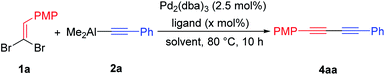Open Access Article
Open Access ArticleHighly selective cross-coupling reactions of 1,1-dibromoethylenes with alkynylaluminums for the synthesis of aryl substituted conjugated enediynes and unsymmetrical 1,3-diynes†
Kun Wu,
Chuan Wu,
Xiao-Ying Jia,
Lin Zhou * and
Qing-Han Li
* and
Qing-Han Li
Key Laboratory of General Chemistry of the National Ethnic Affairs Commission, College of Chemistry and Environment, Southwest Minzu University, Chengdu 610041, P. R. China. E-mail: zhoulin@swun.edu.cn
First published on 3rd May 2022
Abstract
A highly efficient method for the synthesis of aryl substituted conjugated enediynes and unsymmetrical 1,3-diynes via selective cross-coupling reactions of 1,1-dibromoethylenes with alkynylaluminums using the Pd(OAc)2–DPPE and Pd2(dba)3–TFP complexes as catalysts, respectively, has been successfully developed. Though the alkyl substituted conjugated enediynes and unsymmetrical 1,3-diynes were not obtained, this case is also remarkable as the same starting materials could selectively produce either aryl substituted conjugated enediynes or unsymmetrical 1,3-diynes in moderate to excellent yields (up to 99%) in the different Pd–phosphine catalytic systems.
Introduction
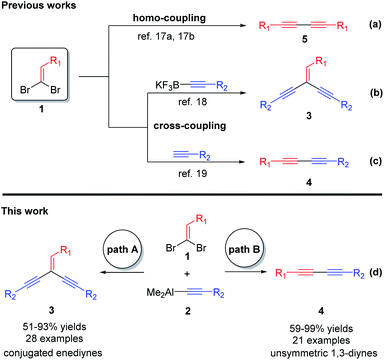
The conjugated enynes1 and diynes,2 which play important roles in organic synthesis, have been widely used in the preparation of natural products,3 pharmaceuticals4 and advanced materials.5 Particularly, the conjugated enediynes are usually used for synthesis of electronic and optical materials.6 Meanwhile, the 1,3-diynes are common structural motifs found in biologically active and pharmaceutical compounds, which are known to have anti-HIV,7 anticancer,8 antibacterial,9 and anti-inflammatory properties.10 Though several kinds of typical synthetic processes, including homo-11 or cross-coupling,12,13 diynone decarbonylation,14 oxidative coupling15 and oxidative decarboxylative homo-coupling,16 have been realized, developing some efficient methods for accessing such frameworks from easily available organic compounds is very desirable and important.The 1,1-dibromoethylenes, which are readily available from aldehydes, have attracted some attention due to their potentialities in the construction of conjugated enediynes and 1,3-diynes. Compared with the mature cases for constructing of symmetric conjugated 1,3-diynes, which are mostly from homo-coupling of 1,1-dibromoethylenes (Scheme 1a),17 the applications of 1,1-dibromoethylenes for synthesizing of conjugated enediynes and unsymmetrical 1,3-diynes are very rare. In 2005, Kabalka et al. reported a highly efficient Suzuki–Miyaura coupling of 1,1-dibromo-1-olefin with potassium alkynyl trifluoroborate to provide conjugated enediynes (Scheme 1b).18 Besides, they have been successfully applied to the cross-coupling reaction of terminal alkynes to produce unsymmetrical conjugated 1,3-diynes (Scheme 1c).19 Obviously, the cross-coupling reactions of 1,1-dibromoethylenes with organometal reagents is one of the most generally useful. However, this type of reactions has been less explored due to a complication of three competitive pathways (Scheme 1a–c). A key success of this reaction relies mainly on suitable catalytic systems and/or appropriate organometallic reagents that can selectively produce either conjugated enediynes or 1,3-diynes.
To the best of our knowledge, the cross-coupling reaction of 1,1-dibromoethylenes with alkynylaluminums, which have been extensively used in organic synthesis,20 has not been achieved. Herein, we would like to describe the novel Pd–phosphine complexes catalyzed selective cross-coupling reactions of 1,1-dibromoethylenes 1 with alkynylaluminums 2 to provide the aryl substituted conjugated enediynes 3 and unsymmetrical 1,3-diynes 4, respectively (Scheme 1d).
Results and discussion
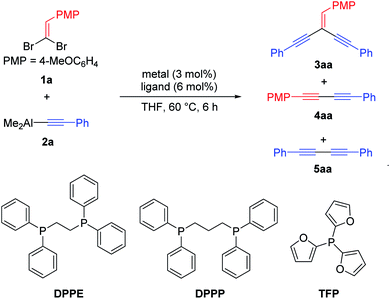
In the initial study, dimethyl(phenylethynyl)aluminum 2a and 1,1-dibromoethylene 1a were chosen as the model substrates for the synthesis of aryl substituted conjugated enediynes via cross-coupling reaction.21 Various palladium salts were surveyed in THF at 60 °C and PdCl2, Pd(PPh3)2Cl2 or Pd(PPh3)4 could afford 3aa in lower yield, meanwhile, the homo-coupling byproduct 1,4-diphenylbuta-1,3-diyne 5aa was observed (Table 1, entries 1–3). When the reaction was performed with Pd(OAc)2, an acceptable yield of 3aa (36%) was obtained, and also the homo-coupling byproduct 5aa was isolated in 16% yield (Table 1, entry 4). Unexpectedly, the cross-coupling product 1,3-diyne 4aa as the major compound was achieved in 13% yield under Pd2(dba)3 catalysis (Table 1, entry 5). Further optimization of the reaction conditions was then aimed at exploring the efficiency of Pd(OAc)2 with various P-ligands. Among them, the diphosphine ligands benefited the reactivity (Table 1, entries 6–9). As for the backbone moiety, the 1,2-bis(diphenylphosphanyl)ethane (DPPE) exhibited a slight superiority in reactivity toward this cross-coupling compared with 1,3-bis(diphenylphosphanyl)propane (DPPP) (Table 1, entry 8 vs. 9). To further improve the conversion, the efficiency of additives was then examined (Table 1, entries 10–12). We were delighted to find that the addition of 10 mol% of K3PO4 as additive could improve the yield of 3aa to 80% and only trace of byproducts 4aa and 5aa were observed (Table 1, entry 11). Therefore, the optimal conditions were identified as 3 mol% of Pd(OAc)2 with 6 mol% of DPPE, 10 mol% of K3PO4 as additive in THF at 60 °C for 6 h.| Entry | Metal/ligand/base | Yieldb (%) | ||
|---|---|---|---|---|
| 3aa | 4aa | 5aa | ||
| a Reaction conditions: 1a (0.5 mmol), 2a (1.0 mmol), metal (3 mol%), ligand (6 mol%), base (10 mol%), THF (1.0 mL), 60 °C, 6 h, under Ar.b Isolated yield. | ||||
| 1 | PdCl2 | 17 | Trace | 22 |
| 2 | Pd(PPh3)2Cl2 | 15 | Trace | 26 |
| 3 | Pd(PPh3)4 | 14 | Trace | 24 |
| 4 | Pd(OAc)2 | 36 | Trace | 16 |
| 5 | Pd2(dba)3 | Trace | 13 | Trace |
| 6 | Pd(OAc)2/PPh3 | 56 | Trace | 7 |
| 7 | Pd(OAc)2/PCy3 | 45 | Trace | 5 |
| 8 | Pd(OAc)2/DPPE | 70 | Trace | Trace |
| 9 | Pd(OAc)2/DPPP | 55 | Trace | 10 |
| 10 | Pd(OAc)2/DPPE/Cs2CO3 | 32 | Trace | Trace |
| 11 | Pd(OAc)2/DPPE/K3PO4 | 80 | Trace | Trace |
| 12 | Pd(OAc)2/DPPE/Et3N | 75 | Trace | Trace |
Under the optimal conditions (Table 1, entry 11), various 1,1-dibromoethylenes 1 and alkynylaluminum reagents 2 were evaluated, affording the corresponding aryl substituted conjugated enediynes 3 with moderate to good yields (up to 93%) and the trace of 1,3-diyne byproducts 4 and 5 were not isolated. As shown in Table 2, the reactivity of the cross-coupling was sensitive to the steric hindrance rather than to the electronic property of substituents on the phenyl ring of 1,1-dibromoethylenes 1. The substrates 1 with ortho-substituents gave lower yields than those with para ones (Table 2, entries 2 vs. 1, 6 vs. 5 and 8 vs. 7). Meanwhile, the fused-ring and heteroaromatic substrates (1m, 1n and 1o) were also tolerable, giving the desired products with 51% to 93% yields (Table 2, entries 11, 12, 13, 16, 19, 20, 24 and 28). On the other hand, the reactivity of this reaction was sensitive to neither the electronic properties nor the steric hindrance of substituents on the phenyl ring of alkynylaluminums 2. Generally, the desired conjugated enediynes 3 were isolated with good to excellent yields (up to 93%) except 3fa and 3oa (53% and 51% yields, Table 2, entries 6 and 13). Moreover, the 2-thienyl substituted substrate 2g also successfully afforded the desired products with good yields (Table 2, entries 26–28).22
| Entry | R1 | R2 | 3 | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.5 mmol), 2 (1.0 mmol), Pd(OAc)2 (3 mol%), DPPE (6 mol%), K3PO4 (10 mol%), THF (1.0 mL), 60 °C, 6 h, under Ar.b Isolated yield. | ||||
| 1 | 4-MeOC6H4 | Ph | 3aa | 80 |
| 2 | 2-MeOC6H4 | Ph | 3ba | 61 |
| 3 | 4-MeC6H4 | Ph | 3ca | 83 |
| 4 | 3-MeC6H4 | Ph | 3da | 91 |
| 5 | 4-FC6H4 | Ph | 3ea | 72 |
| 6 | 2-FC6H4 | Ph | 3fa | 53 |
| 7 | 4-ClC6H4 | Ph | 3ga | 83 |
| 8 | 2,4-Cl2C6H3 | Ph | 3ha | 76 |
| 9 | 4-BrC6H4 | Ph | 3ja | 75 |
| 10 | 4-F3CC6H4 | Ph | 3la | 71 |
| 11 | 1-Naphthyl | Ph | 3ma | 66 |
| 12 | 2-Thienyl | Ph | 3na | 69 |
| 13 | 2-Furyl | Ph | 3oa | 51 |
| 14 | 4-MeOC6H4 | 4-MeC6H4 | 3ab | 77 |
| 15 | 4-BrC6H4 | 4-MeC6H4 | 3jb | 88 |
| 16 | 1-Naphthyl | 4-MeC6H4 | 3mb | 74 |
| 17 | 4-MeOC6H4 | 3-MeC6H4 | 3ac | 75 |
| 18 | 3-BrC6H4 | 3-MeC6H4 | 3kc | 89 |
| 19 | 1-Naphthyl | 3-MeC6H4 | 3mc | 93 |
| 20 | 2-Thienyl | 3-MeC6H4 | 3nc | 69 |
| 21 | 4-MeC6H4 | 4-FC6H4 | 3cd | 72 |
| 22 | 4-BrC6H4 | 4-FC6H4 | 3jd | 89 |
| 23 | 3-BrC6H4 | 4-FC6H4 | 3kd | 84 |
| 24 | 2-Thienyl | 4-FC6H4 | 3nd | 83 |
| 25 | 4-BrC6H4 | 3-FC6H4 | 3je | 66 |
| 26 | 4-MeOC6H4 | 2-Thienyl | 3ag | 65 |
| 27 | 4-MeC6H4 | 2-Thienyl | 3cg | 70 |
| 28 | 2-Thienyl | 2-Thienyl | 3ng | 71 |
Inspired by the previous discovery (Table 1, entry 5), it was envisioned that the aryl substituted unsymmetrical 1,3-diynes 4 could be achieved via cross-coupling reaction of 1,1-dibromoethylenes 1 with alkynylaluminums 2 in the presence of Pd2(dba)3. Thus, we then restarted to optimize the reaction conditions of this Pd2(dba)3 catalyzed cross-coupling using 1a21 and 2a as the model substrates, respectively, in which the conjugated enediyne 3aa and homo-coupling byproduct 1,4-diphenylbuta-1,3-diyne 5aa were not determined. Performing the reaction in THF at higher temperature afforded the desired product 4aa with higher yield [Table 3, entries 2 (80 °C) vs. 1 (60 °C)]. To improve the reactivity, the efficiency of solvent was then examined and it was found that the polar aprotic solvents were beneficial (Table 3, entries 3 and 4). Further optimization of the reaction conditions was then aimed at exploring the efficiency of Pd2(dba)3 with various P-ligands. The addition of DPPE, which had been proved to be the most effective ligand in the synthesis of conjugated enediyne 3aa, could hardly provide the target product 4aa (Table 3, entry 5). Delightedly, when the reaction was carried out with 5.0 mol% of tri(2-furyl)phosphine (TFP, Table 1) as ligand, the desired 1,3-diyne 4aa could be isolated in 45% yield (Table 3, entry 8). Increasing the amount of TFP to 15.0 mol% could greatly improve the yield to 60% (Table 3, entry 9). The addition of 1.5 equiv. of diisopropyl ethylamine (DIPEA) could further enhance the yield to 74% (Table 3, entry 10). Thence, the optimal conditions were identified as 2.5 mol% of Pd2(dba)3 with 15.0 mol% of TFP, 1.5 equiv. of DIPEA in DMF at 80 °C for 10 h.
| Entry | Ligand | x (mol%) | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), 2a (0.8 mmol), Pd2(dba)3 (2.5 mol%), ligand (x mol%), solvent (3.0 mL), 80 °C, 10 h, under Ar.b Isolated yield.c Reaction was performed at 60 °C.d DIPEA (0.75 mmol) was added as additive. | ||||
| 1c | — | 5 | THF | 12 |
| 2 | — | 5 | THF | 18 |
| 3 | — | 5 | DMSO | 26 |
| 4 | — | 5 | DMF | 28 |
| 5 | DPPE | 5 | DMF | Trace |
| 6 | PPh3 | 5 | DMF | 19 |
| 7 | PCy3 | 5 | DMF | 21 |
| 8 | TFP | 5 | DMF | 45 |
| 9 | TFP | 15 | DMF | 60 |
| 10d | TFP | 15 | DMF | 74 |
With the optimal reaction conditions in hand (Table 3, entry 10), the substrate scope of 1,1-dibromoethylenes 1 with alkynylaluminums 2 was next examined and also the conjugated enediynes 3 and homo-coupling byproducts 1,3-diynes 5 were not determined. As shown in Table 4, the electronic or positional nature of the substituents either in 1,1-dibromoethylenes 1 or in alkynylaluminums 2 had nearly no effect on the efficiency of this cross-coupling reaction, affording the aryl substituted unsymmetrical 1,3-diynes 4 with good to excellent yields (up to 99%). For the fused-ring substrate 1m, the expected products 4ma and 4mb were obtained in good yields (Table 4, entries 6 and 11). Especially, the 2-thienyl or 2-furyl substituted substrates successfully afforded the unsymmetrical 1,3-diynes 4ob, 4oc and 4cg in good yields (Table 4, entries 12, 16 and 21).22
| Entry | R1 | R2 | 4 | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.5 mmol), 2 (0.8 mmol), Pd2(dba)3 (2.5 mol%), TFP (15.0 mol%), DIPEA (0.75 mmol), DMF (3.0 mL), 80 °C, 10 h, under Ar.b Isolated yield. | ||||
| 1 | 4-MeOC6H4 | Ph | 4aa | 74 |
| 2 | 4-MeC6H4 | Ph | 4ca | 97 |
| 3 | 3-MeC6H4 | Ph | 4da | 78 |
| 4 | 2-FC6H4 | Ph | 4fa | 82 |
| 5 | 2-ClC6H4 | Ph | 4ia | 78 |
| 6 | 1-Naphthyl | Ph | 4ma | 69 |
| 7 | 4-MeC6H4 | 4-MeC6H4 | 4cb | 68 |
| 8 | 3-MeC6H4 | 4-MeC6H4 | 4db (4cc) | 99 |
| 9 | 4-FC6H4 | 4-MeC6H4 | 4eb (4cd) | 91 |
| 10 | 2-FC6H4 | 4-MeC6H4 | 4fb | 81 |
| 11 | 1-Naphthyl | 4-MeC6H4 | 4mb | 66 |
| 12 | 2-Furyl | 4-MeC6H4 | 4ob | 59 |
| 13 | 4-MeC6H4 | 3-MeC6H4 | 4cc (4db) | 74 |
| 14 | 3-MeC6H4 | 3-MeC6H4 | 4dc | 89 |
| 15 | 2-FC6H4 | 3-MeC6H4 | 4fc (4df) | 77 |
| 16 | 2-Furyl | 3-MeC6H4 | 4oc | 61 |
| 17 | 4-MeC6H4 | 4-FC6H4 | 4cd (4eb) | 64 |
| 18 | 3-MeC6H4 | 4-FC6H4 | 4dd | 69 |
| 19 | 3-MeC6H4 | 2-FC6H4 | 4df (4fc) | 70 |
| 20 | 2-FC6H4 | 2-FC6H4 | 4ff | 63 |
| 21 | 4-MeC6H4 | 2-Thienyl | 4cg | 61 |
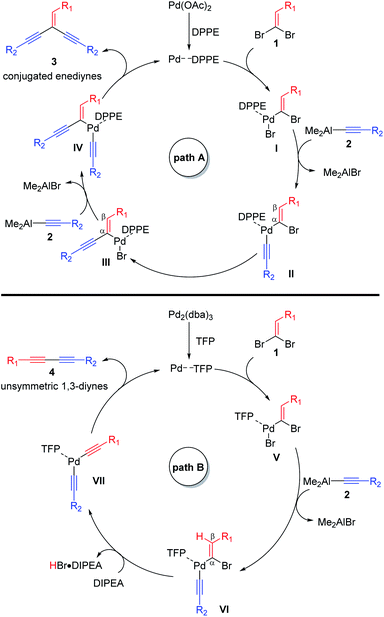
According to the previous studies on the palladium catalyzed cross-coupling reactions11–13 and our previous works about organoaluminums,20g–l two reasonable catalytic cycles are proposed in Scheme 2. The possible mechanism of the cross-coupling for producing enediynes 3 is shown in path A. First, the oxidative additions of 1,1-dibromoethylenes 1 to Pd–DPPE complex generate the organopalladium(II) bromide intermediates I. Then, the transmetalations of alkynylaluminums 2 with intermediates I give complex intermediates II and Me2AlBr. The intermediates II with another alkenyl bromide could isomerize into complex intermediates III via intramolecular PdII-translation. Next, the transmetalation of alkynylaluminums 2 with intermediates III provided complex intermediates IV and another Me2AlBr again. Finally, intermediates IV under goes reductive eliminations to afford the desired aryl substituted conjugated enediynes 3 and regenerate the active Pd–DPPE species for the next catalytic cycle. Similar to path A, the possible mechanism of the cross-coupling for producing unsymmetrical 1,3-diynes 4 is shown in path B. Oxidative additions of 1,1-dibromoethylenes 1 to Pd–TFP generate the organopalladium(II) bromide intermediates V and transmetalations of alkynylaluminums 2 with intermediates V give intermediates VI and Me2AlBr. Elimination of the β-H in intermediates VI, in which the acidities of β-H of intermediates VI could probably be stronger than those of intermediates II so that this elimination could be promoted by an equiv. amount of DIPEA, generate intermediates VII and HBr·DIPEA. Finally, intermediates VII also under goes reductive eliminations to afford the desired aryl substituted unsymmetrical 1,3-diynes 4 and regenerate the active Pd–TFP species for the next catalytic cycle.
Conclusions
Though the specific mechanism and reason why the same starting materials could selectively produce either conjugated enediynes or unsymmetrical 1,3-diynes in analogous Pd–phosphine catalytic system were unclear, we have successfully developed a highly efficient method for the synthesis of aryl substituted conjugated enediynes and unsymmetrical 1,3-diynes via selective cross-coupling reactions of 1,1-dibromoethylenes with alkynylaluminums using Pd(OAc)2–DPPE and Pd2(dba)3–TFP complexes as catalysts, respectively. A series of aryl substituted conjugated enediynes 3 and unsymmetrical 1,3-diynes 4 have been obtained in moderate to excellent yields (up to 99%). To the best of our knowledge, there is no precedent for the application of 1,1-dibromoethylenes and alkynylaluminums in cross-coupling reaction to date. Further mechanistic studies of these selective cross-coupling reactions are still in progress.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the National Natural Science Foundation of China (No. 22001219) and the Fundamental Research Funds for the Central Universities, Southwest Minzu University (No. ZYN2022030) for financial supports.Notes and references
- (a) K. C. Nicolaou, M. W. Dai, S. C. Tsay, V. A. Estevez and W. Wrasidlo, Science, 1992, 256, 1172 CrossRef CAS PubMed; (b) Y. Y. Lin, Y. J. Wang, J. H. Chen and C. F. Lee, Synlett, 2012, 23, 930 CrossRef CAS; (c) V. Sandrine, D. Etienne, A. Muriel, A. Corinne and P. Marc, Adv. Synth. Catal., 2013, 355, 2584 CrossRef; (d) H. K. H. Fong, J. M. Brunel, A. Longeon, M.-L. Bourguet-Kondracki, D. Barker and B. R. Copp, Org. Biomol. Chem, 2017, 15, 6194 RSC; (e) D. Qian and J. Zhang, Acc. Chem. Res., 2020, 53, 2358 CrossRef CAS PubMed; (f) D. Campeau, D. F. L. Rayo, A. Mansour, K. Muratov and F. Gagosz, Chem. Rev., 2021, 121, 8756 CrossRef CAS PubMed; (g) T. Zhao, X. Pu, W. Han and G. Gao, Org. Lett., 2021, 23, 1199 CrossRef CAS PubMed.
- (a) J. Z. Liu, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2009, 109, 5799 CrossRef CAS PubMed; (b) W. Shi and A. Lei, Tetrahedron Lett., 2014, 55, 2763 CrossRef CAS; (c) Y. Matsuda, S. Naoe, S. Oishi, N. Fujii and H. Ohno, Chem.–Eur. J., 2015, 21, 1463 CrossRef CAS PubMed; (d) J. S. Lampkowski, D. M. Uthappa, J. F. Halonski, J. C. Maza and D. D. Young, J. Org. Chem., 2016, 81, 12520 CrossRef CAS PubMed; (e) H. L. Sang, C. Wu, G. G. D. Phua and S. Ge, ACS Catal., 2019, 9, 10109 CrossRef CAS; (f) K. Dhananjaya, V. Nagaraju, G. Raghuram, K. K. Arup, N. Subhashree and C. M. Chandi, Tetrahedron Lett., 2020, 61, 151775 CrossRef; (g) M. W. Sebastian and H. Gerhard, Front. Chem., 2021, 9, 635826 CrossRef PubMed.
- A. L. K. Shi Shun and R. R. Tykwinski, Angew. Chem., Int. Ed., 2006, 45, 1034 CrossRef PubMed.
- H.-Y. Cao, X.-F. Guo, X.-F. Zhu, S.-S. Li and Y.-S. Zhen, Oncol. Rep., 2017, 37, 3329 CrossRef CAS PubMed.
- M. Gholami and R. R. Tykwinski, Chem. Rev., 2006, 106, 4997 CrossRef CAS PubMed.
- G. T. Hwang, H. S. Son, J. K. Ku and B. H. Kim, J. Am. Chem. Soc., 2003, 125, 11241 CrossRef CAS PubMed.
- M. L. Lerch, M. K. Harper and D. J. Faulkner, J. Nat. Prod., 2003, 66, 667 CrossRef CAS PubMed.
- S. Morandi, F. Pellati, S. Benvenuti and F. Prati, Tetrahedron, 2008, 64, 6324 CrossRef CAS.
- D. Lechner, M. Stavri, M. Oluwatuyi, R. Perda-Miranda and S. Gibbons, Phytochemistry, 2004, 65, 331 CrossRef CAS PubMed.
- (a) R. Schmidt, R. Thorwirth, T. Szuppa, A. Stolle, B. Ondruschka and H. Hopf, Chem.–Eur. J., 2011, 17, 8129 CrossRef CAS PubMed; (b) R. A. Xiao, R. Y. Yao and M. Z. Cai, Eur. J. Org. Chem., 2012, 22, 4178 CrossRef; (c) A. Narani, R. K. Marella, P. Ramudu, K. S. R. Rao and D. R. Burri, RSC Adv., 2014, 4, 3774 RSC.
- (a) Z. Chen, H. Jiang, A. Wang and S. Yang, J. Org. Chem., 2010, 75, 6700 CrossRef CAS PubMed; (b) C. Alexis, C. François and E. Gwilherm, Synthesis, 2010, 9, 1500 Search PubMed; (c) C. Longrui, E. L. Betsegaw, S. R. Jenna and M. James, Green Chem., 2014, 16, 1101 RSC; (d) R.-W. Orestes, C. Subrata, J. W. S. Linda, B.-E. Yehoshoa and M. David, Angew. Chem., Int. Ed., 2016, 55, 6942 CrossRef PubMed.
- (a) W. Shi, Y. D. Luo, X. C. Luo, L. Chao, H. Zhang, J. Wang and A. Lei, J. Am. Chem. Soc., 2008, 130, 14713 CrossRef CAS PubMed; (b) K. Park, G. Bae, J. Moon, J. Choe, K. H. Song and S. Lee, J. Org. Chem., 2010, 75, 6244 CrossRef CAS PubMed; (c) X. Feng, Z. Zhao, F. Yang, T. Jin, Y. Ma and M. Bao, J. Organomet. Chem., 2011, 696, 1479 CrossRef CAS; (d) D. Saha, T. Chatterjee, M. Mukherjee and B. C. Ranu, J. Org. Chem., 2012, 77, 9379 CrossRef CAS PubMed; (e) X. Jie, Y. Shang, P. Hu and W. Su, Angew. Chem., Int. Ed., 2013, 52, 3630 CrossRef CAS PubMed; (f) S. Ahammed, D. Kundu and B. C. Ranu, J. Org. Chem., 2014, 79, 7391 CrossRef CAS PubMed.
- (a) Y. Kim, A. Park, K. Park and S. Lee, Tetrahedron Lett., 2011, 52, 1766 CrossRef CAS; (b) Z. Y. Ma, X. Y. Wang, S. Y. Wei, H. L. Yang, F. W. Zhang, P. Wang, M. Xie and J. T. Ma, Catal. Commun., 2013, 39, 24 CrossRef CAS; (c) M. NasrEsfahani, I. Mohammadpoor-Baltork, A. R. Khosropour, M. Moghadam, V. Mirkhani, S. Tangestaninejad, V. Agabekov and H. A. Rudbaria, RSC Adv., 2014, 4, 14291 RSC; (d) A. L. Stein, F. N. Bilheri and G. Zeni, Chem. Commun., 2015, 51, 15522 RSC; (e) X. Li, X. Xie, N. Sun and Y. Liu, Angew. Chem., Int. Ed., 2017, 56, 6994 CrossRef CAS PubMed; (f) J. Schrgenhumer and M. Waser, Org. Biomol. Chem., 2018, 16, 7561 RSC.
- A. Dermenci, R. E. Whittaker and G. Dong, Org. Lett., 2013, 15, 2242 CrossRef CAS PubMed.
- (a) M. S. Maji, S. Murarka and A. Studer, Org. Lett., 2010, 12, 3878 CrossRef CAS PubMed; (b) S. L. Zhang, X. Y. Liu and T. Q. Wang, Adv. Synth. Catal., 2011, 353, 1463 CrossRef CAS; (c) K. Yin, C. J. Li, J. Li and X. S. Jia, Green Chem., 2011, 13, 591 RSC; (d) F. Alonso and M. Yus, ACS Catal., 2012, 2, 1441 CrossRef CAS; (e) Y. G. Zhu and Y. A. Shi, Org. Biomol. Chem., 2013, 11, 7451 RSC; (f) H. L. Li, M. Yang, X. Zhang, L. Yan, J. Li and Y. X. Qi, New J. Chem., 2013, 37, 1343 RSC; (g) H. Peng, Y. Xi, N. Ronaghi, B. Dong, N. G. Akhmedov and X. Shi, J. Am. Chem. Soc., 2014, 136, 13174 CrossRef CAS PubMed; (h) Y. G. Zhu, T. Xiong, W. Y. Han and Y. A. Shi, Org. Lett., 2014, 16, 6144 CrossRef CAS PubMed; (i) J. R. Suarez, D. Collado-Sanz, D. J. Cardenas and J. L. Chiara, J. Org. Chem., 2015, 80, 1098 CrossRef CAS PubMed; (j) L. Su, J. Dong, L. Liu, M. Sun, R. Qiu, Y. Zhou and S.-F. Yin, J. Am. Chem. Soc., 2016, 138, 12348 CrossRef CAS PubMed.
- D. X. Liu, F. L. Li, H. X. Li, J. Gao and J. P. Lang, Tetrahedron, 2014, 70, 2416 CrossRef CAS.
- (a) H. Jin and C. Kuang, Chin. J. Chem., 2011, 29, 592 CrossRef CAS; (b) Z. Huang, R. Shang, Zi-R. Zhang, X.-D. Tan, X. Xiao and Y. Fu, J. Org. Chem., 2013, 78, 4551 CrossRef CAS PubMed; (c) M. L. N. Rao, P. Dasgupta, B. S. Ramakrishna and V. N. Murty, Tetrahedron Lett., 2014, 55, 3529 CrossRef CAS.
- G. W. Kabalka, G. Dong and B. Venkataiah, Tetrahedron Lett., 2005, 46, 763 CrossRef CAS.
- (a) W. Shen and S. A. Thomas, Org. Lett., 2000, 2, 2857 CrossRef CAS PubMed; (b) M. L. N. Rao, S. S. Islam and P. Dasgupta, RSC Adv., 2015, 5, 78090 RSC.
- (a) O. V. Larionov and E. J. Corey, Org. Lett., 2010, 12, 300 CrossRef CAS PubMed; (b) Q. H. Li and H. M. Gau, Synlett, 2012, 5, 747 CrossRef; (c) D. F. Crepin and J. P. A. Harrity, Org. Lett., 2013, 15, 4222 CrossRef CAS PubMed; (d) Q. H. Li, J. W. Liao, Y. L. Huang, R. T. Chiang and H. M. Gau, Org. Biomol. Chem., 2014, 12, 7634 RSC; (e) Q. H. Li, J. Y. Jeng and H. M. Gau, Eur. J. Org. Chem., 2014, 7916 CrossRef CAS; (f) B. Shrestha, S. Thapa, S. K. Gurung, R. A. S. Pike and R. Giri, J. Org. Chem., 2016, 81, 787 CrossRef CAS PubMed; (g) S. Mo, X.-B. Shao, G. Zhang and Q.-H. Li, RSC Adv., 2017, 7, 27243 RSC; (h) Q.-H. Li, Y. Ding, G. Zhang, Z. Zhang and S. Mo, Curr. Org. Chem., 2017, 14, 462 Search PubMed; (i) X.-B. Shao, C. Wen, G. Zhang, K. Cao, L. Wu and Q.-H. Li, J. Organomet. Chem., 2018, 870, 68 CrossRef CAS; (j) X.-B. Shao, Z. Zhang, Q.-H. Li and Z.-G. Zhao, Org. Biomol. Chem., 2018, 16, 4797 RSC; (k) Q.-H. Li, X.-B. Shao, Y. Ding, C. Wen and Z.-G. Zhao, Curr. Org. Chem., 2018, 22, 1523 CrossRef CAS; (l) X.-B. Shao, X. Jiang, Q.-H. Li and Z.-G. Zhao, Tetrahedron, 2018, 74, 6063 CrossRef CAS.
- The p-methoxyphenyl substituted 1,1-dibromoethylene 1a was selected as the model substrate to facilitate purification and distinguish from the homo-coupling product.
- Under the optimal reaction conditions, no products were observed for alkyl or cycloalkyl substituted alkynylaluminums.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, compounds characterization and NMR spectra. See https://doi.org/10.1039/d2ra02127g |
| This journal is © The Royal Society of Chemistry 2022 |