto, X. Shen, T. Kakuchi, H. Kataoka, A. Nomoto, T. Yoshimura and S. Yano, ACS Omega, 2021, 6, 7023–7033 CrossRef CAS PubMed.
- R. Bonnett, R. D. White, U. J. Winfield and M. C. Berenbaum, Biochem. J., 1989, 261, 277–280 CrossRef CAS PubMed.
- X. Fu, Z. Yang, T. Deng, J. Chen, Y. Wen, X. Fu, L. Zhou, Z. Zhu and C. Yu, J. Mater. Chem. B, 2020, 8, 1481–1488 RSC.
- K. Valachová and L. Šoltés, Molecules, 2021, 26, 1195 CrossRef PubMed.
- M. Hassn Mesrati, S. E. Syafruddin, M. A. Mohtar and A. Syahir, Biomolecules, 2021, 11, 1850 CrossRef CAS PubMed.
- G. Mattheolabakis, L. Milane, A. Singh and M. M. Amiji, J. Drug Targeting, 2015, 23, 605–618 CrossRef CAS PubMed.
- G. Borland, J. A. Ross and K. Guy, Immunology, 1998, 93, 139–148 CrossRef CAS PubMed.
- P. Teriete, S. Banerji, M. Noble, C. D. Blundell, A. J. Wright, A. R. Pickford, E. Lowe, D. J. Mahoney, M. I. Tammi, J. D. Kahmann, I. D. Campbell, A. J. Day and D. G. Jackson, Mol. Cell, 2004, 13, 483–496 CrossRef CAS PubMed.
- D.-H. Wan, B.-Y. Zheng, M.-R. Ke, J.-Y. Duan, Y.-Q. Zheng, C.-K. Yeh and J.-D. Huang, Chem. Commun., 2017, 53, 4112–4115 RSC.
- D. Chen, M. Suo, J. Guo, W. Tang, W. Jiang, Y. Liu and Y. Duo, Adv. Healthcare Mater., 2021, 10, 2001577 CrossRef CAS PubMed.
- M. Benej, X. Hong, S. Vibhute, S. Scott, J. Wu, E. Graves, Q.-T. Le, A. C. Koong, A. J. Giaccia, B. Yu, C.-S. Chen, I. Papandreou and N. C. Denko, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 10756–10761 CrossRef CAS PubMed.
- S. Liu, H. Zhang, Y. Li, J. Liu, L. Du, M. Chen, R. T. K. Kwok, J. W. Y. Lam, D. L. Phillips and B. Z. Tang, Angew. Chem., Int. Ed., 2018, 57, 15189–15193 CrossRef CAS PubMed.
- J. Zou, Z. Yin, P. Wang, D. Chen, J. Shao, Q. Zhang, L. Sun, W. Huang and X. Dong, Chem. Sci., 2018, 9, 2188–2194 RSC.
- Y. Wang, L. Shi, D. Ma, S. Xu, W. Wu, L. Xu, M. Panahandeh-Fard, X. Zhu, B. Wang and B. Liu, ACS Nano, 2020, 14, 13056–13068 CrossRef CAS PubMed.
- L. Tang, X. Yang, Q. Yin, K. Cai, H. Wang, I. Chaudhury, C. Yao, Q. Zhou, M. Kwon, J. A. Hartman, I. T. Dobrucki, L. W. Dobrucki, L. B. Borst, S. Lezmi, W. G. Helferich, A. L. Ferguson, T. M. Fan and J. Cheng, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 15344–15349 CrossRef CAS PubMed.
- C.-Y. Shih, P.-T. Wang, W.-C. Su, H. Teng and W.-L. Huang, Biomedicines, 2021, 9, 137 CrossRef CAS PubMed.
- Z. Xie, S. Liang, X. Cai, B. Ding, S. Huang, Z. Hou, P. a. Ma, Z. Cheng and J. Lin, ACS Appl. Mater. Interfaces, 2019, 11, 31671–31680 CrossRef CAS PubMed.
- Q. Xu, G. Zhan, Z. Zhang, T. Yong, X. Yang and L. Gan, Theranostics, 2021, 11, 1937–1952 CrossRef CAS PubMed.
- Y. You, D. Xu, X. Pan and X. Ma, Appl. Mater. Today, 2019, 16, 508–517 CrossRef.
- Q. You, K. Zhang, J. Liu, C. Liu, H. Wang, M. Wang, S. Ye, H. Gao, L. Lv, C. Wang, L. Zhu and Y. Yang, Adv. Sci., 2020, 7, 1903341 CrossRef CAS PubMed.
- H. Cheng, J.-Y. Zhu, S.-Y. Li, J.-Y. Zeng, Q. Lei, K.-W. Chen, C. Zhang and X.-Z. Zhang, Adv. Funct. Mater., 2016, 26, 7847–7860 CrossRef CAS.
- Y. Wang, Z. Luan, C. Zhao, C. Bai and K. Yang, Eur. J. Pharm. Sci., 2020, 142, 105136 CrossRef CAS PubMed.
- X. Zhen, P. Cheng and K. Pu, Small, 2019, 15, e1804105 CrossRef PubMed.
- H.-J. Cai, T.-T. Shen, J. Zhang, C.-F. Shan, J.-G. Jia, X. Li, W.-S. Liu and Y. Tang, J. Mater. Chem. B, 2017, 5, 2390–2394 RSC.
- F. Wang and X. Liu, Chem. Soc. Rev., 2009, 38, 976–989 RSC.
- F. Auzel, Chem. Rev., 2004, 104, 139–174 CrossRef CAS PubMed.
- B. del Rosal and D. Jaque, Methods Appl. Fluoresc., 2019, 7, 022001 CrossRef CAS PubMed.
- Y. Huang, F. Qiu, R. Chen, D. Yan and X. Zhu, J. Mater. Chem. B, 2020, 8, 3772–3788 RSC.
- Y. Zou, S. Long, T. Xiong, X. Zhao, W. Sun, J. Du, J. Fan and X. Peng, ACS Cent. Sci., 2021, 7, 327–334 CrossRef CAS PubMed.
- Y.-C. Ma, Y.-H. Zhu, X.-F. Tang, L.-F. Hang, W. Jiang, M. Li, M. I. Khan, Y.-Z. You and Y.-C. Wang, Biomater. Sci., 2019, 7, 2740–2748 RSC.
- W. He, Y.-T. Zhou, W. G. Wamer, X. Hu, X. Wu, Z. Zheng, M. D. Boudreau and J.-J. Yin, Biomaterials, 2013, 34, 765–773 CrossRef CAS PubMed.
- J. R. Starkey, A. K. Rebane, M. A. Drobizhev, F. Meng, A. Gong, A. Elliott, K. McInnerney and C. W. Spangler, Clin. Cancer Res., 2008, 14, 6564–6573 CrossRef CAS PubMed.
- A. V. Kachynski, A. Pliss, A. N. Kuzmin, T. Y. Ohulchanskyy, A. Baev, J. Qu and P. N. Prasad, Nat. Photonics, 2014, 8, 455–461 CrossRef CAS.
- C. Zhang, K. Zhao, W. Bu, D. Ni, Y. Liu, J. Feng and J. Shi, Angew. Chem., Int. Ed., 2015, 54, 1770–1774 CrossRef CAS PubMed.
- D. Yang, G. Yang, S. Gai, F. He, C. Li and P. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 6829–6838 CrossRef CAS PubMed.
- J. Liu, Y. Liu, N. Liu, Y. Han, X. Zhang, H. Huang, Y. Lifshitz, S. T. Lee, J. Zhong and Z. Kang, Science, 2015, 347, 970–974 CrossRef CAS PubMed.
- J. F. Algorri, M. Ochoa, P. Roldán-Varona, L. Rodríguez-Cobo and J. M. López-Higuera, Cancers, 2021, 13, 3484 CrossRef CAS PubMed.
- F. Bolze, S. Jenni, A. Sour and V. Heitz, Chem. Commun., 2017, 53, 12857–12877 RSC.
- Y. Ji, C. Jones, Y. Baek, G. K. Park, S. Kashiwagi and H. S. Choi, Adv. Drug Delivery Rev., 2020, 167, 121–134 CrossRef CAS PubMed.
- Q. Sun, H. Bi, Z. Wang, C. Li, C. Wang, J. Xu, D. Yang, F. He, S. Gai and P. Yang, ACS Appl. Mater. Interfaces, 2019, 11, 36347–36358 CrossRef CAS PubMed.
- P. Prasad, C. R. Gordijo, A. Z. Abbasi, A. Maeda, A. Ip, A. M. Rauth, R. S. DaCosta and X. Y. Wu, ACS Nano, 2014, 8, 3202–3212 CrossRef CAS PubMed.
- D. Wang, N. Zhang, X. Jing, Y. Zhang, Y. Xu and L. Meng, J. Mater. Chem. B, 2020, 8, 8271–8281 RSC.
- X. Ge, Q. Fu, L. Bai, B. Chen, R. Wang, S. Gao and J. Song, New J. Chem., 2019, 43, 8835–8851 RSC.
- A. B. E. Attia, G. Balasundaram, M. Moothanchery, U. S. Dinish, R. Bi, V. Ntziachristos and M. Olivo, Photoacoustics, 2019, 16, 100144 CrossRef PubMed.
- B. Qu, Y. Han, J. Li, Q. Wang, B. Zhao, X. Peng and R. Zhang, RSC Adv., 2021, 11, 5044–5054 RSC.
- C. Maihöfner, I. Diel, H. Tesch, T. Quandel and R. Baron, Support. Care Cancer, 2021, 29, 4223–4238 CrossRef PubMed.
- P. Smith, A. Lavery and R. C. Turkington, Best Pract. Res., Clin. Gastroenterol., 2020, 48–49, 101691 CrossRef PubMed.
- R. Chen, J. Zhang, Y. Wang, X. Chen, J. A. Zapien and C.-S. Lee, Nanoscale, 2015, 7, 17299–17305 RSC.
- L. Zhang, Y. Gao, S. Sun, Z. Li, A. Wu and L. Zeng, J. Mater. Chem. B, 2020, 8, 1739–1747 RSC.
- Y. Tao, M. Li, J. Ren and X. Qu, Chem. Soc. Rev., 2015, 44, 8636–8663 RSC.
- W. Zhang, S. Li, X. Liu, C. Yang, N. Hu, L. Dou, B. Zhao, Q. Zhang, Y. Suo and J. Wang, Adv. Funct. Mater., 2018, 28, 1706375 CrossRef.
- S.-Z. Ren, B. Wang, X.-H. Zhu, D. Zhu, M. Liu, S.-K. Li, Y.-S. Yang, Z.-C. Wang and H.-L. Zhu, ACS Appl. Mater. Interfaces, 2020, 12, 24662–24674 CrossRef CAS PubMed.
- H. Phan, V. Taresco, J. Penelle and B. Couturaud, Biomater. Sci., 2021, 9, 38–50 RSC.
- Z. Lei, Y. Ju, Y. Lin, X. Bai, W. Hu, Y. Wang, H. Luo and Z. Tong, ACS Appl. Bio Mater., 2019, 2, 3648–3658 CrossRef CAS PubMed.
- A. K. Jacob, R. S. Hotchkiss, S. L. DeMeester, M. Hiramatsu, I. E. Karl, P. E. Swanson, J. P. Cobb and T. G. Buchman, Surgery, 1997, 122, 243–254 CrossRef CAS PubMed.
- X. Feng, Z. Wang, Y. Chen, T. Tao, F. Wu and Y. Zuo, Ind. Eng. Chem. Res., 2012, 51, 7007–7012 CrossRef CAS.
- Y. Chen, Z. Liu, Z. Wang, M. Xue, X. Zhu and T. Tao, J. Hazard. Mater., 2011, 194, 202–208 CrossRef CAS PubMed.
- Y.-T. Qin, H. Peng, X.-W. He, W.-Y. Li and Y.-K. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 34268–34281 CrossRef CAS PubMed.
- A. DeCarlo, C. Malardier-Jugroot and M. R. Szewczuk, Bioconjugate Chem., 2021, 32, 512–522 CrossRef CAS PubMed.
- Y. J. Hou, X. X. Yang, R. Q. Liu, D. Zhao, C. X. Guo, A. C. Zhu, M. N. Wen, Z. Liu, G. F. Qu and H. X. Meng, Int. J. Nanomed., 2020, 15, 6827–6838 CrossRef CAS PubMed.
- S. Cui, D. Yin, Y. Chen, Y. Di, H. Chen, Y. Ma, S. Achilefu and Y. Gu, ACS Nano, 2013, 7, 676–688 CrossRef CAS PubMed.
- H. Kawasaki, S. Kumar, G. Li, C. Zeng, D. R. Kauffman, J. Yoshimoto, Y. Iwasaki and R. Jin, Chem. Mater., 2014, 26, 2777–2788 CrossRef CAS.
- D. Liu, J. Li, C. Wang, L. An, J. Lin, Q. Tian and S. Yang, Nanomedicine, 2021, 32, 102335 CrossRef CAS PubMed.
- J. Feng, W. Yu, Z. Xu and F. Wang, Chem. Sci., 2020, 11, 1649–1656 RSC.
- P. Yang, Y. Tian, Y. Men, R. Guo, H. Peng, Q. Jiang and W. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 42039–42049 CrossRef CAS PubMed.
- B. Russell, J. Villaroel, K. Sapag and A. D. Migone, J. Phys. Chem. C, 2014, 118, 28603–28608 CrossRef CAS.
- W. Cheng, H. Zhang, D. Luan and X. W. Lou, Sci. Adv., 2021, 7, eabg2580 CrossRef CAS PubMed.
- P. Z. Moghadam, T. Islamoglu, S. Goswami, J. Exley, M. Fantham, C. F. Kaminski, R. Q. Snurr, O. K. Farha and D. Fairen-Jimenez, Nat. Commun., 2018, 9, 1378 CrossRef PubMed.
- J. B. DeCoste, M. H. Weston, P. E. Fuller, T. M. Tovar, G. W. Peterson, M. D. LeVan and O. K. Farha, Angew. Chem., Int. Ed. Engl., 2014, 53, 14092–14095 CrossRef CAS PubMed.
- C. Zhang, W. Bu, D. Ni, S. Zhang, Q. Li, Z. Yao, J. Zhang, H. Yao, Z. Wang and J. Shi, Angew. Chem., Int. Ed., 2016, 55, 2101–2106 CrossRef CAS PubMed.
- P. Kuppusamy, H. Li, G. Ilangovan, A. J. Cardounel, J. L. Zweier, K. Yamada, M. C. Krishna and J. B. Mitchell, Cancer Res., 2002, 62, 307–312 CAS.
- Y. Sun, X. Ma and H. Hu, Int. J. Mol. Sci., 2021, 22, 5698 CrossRef CAS PubMed.
- L.-H. Fu, C. Qi, J. Lin and P. Huang, Chem. Soc. Rev., 2018, 47, 6454–6472 RSC.
- C. Wei, Y. Liu, X. Zhu, X. Chen, Y. Zhou, G. Yuan, Y. Gong and J. Liu, Biomaterials, 2020, 238, 119848 CrossRef CAS PubMed.
- X. Wan, L. Song, W. Pan, H. Zhong, N. Li and B. Tang, ACS Nano, 2020, 14, 11017–11028 CrossRef CAS PubMed.
- Y. Ouyang, P. Wang, B. Huang, G. Yang, J. Tian and W. Zhang, ACS Appl. Bio Mater., 2021, 4, 4413–4421 CrossRef CAS PubMed.
- Y. Xue, J. Tian, Z. Liu, J. Chen, M. Wu, Y. Shen and W. Zhang, Biomacromolecules, 2019, 20, 2796–2808 CrossRef CAS PubMed.
- J. Mendelsohn, Clin. Cancer Res., 1997, 3, 2703–2707 CAS.
- H. He, Y. Chen, Y. Li, Z. Song, Y. Zhong, R. Zhu, J. Cheng and L. Yin, Adv. Funct. Mater., 2018, 28, 1706710 CrossRef.
- L. Ding, X. Lin, Z. Lin, Y. Wu, X. Liu, J. Liu, M. Wu, X. Zhang and Y. Zeng, ACS Appl. Mater. Interfaces, 2020, 12, 36906–36916 CrossRef CAS PubMed.
- K. Sinha, J. Das, P. B. Pal and P. C. Sil, Arch. Toxicol., 2013, 87, 1157–1180 CrossRef CAS PubMed.
- C. Roma-Rodrigues, L. Rivas-García, P. V. Baptista and A. R. Fernandes, Pharmaceutics, 2020, 12, 233 CrossRef CAS PubMed.
- P. R. Cullis and M. J. Hope, Mol. Ther., 2017, 25, 1467–1475 CrossRef CAS PubMed.
- H. Wang, Y. Chen, H. Wang, X. Liu, X. Zhou and F. Wang, Angew. Chem., Int. Ed., 2019, 58, 7380–7384 CrossRef CAS PubMed.
- D. A. Baum and S. K. Silverman, Cell. Mol. Life Sci., 2008, 65, 2156–2174 CrossRef CAS PubMed.
- H. Fan, X. Zhang and Y. Lu, Sci. China: Chem., 2017, 60, 591–601 CrossRef CAS.
- A. J. Amali, H. Hoshino, C. Wu, M. Ando and Q. Xu, Chemistry, 2014, 20, 8279–8282 CrossRef CAS PubMed.
- D. Wang, D. Jana and Y. Zhao, Acc. Chem. Res., 2020, 53, 1389–1400 CrossRef CAS PubMed.
- C. Sui, R. Tan, Y. Chen, G. Yin, Z. Wang, W. Xu and X. Li, Bioconjugate Chem., 2021, 32, 318–327 CrossRef CAS PubMed.
- S. Wang, L. Shang, L. Li, Y. Yu, C. Chi, K. Wang, J. Zhang, R. Shi, H. Shen, G. I. Waterhouse, S. Liu, J. Tian, T. Zhang and H. Liu, Adv. Mater., 2016, 28, 8379–8387 CrossRef CAS PubMed.
- Q. Zhang, J. He, W. Yu, Y. Li, Z. Liu, B. Zhou and Y. Liu, RSC Med. Chem., 2020, 11, 427–437 RSC.
- L. Zhang, Z. Yang, W. He, J. Ren and C.-Y. Wong, J. Colloid Interface Sci., 2021, 599, 543–555 CrossRef CAS PubMed.
- B. Wu, J. Fu, Y. Zhou, S. Luo, Y. Zhao, G. Quan, X. Pan and C. Wu, Acta Pharm. Sin. B, 2020, 10, 2198–2211 CrossRef CAS PubMed.
- M. Ma, A. Bétard, I. Weber, N. S. Al-Hokbany, R. A. Fischer and N. Metzler-Nolte, Cryst. Growth Des., 2013, 13, 2286–2291 CrossRef CAS.
- C. Mellot-Draznieks, C. Serre, S. Surblé, N. Audebrand and G. Férey, J. Am. Chem. Soc., 2005, 127, 16273–16278 CrossRef CAS PubMed.
- X. Sun, G. He, C. Xiong, C. Wang, X. Lian, L. Hu, Z. Li, S. J. Dalgarno, Y.-W. Yang and J. Tian, ACS Appl. Mater. Interfaces, 2021, 13, 3679–3693 CrossRef CAS PubMed.
- J.-C. Yang, Y. Shang, Y.-H. Li, Y. Cui and X.-B. Yin, Chem. Sci., 2018, 9, 7210–7217 RSC.
- G. K. Gupta and D. K. Agrawal, BioDrugs, 2010, 24, 225–235 CrossRef CAS PubMed.
- G. Turgeon, A. Weickhardt, A. Azad, B. Solomon and S. Siva, Med. J. Aust., 2019, 210, 47–53 CrossRef PubMed.
- W.-D. Yu, G. Sun, J. Li, J. Xu and X. Wang, Cancer Lett., 2019, 452, 66–70 CrossRef CAS PubMed.
- R. Xie, P. Yang, S. Peng, Y. Cao, X. Yao, S. Guo and W. Yang, J. Mater. Chem. B, 2020, 8, 6128–6138 RSC.
- M. P. Cuajungco, M. S. Ramirez and M. E. Tolmasky, Biomedicines, 2021, 9, 208 CrossRef CAS PubMed.
- P. Chen, M. He, B. Chen and B. Hu, Ecotoxicol. Environ. Saf., 2020, 205, 111110 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |






