Open Access Article
Open Access ArticleCatalytic behaviour of the Cu(I)/L/TEMPO system for aerobic oxidation of alcohols – a kinetic and predictive model†
Afnan Al-Hunaiti *a,
Batool Abu-Radahaa,
Darren Wraithb and
Timo Repo
*a,
Batool Abu-Radahaa,
Darren Wraithb and
Timo Repo c
c
aDepartment of Chemistry, School of Science, The University of Jordan, Amman 11942, Jordan. E-mail: a.alhunaiti@ju.edi.jo
bSchool of Public Health and Social Work, Queensland University of Technology, Queensland 4000, Australia
cDepartment of Chemistry, Laboratory of Inorganic Chemistry, University of Helsinki, 00014 Helsinki, Finland
First published on 10th March 2022
Abstract
Here, we disclose a new copper(I)-Schiff base complex series for selective oxidation of primary alcohols to aldehydes under benign conditions. The catalytic protocol involves 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO), N-methylimidazole (NMI), ambient air, acetonitrile, and room temperature. This system provides a straightforward and rapid pathway to a series of Schiff bases, particularly, the copper(I) complexes bearing the substituted (furan-2-yl)imine bases N-(4-fluorophenyl)-1-(furan-2-yl)methanimine (L2) and N-(2-fluoro-4-nitrophenyl)-1-(furan-2-yl)methanimine (L4) have shown excellent yields. Both benzylic and aliphatic alcohols were converted to aldehydes selectively with 99% yield (in 1–2 h) and 96% yield (in 16 h). The mechanistic studies via kinetic analysis of all components demonstrate that the ligand type plays a key role in reaction rate. The basicity of the ligand increases the electron density of the metal center, which leads to higher oxidation reactivity. The Hammett plot shows that the key step does not involve H-abstraction. Additionally, a generalized additive model (GAM, including random effect) showed that it was possible to correlate reaction composition with catalytic activity, ligand structure, and substrate behavior. This can be developed in the form of a predictive model bearing in mind numerous reactions to be performed or in order to produce a massive data-set of this type of oxidation reaction. The predictive model will act as a useful tool towards understanding the key steps in catalytic oxidation through dimensional optimization while reducing the screening of statistically poor active catalysis.
Introduction
Due to the use of aldehydes and carboxylic acids in various applications, a diverse effort to develop efficient, selective and greener oxidation systems using environmentally friendly catalysts, oxidants, and solvents has become a critical drive for catalysis.1 Thus, aerobic oxidation at ambient temperature is most preferred. Among numerous methods in the literature, copper (Cu) shows prominent activities for aerobic oxidation,.2–5 Specifically, Cu (I, II) have been employed in the mediation of nitroxyl-radicals; for example, 2,2,6,6-tetramethylpiperidinyl-1-oxyl (TEMPO) in the past few decades.6–9 Besides that, being inspired by the catalytic chemistry of the copper-containing enzyme, galactose oxidase (GOase), copper complexes have attracted attention as catalysts in alcohol oxidations.10–14 It has been reported that the Cu(I)/nitroxyl catalyst systems can catalyse the aerobic oxidations of a variety of substrates, including aromatic alcohols, secondary and sterically hindered alcohols as well as even more complex substrates such as polysaccharides.15,16Recently, it was found by Stahl and co-workers17,18 that NMI acts both as a base and as a ligand during the catalysis. As an additive, it was found that a certain molar ratio of NMI/Cu alongside another bidentate ligand, for example, 2,2′-bipyridine (bpy), gave the best catalytic performance. By carefully considering the catalyst optimization reported by Stahl and co-workers, the Cu/nitroxyl catalyst system using NMI as the only ligand added into the system could lead to moderate yield (68%).18,19
A five member ring Schiff base [(N-(4-fluorophenyl)-1-(pyroll-2-yl)methanimine] was recently applied and showed a high reactivity for the primary alcohol oxidation. These Schiff bases have a five-ring bearing oxygen which will behaves as a second donor atom in a bidentate ligand.18,28–31 Therefore, in this work we focused at studying the substitution effect on such ligands, and subsequently highlight the role of chelating nitrogen ligands on the reaction mechanistic understanding via kinetic approach. In our studies, CuI sources (Cu(OTf) and CuBr) were employed for comparison. The correlation between the ligand type and various catalytic reaction parameters were also analysed. To minimize the number of trials required to obtain optimum yield, a predictive model relating reactivity of catalytic system (Cu/L/NMI/TEMPO) to its product selectivity was developed. This would shows the usefulness of modelling approach consists not only in their capability to establish an approximate dependence of the performance of a catalytic material on its properties, but also in the possibility to use them for prediction the reactivity for various substrates.
Experimental
Synthesis and oxidation
Generalized additive model (GAM)
We undertook an exploratory data analysis to first examine potential relationships and to identify anomalies in the data or experiments conducted. As some of the experiments were only run once for a particular combination of variables, we restricted the available data to include combinations of variables where a number of experiments were performed. After filtering out these cases, for ligand type (L2 and L4): there were only two different types of growth/yield curves of ligand level available for L2 (levels 4 and 5) and one for L4 (level 4); only one value for TEMPO (equal to 5); two different types of alcohol for both ligand types (alcohol equal to Aliph1 and BOH1); three different levels of pressure (0.95, 0.89, 0.50); one temperature level (equal to 21); and two different values for NMI (5 and 10).For the main results, we used a Generalised Additive Model (GAM) using a beta distribution and logit link to establish associations/predictors of yield growth for ligand type (L2 and L4). Using a beta distribution naturally constrains the predicted yield to be between 0 and 100% (or 0 and 1). These models can effectively and flexibly allow for non-linear relationships between variables, in this case between yield and time.32 Other explanatory variables for these observed changes in yield over time (growth curves) can also be included in the regression model in different forms including allowing for interaction terms. Based on the data from the experiments, interaction terms for ligand type were only possible for ligand level, alcohol and NMI. Interaction between these potential predictors (as a three-way interaction) was more limited: alcohol has different levels for NMI (only one experiment for Aliph1); and two different levels for ligand level and NMI. Random effects were also included to allow for heterogeneity between observed growth curves present in the data.
Results and discussion
Optimization of reaction conditions
To examine the optimal reaction conditions for the oxidation, 1-octanol was employed as a model substrate for aerobic catalytic oxidation, Scheme 1. The oxidation is highly solvent dependent as shown in Table 1. Among the common solvents, acetonitrile (MeCN) is the most suitable solvent. It does not seem that there is a straight correlation between the reaction progress and both the polarity, and the coordination character of the solvent can be established. In this work acetonitrile was employed as the reaction medium. The use of water in such systems leads to decrease reactivity and catalyst deactivation.| Entry | Solvent (3 mL) | Conversion (%) |
|---|---|---|
| 1 | Toluene | 5 |
| 2 | DCM | 45 |
| 3 | MeCN | 100 |
| 4 | Water | 18 |
| 5 | Acetone | 15 |
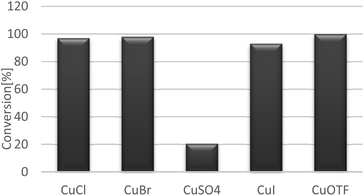
Pyridine is good base in numerous reactions, however in our reaction condition it diminish the reaction conversion, possibly due to the coordination with metal salt as ligand, therefore, blocking the chance to the reaction to occur (entry 6, Table 2) whereas inorganic bases are poorly soluble in organic solvents. Therefore, presence of a such base may cause a loss of the reactivity. The low yield can be by forming copper species such Cu(II)-hydroxides which may altering the reaction pathway.25,27 The observation suggests that either deprotonation is a fast step in the oxidation or there has another pathway involved the need of base in the system, and in this context the NMI has shown the highest reactivity (Fig. 2).
| Parameter | K | R | ||
|---|---|---|---|---|
| L2 | L4 | |||
| TEMPO | 0.0015 | 0.0016 | 0.8990 | 0.9210 |
| Cu salt | 0.0959 | 0.0204 | 0.9794 | 0.9470 |
| O2 | 0.0656 | 0.0945 | 0.7890 | 0.6701 |
| NMI | 0.0049 | 0.0050 | 0.9794 | 0.9658 |
| Ligand type | 0.0832 | 0.0388 | 0.8539 | 0.9585 |
Kinetic studies
Our initial mechanistic studies focused on probing mechanistic differences between two Schiff bases and their effect on the overall mechanism (L2 and L4 Scheme 1). The reaction rate was studied in combination with the other reaction parameters (TEMPO, NMI, alcohol type, copper salts and temperature). First, reaction time plots with different concentrations of aldehyde, determined by GC spectroscopy during the oxidation of benzylic and aliphatic alcohols (Fig. 4). Regarding the benzylic alcohols oxidations, the formation of benzaldehyde from benzyl alcohol (BOH2) exhibits a linear relation with time course. Furthermore, the oxidation of other benzylic show similar behaviour with lower reaction rate Fig. 5. Interestingly, the obtained results from aliphatic alcohols when compared to benzylic ones behave differently. | ||
| Fig. 4 Time course data for the oxidation of aliphatic (1-octanol, 2-octene-ol), benzyl alcohols (BOH2 benzyl alcohol, BOH3 cinnamyl alcohol, BOH4 diphenylmethanol) using L4. | ||
The tested aliphatic alcohols showed similar reaction rate but with slower reaction rate when compared to benzylic alcohol. This observation indicate that the substrate type has the effect on turnover rate with more reactive benzylic alcohols substrate the faster reaction rate, while with less reactive aliphatic substrates shows that catalyst oxidations contribution into the turnover rate only.
The data presented here provided a valuable insight into the roles of each component in the reaction system. The first order dependence on [O2] and mixed second-order/first-order dependence [Cu] in L2 resembles reactions of O2 with biomimetic nitrogen-chelated CuI complexes,21,22,26,27 and the data is in consistence of the mechanism where O2 is reacted with CuI to produce CuII superoxide species.22,26
The rate corresponding to a two-step sequence accounts for the second-order dependence on [Cu] at low [Cu], and first-order dependence at high [Cu]. However, a one sequence rate (first order) dependence on metal complex using L4 shows that the ligand type plays a key role in reaction rate. The ligand L4 weaker base than L2 that leads to a slower rate. Further studies will be needed to understand the origin of this difference. Noteworthy, the use of ancillary ligands it is tentatively attributed as base that should stabilize CuI–CuII oxo species. Table 2 summarise the reaction rate constant of all parameters control experiments show that oxidation of CuI to CuII by O2 does not require TEMPOH. However, TEMPOH appears to be required to achieve a kinetically competent rate. At the start of the reaction, when no TEMPOH is available, we speculate that the substrate reacts with the peroxo complex species to afford CuII–OOH and Cu II– substrate species.19–21
We obtained the UV-Vis of CuIOTf/L4 to study the interaction between different components in the reaction mixture (Fig. 6), which is useful to learn more about unclear aspects from the kinetic approach. The metal-to-ligand charge-transfer MLCT sharp peaks was observed between 310 and 360 nm. This indicates that the replacement of coordinated solvent molecules is at the CuI site. Meanwhile the bubbling with O2 through the solution shifted the band from 360 to 400 nm, which is attributed to dipolar interaction between the complex and the O2 instead of the complex oxidation.30 Broadening of the 400 nm band indicates a LMCT between NMI and the complex. New d–d transition band is formed at 510 nm after the NMI addition attributes to oxidation of the CuI to CuII species as postulated in other papers.30 TEMPO addition gave similar bands between 410 and 510 nm. Therefore, we believe that the role of TEMPO is the activation and stabilization of O2 in the active CuII species. Alcohol addition to the solution shows a disappearance of d–d band at 510, no broadening on band 410, and a negligible shift of the 410 band is observed.
Substrates expansion for the catalytic system
After investigating the reaction mechanism, the alcohol substrates scope was examined using the catalytic system (L4) Table 3. The results showed that the system has an excellent applicability with no over oxidation by-product. It aerobically oxidizes benzylic type alcohols quantitatively with no significant effect of the substituent on the phenyl ring, either electron with-drawing or donating groups on the product yield.Furthermore, the catalytic system showed good activity toward primary aliphatic alcohols oxidation with good yields. A moderate activity was observed for secondary alcohols such as 2-phenylethanol (entry 8), however, barely any activity to other secondary alcohol. It is noteworthy that the yield for the oxidation of cyclohexanol was negligible. This may be attributed to the volatile nature rather than poor catalytic efficiency.
In comparison of our results with previously reported Cu/TEMPO systems we found that the systems have shown a significantly preference to primary alcohols over secondary alcohols and successfully discriminate between the benzylic and aliphatic primary alcohols. The resutls were in consistance with previously reported Cu/TEMPO catalysts.7–13 In oxidizing diols such 1,4-butandiol (entry 14), this system afforded the lactones in good yield comparable to Cu/ABNO.31
A simple model for oxidation reaction prediction – GAM
The simple model prediction was developed for the yield curves with a particular focus on predictors for L2 and L4 (Fig. S13–S19†). Using the results from a generalized additive model (GAM, including random effects), there is some evidence of a difference in yield curves between NMI 5 and 10 (p = 0.0475) (Fig. 8a) and also for alcohol (Aliph1 versus BOH1) (p < 0.001) but not for the other variables examined (see Fig. 8b). A significantly lower yield curve over most of the time period for the experiment is observed for BOH1 compared to Aliph1. A lower yield curve is observed for NMI 5 compared to NMI 10 only over the middle time period of the experiment. In terms of interaction with ligand type (L2 and L4) there appears to be some evidence of an interaction only between ligand type and alcohol over time (p < 0.001) and not for the other variables. | ||
| Fig. 8 (a) Predicted yield over time for NMI (5 and 10) using GAM results. (b) Predicted yield over time for alcohol (Aliph1 and BOH1) using GAM results. | ||
There is evidence of a different effect of alcohol on ligand type L2 compared to ligand type L4 with a significant effect of alcohol on L2 but not L4 (Fig. 9). This difference in effect could be due to a small number of experiments/yield curves over the entire time for L4 compared to L2. The result from the predictive model inconsistent with results we obtained from the kinetic studies. This shows the possibility to use such simple model to predict the results of multi variable component in the reaction with minimal statistically unnecessary screening reactions. We have to keep in mind the32 necessity of large number of data to achieve a better understanding of all reaction components to reduce the level of uncertainty of the predictive model.
Conclusion
The results provide insights into catalytic mechanism of furan ligand type in Cu/TEMPO/NMI that show a simple system can achieve the desired reactivity with ligand designing and benign reaction condition. The substitution of furan moity such L2 and L4 would change basicity of the ligand, thus increases the electron density of the metal centre; therefore, a higher oxidation reactivity is expected. Currently, the catalytic systems are aerobically oxidizing primary alcohols into aldehydes in excellent yields and selectivity. The kinetic studies shows that the base effect NMI exhibited a first order dependence in both systems using L2 and L4. Similarly, a first order reaction was also observed on TEMPO with non-zero intercept. The Cu(OTf) loading amount reveals a varied-order dependence, with a second-order dependence at low [Cu] and a first-order dependence at high [Cu] using L2. However, L4 showed a first order dependence for [Cu]. This variation of the rate comes from the difference in the basicity moity of ligand and consequently leads to a slower rate. The control experiments show that oxidation of CuI to CuII by O2 does not require TEMPOH. However, TEMPOH appears to be required to achieve a kinetically competent rate. At the start of the reaction, when no TEMPOH is available, we speculate that the substrate reacts with the peroxo complex species to afford CuII–OOH and CuII– substrate species. The Hammett plot in our case oxidation reaction for both ligands L2 and L4 are ρ = +0.28 and 0.33 respectively. Therefore, the key step in the mechanism is not controlled by H-abstraction. The kinetic study in this work provides clear insight regarding the factors that play key role in reaction mechanism, which closely mimic galactose oxidase enzyme. The generalized additive model (GAM, including random effect) study allowed us to study simultaneously the four synthesis variables (reactant concentrations) and to map the nonlinear space. By means of clustering and factorial analysis of the multiparametric results, it was possible to correlate reaction composition with catalytic activity, ligand structure, and substrate behaviour. This hybrid system seems to be a useful tool in catalyst development through dimensional optimisation while reducing the screening of statistically poor active catalysis. This can be developed in the form a predictive model bearing in mind numerous numbers of reactions to be performed or to produce a massive dataset of this type of oxidation reactions. The predictive model will act as a useful tool towards understanding the key steps in the catalytic oxidation.Author contributions
Conceptualization, A. A. H.; methodology, A. A. H., D. W.; validation, A. A. H. and D. W. T. R.; formal analysis, A. A. H.; investigation B. A., A. A. H.; resources, A. A. H.; data curation, A. A. H. and D. W.; writing—original draft preparation, A. A. H. and D. W. T. R.; writing—review and editing, A. A. H., D. W. and T. R.; visualization, A. A. H.; supervision, A. A. H.; project administration, A. A. H.; funding acquisition, A. A. H.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Deanship of Scientific Research at the University of Jordan.Notes and references
- M. Hudlicky, Oxidations in Organic Chemistry, American Chemical Society, Washington DC, 1990 RSC; Q. Cao, L. M. Dornan, L. Rogan, N. L. Hughes and M. J. Muldoon, Chem. Commun., 2014, 50, 4524 RSC; M. T. Räisänen, A. Al-Hunaiti, E. Atosuo, M. Kemell, M. Leskelä and T. Repo, Catal. Sci. Technol., 2014, 4, 2564–2573 RSC; A. Al-Hunaiti, Q. Mohaidat, I. Bsoul, S. Mahmood, D. Taher and T. Hussein, Catalysts, 2020, 10, 839 CrossRef CAS.
- G. Tojo and M. I. Fernandez, Oxidation of Alcohols to Aldehydes and Ketones, Springer Verlag, New York, 2006 Search PubMed.
- G. Tojo and M. I. Fernandez, Oxidation of Primary Alcohols to Carboxylic Acids, Springer, New York, NY, 2007 Search PubMed.
- S. Caron, R. W. Dugger, S. G. Ruggeri, J. a Ragan and D. H. B. Ripin, Chem. Rev., 2006, 106, 2943 CrossRef CAS PubMed.
- D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. L. Leazer Jr, R. J. Linderman, K. Lorenz, J. Manley, B. Pearlman, A. Wells, A. Zaks and T. Y. Zhang, Green Chem., 2007, 9, 411 RSC.
- H. Liu, D. Ramella, P. Yu and Y. Luan, RSC Adv., 2017, 7, 22353 RSC.
- R. Jlassi, A. P. C. Ribeiro, M. Mendes, W. Rekik, G. A. O. Tiago, K. T. Mahmudov, H. Naïli, M. F. C. Guedes da Silva and A. J. L. Pombeiro, Polyhedron, 2017, 129, 182 CrossRef CAS.
- J. Rabeah, U. Bentrup, R. Stößer and A. Brückner, Angew. Chem., Int. Ed., 2015, 127, 11957 CrossRef.
- S. E. Kesler and B. H. Wilkinson, Geology, 2008, 36, 255–258 CrossRef.
- G. R. Hemsworth, E. J. Taylor, R. Q. Kim, R. C. Gregory, S. J. Lewis, J. P. Turkenburg, A. Parkin, G. J. Davies and P. H. Walton, J. Am. Chem. Soc., 2013, 135, 6069 CrossRef CAS PubMed.
- R. A. Festa and D. J. Thiele, Curr. Biol., 2011, 21, R877 CrossRef CAS PubMed.
- R. R. Conry, Copper: inorganic & coordination chemistry based in part on the article copper: inorganic & coordination chemistry by Rebecca R. Conry & Kenneth D. Karlin which appeared in the encyclopedia of inorganic chemistry, Encycl. Inorg. Chem, John Wiley & Sons, Ltd, Chichester, UK, 1st edn, 2006 Search PubMed.
- M. M. Whittaker and J. W. Whittaker, J. Biol. Chem., 1988, 263, 6074 CrossRef CAS; L. Marais and A. J. Swarts, Catalysts, 2019, 9, 395 CrossRef; M. J. Muldoon, Catal. Sci. Technol., 2014, 4, 1720–1725 RSC.
- P. J. Filial, A. Sibaouih, J. U. Ahmad, M. Nieger, M. T. Räisänen, M. Leskelä and T. Repo, Adv. Synth. Catal., 2009, 351, 2625 CrossRef.
- E. Safaei, L. Hajikhanmirzaei, B. Karimi, A. Wojtczak, P. Cotič and Y.-I. Lee, Polyhedron, 2016, 106, 153 CrossRef CAS.
- S. Adomeit, J. Rabeah, A. E. Surkus, U. Bentrup and A. Brückner, Inorg. Chem., 2017, 56, 684–691 CrossRef CAS PubMed.
- S. L. Zultanski, J. Zhao and S. S. Stahl, J. Am. Chem. Soc., 2016, 138, 6416 CrossRef CAS PubMed.
- J. M. Hoover, B. L. Ryland and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 2357 CrossRef CAS PubMed.
- J. E. Steves and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 15742 CrossRef CAS PubMed.
- S. S. Stahl, J. Am. Chem. Soc., 2011, 133, 16901 CrossRef PubMed.
- P. Gamez, I. W. C. E. Arends, J. Reedijk and R. A. Sheldon, Chem. Commun., 2003, 2414 RSC.
- M. del Mar Conejo, J. Cantero, A. Pastor, E. Álvarez and A. Galindo, Inorg. Chim. Acta, 2018, 470, 113–118 CrossRef.
- W. Czepa, M. A. Fik, S. Witomska, M. Kubicki, G. Consiglio, P. Pawluć and V. Patroniak, ChemistrySelect, 2018, 3, 9504–9509 CrossRef CAS.
- G. L. Tripodi, M. M. J. Dekker, J. Roithova and L. Que Jr, Angew. Chem., Int. Ed., 2021, 60, 7126–7131 (Angew. Chem., 2021, 133, 7202–7207) CrossRef CAS PubMed.
- Z. Liu, Z. Shen, N. Zhang, W. Zhong and X. Liu, Catal. Lett., 2018, 148, 2709–2718 CrossRef CAS; E. T. Kumpulainen and A. M. Koskinen, Chem.–Eur. J., 2009, 15, 10901–10911 CrossRef PubMed.
- E. Lagerspets, K. Lagerblom, E. Heliövaara, O.-M. Hiltunen, K. Moslova, M. Nieger and T. Repo, Mol. Catal., 2019, 468, 75 CrossRef CAS.
- C. Feng, L. Cheng, H. Ma, L. Ma, Q. Wu and J. Yang, Inorg. Chem., 2021, 60, 14132–14141 CrossRef CAS PubMed.
- A. Ochen, R. Whitten, H. E. Aylott, K. Ruffell, G. D. Williams, F. Slater, A. Roberts, P. Evans, J. E. Steves and M. J. Sanganee, Organometallics, 2019, 38, 176–184 CrossRef CAS.
- E. O. Nutting, K. Mao and S. S. Stahl, J. Am. Chem. Soc., 2021, 143, 10565–10570 CrossRef PubMed.
- L. Marais, J. Bures, J. H. L. Jordaan, S. Mapolie and A. J. A. Swarts, Org. Biomol. Chem., 2017, 15, 6926–6933 RSC.
- X. Xie and S. S. Stahl, J. Am. Chem. Soc., 2015, 137, 3767–3770 CrossRef CAS PubMed; T. Y. Chen, Z. H. Wang, J. C. Xiao, Z. Cao, C. F. Yi and Z. H. Xu, Asian J. Org. Chem., 2019, 8, 1321–1324 CrossRef.
- M. Holena and M. Baerns, Catal. Today, 2003, 81, 485–494 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra09359b |
| This journal is © The Royal Society of Chemistry 2022 |