Open Access Article
Open Access ArticleLow-temperature thermal conversion of Al-substituted goethite in gibbsitic bauxite for maximum alumina extraction
Guotao Zhou ,
Yilin Wang*,
Tiangui Qi,
Qiusheng Zhou
,
Yilin Wang*,
Tiangui Qi,
Qiusheng Zhou ,
Guihua Liu
,
Guihua Liu ,
Zhihong Peng and
Xiaobin Li*
,
Zhihong Peng and
Xiaobin Li*
School of Metallurgy and Environment, Central South University, No. 932, Lushan Road, Changsha 410083, Hunan, China. E-mail: wang.yi.lin@outlook.com; x.b.li@csu.edu.cn; Fax: +86 731 88830453
First published on 2nd February 2022
Abstract
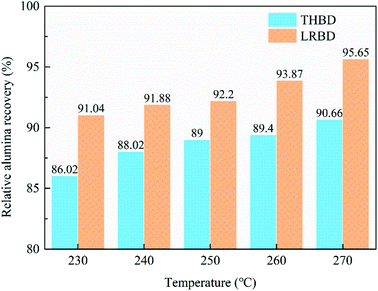
The conversion of Al-substituted goethite (Al-goethite) to hematite in gibbsitic bauxite is conducive to alumina extraction during the Bayer process and the enrichment of iron minerals in red mud. In this work, mineralogical characteristics of gibbsitic bauxite were identified by AMICS analysis, and the low-temperature thermal conversion behavior of both synthetic Al-goethite and natural Al-goethite in gibbsitic bauxite were investigated through thermal gravity analysis, phase transformation, and microstructure studies. Results show that the proportion of aluminum in Al-goethite reached 12.68% of the total aluminum content in gibbsitic bauxite. The conversion of synthetic Al-goethite to hematite starts at ∼280 °C, while that of natural Al-goethite starts at ∼320 °C, and the addition of NaOH can accelerate the conversion. The formed hematite inherits the needle-like appearance of the original Al-goethite, has many holes on the surface due to dehydroxylation, and no migration of aluminum elements occurs during the roasting process, indicating that Al-goethite transformed into porous Al-substituted hematite (Al-hematite), which is beneficial to the extraction of the aluminum retained in the hematite structure during Bayer digestion. To confirm the above results, digestion experiments (without or with roasting for typical Bayer digestion or low-temperature roasting-Bayer digestion) were carried out with gibbsitic bauxite and the one roasted at 400 °C for 30 min as raw materials, respectively. Compared to the typical Bayer digestion, the relative alumina recovery of low-temperature roasting-Bayer digestion increased from 90.06% to 95.65%, the red mud yield decreased from 36.32% to 34.08%, and the grade of Fe in red mud increased from 48.45% to 52.88% at 270 °C for 60 min. Enhanced transformation of Al-goethite significantly improves alumina recovery and the resultant iron-rich red mud can be easily co-processed in the steel industry, thus significant emission reduction of red mud from the Bayer system might be achieved.
1. Introduction
The high-iron gibbsitic bauxite in the Boké region of Guinea has become an important raw material for the global alumina industry.1 Generally, gibbsitic bauxite contains an appreciable amount of Al-goethite,2,3 causing alumina loss due to the isomorphous substitution of aluminum in place of the Fe atoms with a maximum of 33 mol% in the goethite lattice.4,5 Furthermore, the sedimentability, filterability, and washability of the formed red mud slurry deteriorate owing to the non-compact crystal structure of goethite.6–8 The conversion of Al-goethite is of great significance to the efficient utilization of gibbsitic bauxite and has therefore received great attention. Currently, a wide variety of processes have been proposed for the conversion of Al-goethite and can be categorized into hydrometallurgy and pyrometallurgy.In the hydrometallurgical process (mainly referring to the Bayer process), the conversion of Al-goethite can be promoted by increasing the Bayer digestion temperature and the caustic concentration,9,10 but in the commonly adopted digestion temperature range (<280 °C) and caustic concentration (<240 g L−1), the complete conversion of Al-goethite is still difficult. The addition of lime or calcium-containing additives can improve the hydrothermal conversion,11–14 but excessive lime addition is necessary for the industrial digestion process, leading to detrimental consequences for alumina recovery, the discharge of red mud, and the iron mineral sorting performance.15,16 The addition of reducing agents, such as saccharide,17 iron powder,18 and glycerol,19 can also promote the conversion of Al-goethite to hematite or magnetite. However, the use of a large amount of reducing agent greatly affects the balance of organic matter in the Bayer digestion system, which needs further optimization.
In pyrometallurgical methods, the conversion of goethite or Al-goethite usually refers to dehydration conversion due to the temperature increase during heating or mechanical grinding.20,21 A general agreement exists on the fact that the temperature of thermal conversion increases with the Al substitution in Al-goethite.22 With the Al substitution for Fe of up to 14 mol%, the conversion temperature range of Al-goethite shifted to 247–320 °C.23 In addition, the difficulty of converting Al-goethite increases with the crystallinity of Al-goethite, and the conversion temperature can increase from 260 °C to 320 °C.24–26 However, the mechanism of thermal conversion of Al-goethite has two points. Wolska used X-ray diffraction (XRD) and infrared spectroscopy (IR) to observe that intermediate structures referred to as “proto-hematite” and/or “hydro-hematite” were formed by the dehydration of goethite in the temperature range of 180–250 °C and completely transformed into hematite at 800–1050 °C.27 Other researchers insist that the process is a direct conversion from goethite to hematite and does not form any intermediate.28,29 The addition of reducing agents during the thermal conversion process to induce the conversion of goethite and Al-goethite to magnetite has also been widely reported, with the conversion temperature varying depending on the type of reducing agents.30–32 Processing goethite-rich iron ores using reduction roasting followed by low-intensity magnetic separation can obtain an iron concentrate with a total iron grade of ∼66.6% and iron recovery of ∼90.4% at 800 °C for 30 min using 10% coal.33 According to the literature,34 a good temperature for converting goethite to magnetite through reduction roasting was found to be between 650 °C and 700 °C in 50%/50% CO/CO2 mixtures. Furthermore, the reduction of goethite ore with carbohydrates starts at ∼450 °C, with the maximum at ∼520 °C.35 However, the thermal conversion process of Al-goethite still faces the problems of high energy consumption and high cost. Moreover, no report has been published on the mechanism of the conversion of Al-goethite in bauxite at low-temperature roasting and the digestion performance in the Bayer process.
In this work, the distribution characteristics of Al-goethite in gibbsitic bauxite in the Boké region of Guinea were first identified by the advanced mineral identification and characterization system (AMICS). Then, the thermal conversion behavior of both synthetic Al-goethite and natural Al-goethite in gibbsitic bauxite at temperatures between 200 °C and 400 °C were investigated through thermal gravity analysis, phase transformation, and microstructure. The effect of NaOH on the roasting process was also studied. Finally, the effect of the low-temperature roasting process on Bayer digestion (relative alumina recovery, degree of iron enrichment, and organic matter content of the digestion Bayer liquid) was clarified. The results of this study can help in the better understanding of the phase structure changes of Al-goethite during the roasting process and provide a theoretical basis for the efficient conversion of Al-goethite to hematite in gibbsitic bauxite.
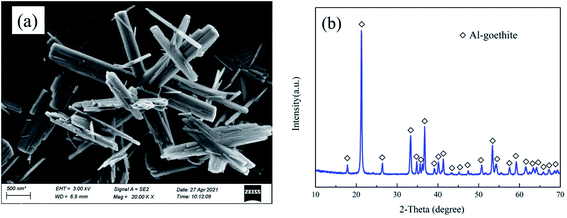
2. Experimental
2.1 Materials
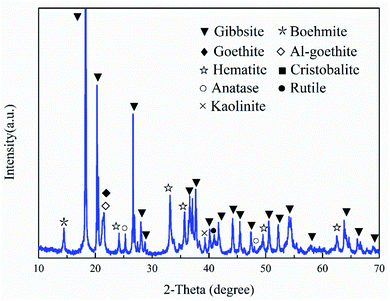
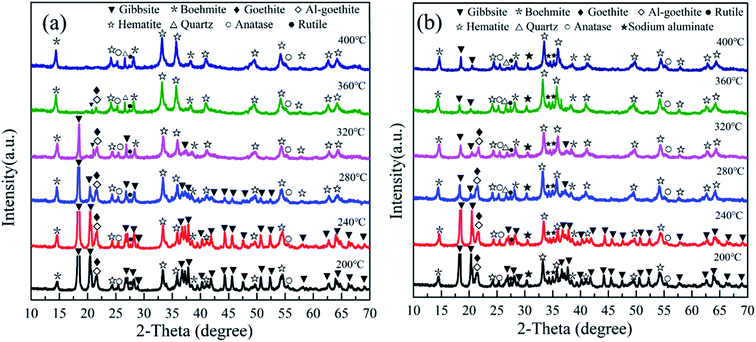
The Fe(NO3)3·9H2O, Al(NO3)3·9H2O, KOH, NaOH, and Al(OH)3 were of analytical grade (>99%) and purchased from Shanghai Macklin Biochemical Co., Ltd in China. The preparation of synthetic Al-goethite was based on a previous report.36 Briefly, Fe(NO3)3·9H2O and Al(NO3)3·9H2O were dissolved in 400 mL of deionized water by continuous stirring to form a transparent yellow solution. Subsequently, 5 and 0.5 mol L−1 KOH solutions were used to adjust the pH to 12.5 ± 0.1. The suspensions were stirred for 60 min and then aged for 14 days at 70 °C. The final product was washed with deionized water. Finally, the samples were dried in an oven at 50 °C for 24 h and then cooled to room temperature for further use. The SEM image and XRD pattern of the synthetic Al-goethite particles are shown in Fig. 1. From Fig. 1(a), the SEM image of synthetic samples shows typical acicular and rod-like morphologies, which is consistent with the literature report of goethite morphology,37 suggesting that the synthetic samples may be goethite. In addition, the XRD patterns in Fig. 1(b) indicate that the characteristic peaks of the synthetic samples matched well with the standard card of goethite [JCPDS (29-0713)], revealing that goethite nanoparticles were successfully synthesized. The composition of synthetic samples was analyzed by using an inductively coupled plasma emission spectrometer (ICP-OES), and the results show an alumina content of 3.05%, a ferric oxide content of 85.84%, and the Al/(Fe + Al) molar ratio of 5.27, implying that 5.27 mol% aluminum was built into the goethite lattice in place of Fe atoms, forming Al-goethite with the formula Fe0.9473Al0.0527OOH.The Al-goethite containing gibbsitic bauxite was obtained from Boké region of Guinea, and its composition was analyzed by ICP-OES. The results indicate that the alumina, silicon dioxide content, and ferric oxide were 44.69%, 2.11%, and 25.21%, respectively. In addition, the total organic carbon content was 0.25%. The XRD analysis in Fig. 2 indicates that gibbsite and boehmite are the primary aluminum minerals. The silicate minerals include kaolinite and quartz. Goethite, Al-goethite, and hematite are the main iron minerals. Titanium minerals include anatase and rutile.
The Bayer liquor containing [Na2Ok] = 230 g L−1 and [Al2O3] = 126 g L−1 was prepared by dissolving NaOH and Al(OH)3. Na2Ok denotes the caustic alkali in Na2O.
2.2 Low-temperature roasting
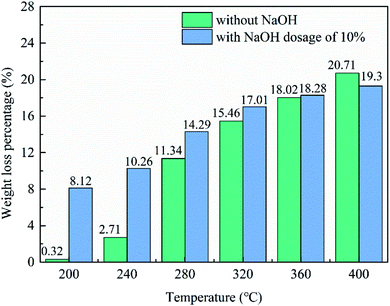
The synthetic Al-goethite or gibbsitic bauxite was sufficiently mixed with 10% NaOH (mixture). Predetermined 10 g of starting materials, namely, synthetic Al-goethite, gibbsitic bauxite, or mixture, were weighed into a flat bottom crucible before they were placed in a muffle furnace at the desired roasting temperature. The roasting temperature varied between 200 °C and 400 °C, and the roasting time was 30 min. After roasting, the samples were removed from the muffle furnace, cooled in air, and weighed before subsequent analysis. Meanwhile, the quality change and the roasting loss rate of the samples before and after roasting were determined under different conditions.2.3 The Bayer digestion
The Bayer digestion experiments were carried out in a molten mixed nitrate salt cell (YYL-150ML/6, Dingda Chemical Machinery Co. Ltd, China). Starting materials, namely, 31.25 g bauxite or 24.78 g roasted bauxite (at 400 °C for 30 min, weight loss rate of 79.24% based on LOI analysis), were digested with 100 mL of Bayer liquor in a 150 mL sealed rotating steel reactor immersed in a molten mixed nitrate salt cell at a preset temperature. To enhance stirring, 2 × Φ15 mm and 4 × Φ15 mm steel balls were added into the reactor in advance. After the reaction, the reactors were taken out of the cell and then immediately cooled in tap water. The obtained slurry was subsequently filtered, and the filter cake was washed with hot water and then dried at 100 °C for 6 h before analysis.The relative alumina recovery during Bayer digestion is calculated using eqn (1):
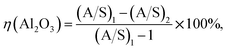 | (1) |
The removal rate of TOC during Bayer digestion is calculated using eqn (2):
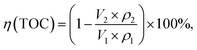 | (2) |
2.4 Characterization of the samples
All the samples have been dried at 100 °C for 12 h before characterization analysis. Automated quantitative mineralogy analyses were performed on gibbsitic bauxite by AMICS, which consists of a scanning electron microscope (Sigma 300, Carl Zeiss AG, Germany), an energy spectrometer (Quantax 400, Bruker, Germany), and a mineral analysis software (AMICS analysis software, Bruker, Germany). The thermogravimetry and derivative thermal gravimetry curves of samples at the temperature range from 30 °C to 820 °C were determined using a thermal analyzer (STD650, Waters, USA) at the heating rate of 10 °C min−1 in an air stream under atmospheric pressure. The mineral phases were characterized by XRD (TTR III, Rigaku, Japan) using Cu Kα radiation at a scan rate of 5° min−1. The microscopic surface morphology and the microscale composition were analyzed through SEM (Zeiss sigma 300, Carl Zeiss AG, Germany; MIRA3-LMH, Tescan, Czech Republic) and X-ray energy spectrometry (SmartEDX, EDAX Inc, USA; EDX-MAX20, Oxford, England). Chemical analysis of the samples was performed via fusion method (750 °C for 15 min with a mixture of NaOH followed by direct dissolution in boiling deionized water) through ICP-OES (ICAP7400 Radial, Thermo Fisher Scientific, USA). The organic matter content of the samples was determined by the potassium dichromate capacity external heating method.38 The total concentration of organic matter in the Bayer liquid was measured using the total organic carbon (TOC-L CPH, Shimadzu (Suzhou) Instruments Manufacturing Co., Ltd, China).3. Results and discussion
3.1 AMICS of gibbsitic bauxite
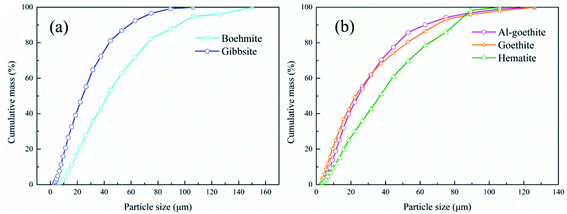
To illustrate the dissemination characteristics and relations among the minerals, the gibbsitic bauxite was scanned for quantitative image analysis using AMICS, and the results are presented in Fig. 3. The mineral maps in Fig. 3(a) show that gibbsite, boehmite, hematite, goethite, and Al-goethite are irregular granular, plate-like, or elongated and mostly have obvious dissociation characteristics and exist independently. However, there is also intercalation between minerals. From Fig. 3(b), gibbsite occasionally occurs as inclusions within goethite and Al-goethite in the form of aggregates, thus possibly hindering gibbsite digestion during the Bayer process. From Fig. 3(c), the main minerals in bauxite are gibbsite, boehmite, hematite, goethite, and Al-goethite and the proportions of them are 60.06%, 2.74%, 9.35%, 5.05%, and 15.90%, respectively. The minerals in the gibbsitic bauxite compare well with the XRD in Fig. 2 for major minerals but identify a much wider range of minor minerals that were below the detection limit for XRD, such as ilmenite, chlorite, iron garnet, and muscovite. From Fig. 3(d), the aluminum contents of the gibbsite and boehmite in bauxite account for approximately 77.93% and 5.21%. The main forms of iron minerals are hematite, goethite, and Al-goethite with an iron content of 28.19%, 13.70%, and 51.43% of the iron content in bauxite, respectively, which exist in granular or platelike single-crystal aggregates along with each other. The proportion of aluminum in Al-goethite is up to 12.68% of the aluminum content in bauxite because Al incorporation into the crystal structure of goethite occurs via the isomorphous ionic substitution of Al for Fe in goethite formation. The mass distribution data of different sized fractions of gibbsitic bauxite are presented in Fig. 4. As is shown in Fig. 4, the gibbsitic bauxite is a mixture of particles of different sizes, from fine (−2.79 μm) to coarse (−150 μm). The boehmite particles are coarser than those of the gibbsite, and the goethite/Al-goethite particles are also coarser than those of hematite.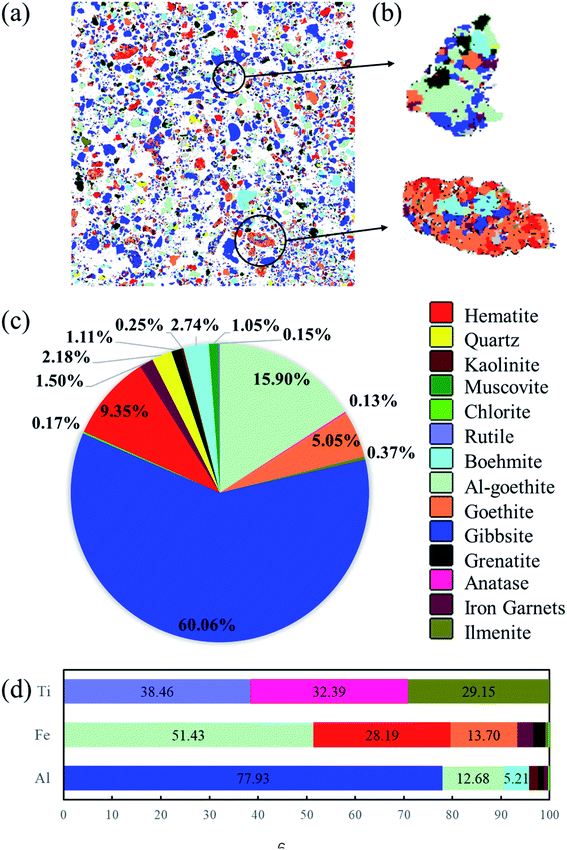 | ||
| Fig. 3 The AMICS results for gibbsitic bauxite (a) and (b) minerals distribution, (c) minerals proportions, and (d) proportions of Ti, Fe, Al in minerals. | ||
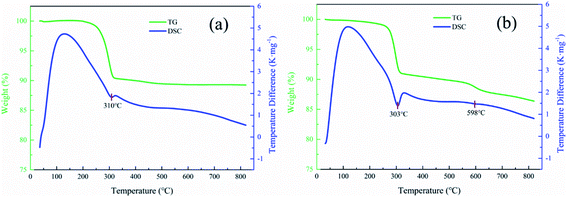
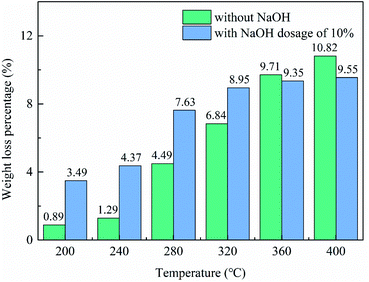
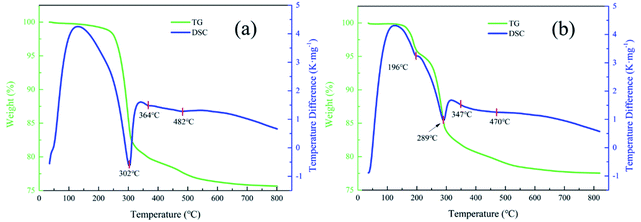
3.2 Thermal conversion of synthetic Al-goethite
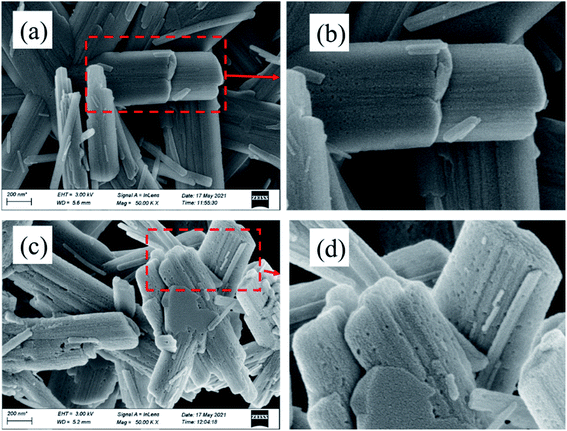 | ||
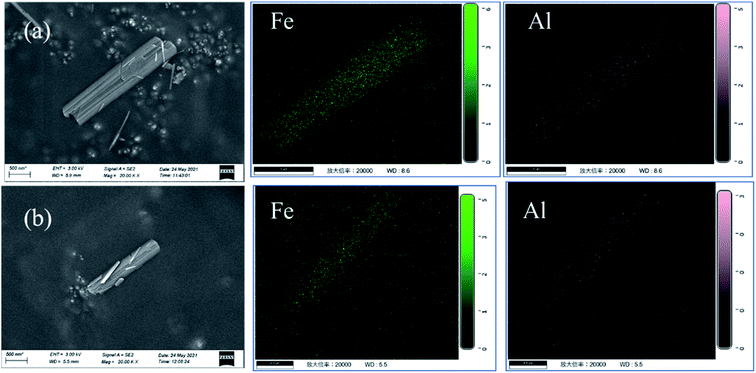
| Fig. 8 SEM images of the roasted products of synthetic Al-goethite at 360 °C for 30 min (a) and (b) without NaOH and (c) and (d) mixed with 10 wt% NaOH. | ||
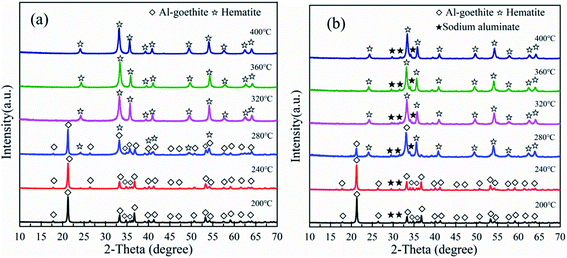
3.3 Thermal conversion of Al-goethite containing gibbsitic bauxite
3.4 Effect of thermal conversion gibbsitic bauxite in Bayer digestion
4. Conclusions
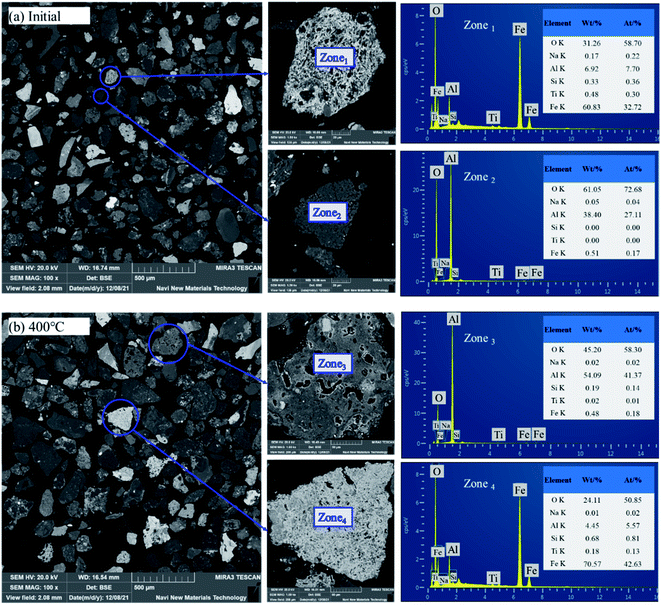
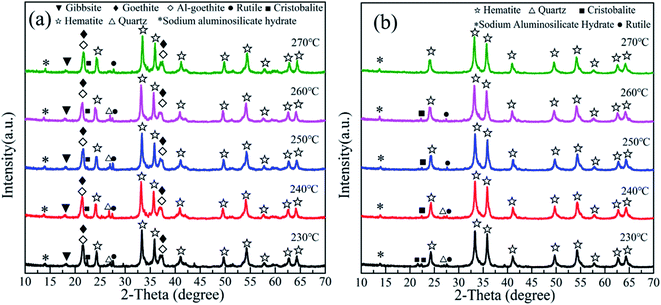
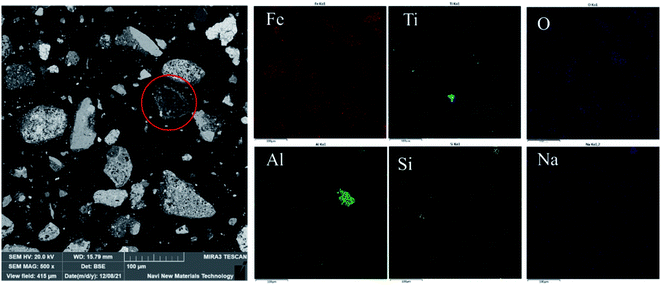
(1) The goethite and Al-goethite in Guinea gibbsitic bauxite account for approximately 5.05% and 15.90%, respectively, and the latter's aluminum content proportion is as high as 12.68%, which exists in the form of irregular, plate-like, or long strip granules. Portions of goethite and Al-goethite cover the surface of the gibbsite particles in the form of aggregates.(2) The conversion temperature of synthetic Al-goethite starts at a rather low temperature, ∼280 °C, while that of natural Al-goethite starts at ∼320 °C. We could attribute this difference in conversion temperature to the shielding effect of the admixtures, such as gibbsite, quartz, and hematite, in bauxite. The roasting process experiences a phase change from Al-goethite to Al-hematite without any morphological change in the original Al-goethite-like appearance, which has many pores due to de-hydroxylation.
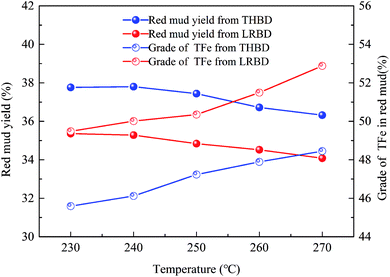
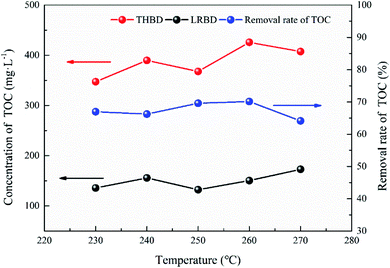
(3) Low-temperature roasting Bayer process can improve the thermal conversion of Al-goethite to hematite and remove a certain amount of organic matter in gibbsitic bauxite, increasing the relative digestion ratio of alumina and the grade of TFe in red mud by approximately 6% and 4%, respectively, and decreasing the yield of red mud and the content of organic matter in the Bayer liquor by about 6% and 67%, respectively.
Author contributions
Guotao Zhou: investigation, experimental, formal analysis and writing–original draft. Yilin Wang: funding acquisition, conceptualization, methodology, review and editing. Tiangui Qi: review and editing. Qiusheng Zhou: review and editing. Guihua Liu: review and editing. Zhihong Peng: review and editing. Xiaobin Li: conceptualization, review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China [grant number 52104353].References
- International Aluminium Institute, 2020, https://www.world-aluminium.org.
- A. R. Hind, S. K. Bhargava and S. C. Grocott, Colloids Surf., A, 1999, 146, 359–374 CrossRef CAS.
- F. Feret, M. Authier-Martin and I. Sajo, Clays Clay Miner., 1997, 45, 418–427 CrossRef CAS.
- P. G. Fazey, B. H. O'Connor and L. C. Hammond, Clays Clay Miner., 1991, 39, 248–253 CrossRef CAS.
- D. G. Schulze, Clays Clay Miner., 1984, 32, 36–44 CrossRef CAS.
- N. Brown and R. J. Tremblay, Light Met., 1974, 3, 825–844 CAS.
- D. Lawson, A. Rijkeboer, D. Dajkovich, M. Jackson and H. Lawrence, Light Met., 2014, 11–18 CAS.
- K. Solymar, I. Sajo, J. Steiner and J. Zoldi, Light Met., 1992, 209–223 CAS.
- J. Murray, L. Kirwan, M. Loan and B. K. Hodnett, Hydrometallurgy, 2009, 95, 239–246 CrossRef CAS.
- X. L. Pan, H. Y. Yu, K. W. Dong, G. F. Tu and S. W. Bi, Int. J. Miner., Metall. Mater., 2012, 19, 973–977 CrossRef CAS.
- B. I. Whittington, Hydrometallurgy, 1996, 43, 13–35 CrossRef CAS.
- B. Xu, P. Smith, C. Wingate and L. D. Silva, Hydrometallurgy, 2010, 105, 75–81 CrossRef CAS.
- H. Arkan, G. K. Demir and S. Vural, Int. J. Ind. Chem., 2019, 10, 57–66 CrossRef.
- B. I. Whittington, Hydrometallurgy, 1996, 43, 13–35 CrossRef CAS.
- S. G. Xue, F. Zhu, X. F. Kong, C. Wu, L. Huang, N. Huang and W. Hartley, Environ. Sci. Pollut. Res., 2016, 23, 1120–1132 CrossRef CAS PubMed.
- G. Power, M. Gräfe and C. Klauber, Hydrometallurgy, 2011, 108, 33–45 CrossRef CAS.
- L. Y. Li, Waste Manag., 2001, 21, 525–534 CrossRef CAS PubMed.
- X. B. Li, L. L. Kong, T. G. Qi, Q. S. Zhou, Z. H. Peng and G. H. Liu, Chin. J. of Nonferrous Met., 2013, 23, 544–548 Search PubMed.
- X. B. Li, Z. Y. Zhou, Y. L. Wang, Q. S. Zhou, T. G. Qi, G. H. Liu and Z. H. Peng, Trans. Nonferrous Met. Soc. China, 2020, 30, 1980–1990 CrossRef CAS.
- L. Diamandescu, D. Mihàilà-Tàràbàşanu and M. Feder, Mater. Lett., 1993, 17, 309–311 CrossRef CAS.
- E. Wolska, W. Szajda and P. Piszora, Solid State Ionics, 1994, 70, 537–541 CrossRef.
- H. D. Ruan, R. L. Frost, J. T. Kloprogge and L. Duong, Spectrochim. Acta, Part A, 2002, 58, 479–491 CrossRef CAS.
- E. Wolska, W. Szajda and P. Piszora, J. Therm. Anal., 1992, 38, 2115–2122 CrossRef CAS.
- U. Schwertmann, Thermochim. Acta, 1984, 78, 39–46 CrossRef CAS.
- C. A. Barrero, R. E. Vandenberghe, E. D. Grave, A. L. Morales and G. Pérez, Hyperfine Interact., 2003, 148, 337–344 CrossRef.
- P. S. R. Prasad, K. Shiva Prasad, V. Krishna Chaitanya, E. V. S. S. K. Babu, B. Sreedharc and S. Ramana Murthy, J. Asian Earth Sci., 2006, 27, 503–511 CrossRef.
- E. Wolska, Solid State Ionics, 1988, 28, 1349–1351 CrossRef.
- R. L. Frost, Z. Ding and H. D. Ruan, J. Therm. Anal. Calorim., 2003, 71, 783–797 CrossRef CAS.
- G. González, A. Sagarzazu and R. Villalba, Mater. Res. Bull., 2000, 35, 2295–2308 CrossRef.
- V. Nunna, S. Hapugoda, M. I. Pownceby and G. J. Sparrow, Miner. Eng., 2021, 166, 106826 CrossRef CAS.
- Y. Zhang, Q. Gao, J. Zhao, M. Li and Y. Qi, Minerals, 2019, 9, 223 CrossRef CAS.
- V. P. Ponomar, Miner. Eng., 2018, 127, 143–152 CrossRef CAS.
- V. Ravisankar, R. Venugopal and H. Bhat, Miner. Process. Extr. Metall., 2019, 128, 175–182 CAS.
- K. O. Jang, V. R. M. Nunna, S. Hapugoda, A. V. Nguyen and W. J. Bruckard, Miner. Eng., 2014, 60, 14–22 CrossRef CAS.
- N. O. Dudchenko and V. P. Ponomar, Vìsnik Dnìpropetrovs'kogo Unìversitetu: Serìâ Geologìâ, 2015, 23, 25–32 Search PubMed.
- T. Hiemstra, J. C. M. D. Wit and W. H. V. Riemsdijk, J. Colloid Interface Sci., 1989, 133, 105–117 CrossRef CAS.
- M. L. Li, H. B. Liu, T. H. Chen, L. Wei, C. Wang, W. Hu and H. L. Wang, Chem. Geol., 2019, 524, 368–382 CrossRef CAS.
- H. Wang, Z. Zhang, K. Arnon, J. Chen and W. Han, Trans. Chin. Soc. Agric. Eng., 2018, 34, 124–131 Search PubMed.
- R. Derie, M. Ghodsi and C. Calvo-Roche, J. Therm. Anal., 1976, 9, 435–440 CrossRef CAS.
- Y. Mochizuki and N. Tsubouchi, ACS Omega, 2019, 4, 19723–19734 CrossRef CAS PubMed.
- V. J. Ingram-Jones, R. Slade, T. W. Davies and S. Salvador, J. Mater. Chem., 1996, 6, 73 RSC.
- A. Atasoy, J. Therm. Anal. Calorim., 2007, 90, 153–158 CrossRef CAS.
- C. Z. Liao, L. Zeng and K. Shih, Chemosphere, 2015, 131, 171–177 CrossRef CAS PubMed.
- S. J. Kim, J. I. Lee, K. S. Han, S. Y. Byun, T. Tran and M. J. Kim, Korean J. Met. Mater., 2018, 56, 49–58 CrossRef.
- M. A. Wellington, Ind. Eng. Chem. Res., 2013, 52, 2013 CrossRef.
- K. Rao and R. Goyal, Light Met., 2006, 71–74 Search PubMed.
- G. Power, Hydrometallurgy, 2010, 105, 1–29 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |