Open Access Article
Open Access ArticleExtraction of insoluble fibrous collagen for characterization and crosslinking with phenolic compounds from pomegranate byproducts for leather tanning applications†
Sara El Moujahed *ad,
Faouzi Errachidib,
Hicham Abou Oualid
*ad,
Faouzi Errachidib,
Hicham Abou Oualid c,
Andreea-Veronica Botezatu-Dediu
c,
Andreea-Veronica Botezatu-Dediu d,
Fouad Ouazzani Chahdia,
Youssef Kandri Rodia and
Rodica Mihaela Dinica
d,
Fouad Ouazzani Chahdia,
Youssef Kandri Rodia and
Rodica Mihaela Dinica d
d
aLaboratory of Applied Organic Chemistry, Faculty of Sciences and Technologies, Sidi Mohamed Ben Abdellah University, Imouzzer Street, B.P. 2202, Fez, Morocco. E-mail: sara.elmoujahed@gmail.com
bLaboratory of Functional Ecology and Engineering Environment, Faculty of Sciences and Technologies, Sidi Mohamed Ben Abdellah University, Imouzzer Street, B.P. 2202, Fez, Morocco
cGreen Energy Park, IRESEN-UM6P, Benguerir, Morocco
dLaboratory of Organic Chemistry, Faculty of Sciences and Environment, Dunarea de Jos University, Domneasca Street 47, 800008, Galati, Romania
First published on 2nd February 2022
Abstract
An environmental approach for leather manufacturing is primordial to provide a global strategy towards more sustainable biomaterials and greener tanning processing methods. The ability of collagen as a principal component of skin to combine natural phenolic compounds, especially vegetable tannins, has been proven to be eco-friendly and manageable, while making good improvement to leather properties in the tanning process. In this study, we have used pomegranate phenolic compounds and insoluble collagen as a model system to examine the effects of tanning steps on the conformation of collagen. In detail, efficient modified extraction of pure insoluble collagen (IC) was presented. The IC was successfully identified using XRD, FTIR, SEM-EDS and TGA-DSC to verify the triple helix structure, morphology and thermal properties. As a result, the as-extracted collagen exhibits a high purity, preserving the triple helix collagen structure. Besides, the IC was modified using extracted pomegranate phenolic compounds, resulting in Crosslinked Insoluble Collagen (CIC). Characterization techniques were also performed to confirm the crosslinking process. Indeed, by comparing the FTIR vibrational spectra of IC and CIC, slight shifts of amide groups were observed, indicating the presence of inter and intramolecular interaction between IC functional groups and pomegranate phenolic compounds. Moreover, the morphology of CIC was changed. XRD analysis confirms collagen conformational integrity. Finally, thermal properties were improved. The temperature at 50% weight loss (T°50) increases from 344.54 °C to 375.53 °C. CIC multifunctionality allowed utilizing pomegranate phenolic compound extracts as a tanning agent in leather processing.
1. Introduction
Taking environmental aspects into consideration is actually the major objective of many industrial producers. It is now mandatory to minimize the use of toxic chemicals due to their hazardous effect on flora and fauna in air, soil, and aqueous media. The role of researchers worldwide is becoming crucial to disclose more how industries could adopt green, safe and eco-friendly ways. International leather manufacturing tanning is known for the huge production quantity (about 6.5 Mt) from raw hides.1 Tanning is a multistep process that converts a putrescible animal hide (or skin) into resistant leather which may last an unlimited period of time. Skin is a connective tissue mostly made up of a fibrous collagen structure chained in the extracellular matrix. Tanning is the collagen matrix protection, making it resistant to humidity, heat and microbial attacks.More than a century earlier, chromium salts (Cr III) were discovered to be relatively close tanning chemicals for fast manufacturing of good leather and have become preferred industrially.2 Large amounts of chrome-containing waste are produced every year which requires a unique elimination due to the carcinogenic and mutagenic potential of hexavalent chromium Cr(VI), resulting easily from Cr(III) in the presence of oxidizing agents,3 being amongst the world's top 10 toxic polluters.4 So further, it has raised serious concerns about leather industry sustainability.
Several studies have presented technologies concerning ecological steps in the tanning process5–7 enzymatic soaking,8 green dehairing or unhairing,9 pickle-free tanning process enhancing chrome tanning process,10–13 waterless tanning14 and utilization techniques for food processing solid waste.15,16 However, these techniques were not prioritized in the production and didn't drive until recently to cleaner products. As a consequence, efficient and low-cost effective solutions using environmentally friendly methods are required in all pre-tanning and tanning operations, particularly unhairing, pickling and tanning steps. The unhairing step is one of the most polluting operations in the leather production process. Strong chemicals, such as sodium sulphide and lime, are utilized in particular during this process step, representing around 80–90% of total leather production pollution.17 In this study, the greener oxidative unhairing approach of Kanagaraj et al.18 was considered and adapted to our conditions. The pickling step was carried out using bio-organic acids such as citric acid. The extracted insoluble collagen was taken as a starting material model for the tanning process.
Studies on collagen interactions with phenolic compounds have been widely reported.6,19–21 However, the choice of an ecological process for insoluble collagen extraction has not been supported by solid scientific proof, but rather on the common processes in the leather industry. Bearing that in mind, the objective of this work was to investigate a new process of insoluble collagen extraction, used as a skin model to study crosslinking interactions with vegetable phenolic compounds from pomegranate juice processing solid waste, which is produced in Morocco in huge quantities ranging up to more than 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year as known that rinds occupy 40% of the total fruit weight.22 Converting this waste into a high-value end-product positively impacts the environment as well as the leather-processing industry.
000 tons per year as known that rinds occupy 40% of the total fruit weight.22 Converting this waste into a high-value end-product positively impacts the environment as well as the leather-processing industry.
In this study, a fruitful association of extracted insoluble collagen and pomegranate phenolic compounds was conducted. The extraction processes were used for the hide pre-treatment and purification. Using an eco-friendly modified extraction method of collagen, a fibrous collagen was successfully extracted and characterized. Structural and thermal properties were analyzed using XRD, FTIR, SEM-EDS and TGA-DSC, before and after cross linking with pomegranate phenolic compounds from Moroccan local cultivar, in light of their potential utility for leather tanning applications.
2. Materials and methods
2.1. Chemicals and materials
All chemicals (hydrogen peroxide, sodium hydroxide, citric acid, sodium chloride, petroleum ether, acetone, methanol, ethanol, sodium carbonate, Folin–Ciocalteu reagent, and gallic acid) used were of analytical grade and used without further purification. The McIlvaine buffer was prepared at pH = 5 using a mixture of 0.1 M citric acid and 0.2 M disodium hydrogen phosphate (Sigma Aldrich). The buffer prepared was stored at 4 °C for further use. Collagen type I (TH) purchased from Sigma Aldrich was used as a reference. Crosslinker solution was lyophilized in a freeze dryer (Christ Alpha 1–4 LD plus (Martin Christ, Germany)) and microplate determination of crosslinked phenolic compounds was performed using a microplate reader (Tecan Pro 200, Tecan Trading AG, Männedorf, Switzerland).2.2. Raw materials
Hide samples made by pieces of dried skin were collected from a local abattoir and stored at −20 °C until used. Usually, hide samples would not need to be stored at −20 °C if sample collection is followed by extraction immediately.23 The pieces came from a Moroccan sheep breed (one-year-old) slaughtered a year ago.A byproduct sample, from a largely consumed pomegranate (Punica granatum L.) commercial variety in Morocco, was collected during the month of October 2020 at the maturity stage. Fruits were peeled and peels covering arils were removed manually. Pomegranate rinds (PR) were water washed, disinfected, rinsed with distilled water and dried in shade, until weight constancy, away from humidity and light and at ambient temperature. After drying, rinds were crushed using an automated grinder and kept hermetically in closed containers at 4 °C until assay achievement. Each analysis was performed in triplicate.
2.3. Sheepskin collagen sample extraction
2.3.1.1. Unhairing. Dried sheepskins were cut into small pieces of 1 × 1 cm2 with uniform size and weight. Based on methods used in industry, pieces of skin were soaked in 300% of distilled water for 24 h. After washing for 1 h at a rotation speed of 10 rpm, skin samples were drained and water was discharged. Under stationary conditions, the oxidative unhairing step was carried out with the optimized green method of Kanagaraj et al.18 using reagent proportions giving 100% unhairing effect, with slight modifications based on the method applied by Puccini et al.24 Unhairing experiments were performed using hydrogen peroxide (5%), sodium hydroxide (5%) and distilled water (200%). Skin samples were soaked in a bath containing reagents mentioned above under constant conditions (T = 20 °C, pH = 13) for a period of 16 h. In all pre-treating steps, mass percentages were determined from the soaked hide mass.25
2.3.1.2. Pickling. After hide fleshing, hair removal and washing, our pickling method consists of reducing the pH to 5 by soaking samples in a pickling bath containing citric acid (2%), sodium chloride (10%) and distilled water (300%), at 15 rpm stirring speed for 1 h. Samples were additionally washed and dipped in distilled water (300%) overnight.
2.3.2.1. Removing non-collagenous proteins. Pickled hide samples were thoroughly soaked in water (200%) for 15 min and water was discharged. Under continuous stirring (10 rpm), residues were removed by degreasing unhaired hide samples with base treatment20 using sodium hydroxide (300% of 0.1 M) and sodium chloride (0.1 M) for 24 h at 20 °C, and then washed with three baths of distilled water (AS & AES1 Methods, Safiya et al.26). The pH was measured as that of the bath and brought to pH = 5 with several consequential additions of citric acid solution. Finally, samples were washed several times with distilled water and drained overnight. Mass percentages were determined from the initial dipped hide mass.
2.3.2.2. Insoluble collagen purification. Considering that all non-collagenous proteins were removed, hide samples were defatted with petroleum ether27 and extracted with acetone followed by methanol to remove moisture. These resultant hide samples, consisting of mostly collagen protein,28 were further processed by drying under vacuum for 24 h at room temperature, and cut milling to a fine powder that was used as an insoluble collagen sample for further tests.
2.4. Insoluble-collagen@Phenolic extract (IC@PE) crosslinking
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/v) rind powder-to-heated distilled water (100 °C) was employed and extraction time was 12 hours away from light at 20 °C. Extractions were performed using ethanol as an additive to increase the phenolic yield extracted as described by previous studies29 and also to prevent microorganism growth in our ecological process. Afterwards, extracts obtained were first centrifuged at 5000 rpm for 15 min at 5 °C, and the supernatant was vacuum filtered, frozen at −80 °C for 24 h and finally lyophilized in a freeze dryer (Christ Alpha 1–4 LD plus (Martin Christ, Germany)) for 3 days. Powdered aqueous extract was dissolved in ultra-pure water to prepare 10% (W/V) of crosslinker solution (PE). In the following experiment, the freshly prepared PE solution pH was ranging between 3 and 3.5.
10 (w/v) rind powder-to-heated distilled water (100 °C) was employed and extraction time was 12 hours away from light at 20 °C. Extractions were performed using ethanol as an additive to increase the phenolic yield extracted as described by previous studies29 and also to prevent microorganism growth in our ecological process. Afterwards, extracts obtained were first centrifuged at 5000 rpm for 15 min at 5 °C, and the supernatant was vacuum filtered, frozen at −80 °C for 24 h and finally lyophilized in a freeze dryer (Christ Alpha 1–4 LD plus (Martin Christ, Germany)) for 3 days. Powdered aqueous extract was dissolved in ultra-pure water to prepare 10% (W/V) of crosslinker solution (PE). In the following experiment, the freshly prepared PE solution pH was ranging between 3 and 3.5.2.5. Characterization techniques
2.5.4.1. Thermogravimetric analysis (TGA) measurement. Non-crosslinked and crosslinked IC samples from hide powder assay treated with TE were subjected to thermogravimetric analysis conducted using a TGA from TA Instruments to characterize the overall thermal denaturation/degradation processes. Samples (10 mg) were analyzed over the temperature range between 25 and 500 ∘C, at 10 °C min−1 heating rate under N2 atmosphere flow (60 mL min−1).
2.5.4.2. Differential scanning calorimetry (DSC) measurement. DSC measurements were carried out with a Discovery DSC Instrument. About 5 mg of IC sample were analyzed using a sealed pan under nitrogen, from 0 °C to 400 °C with 5°C min−1 heating rate. All thermal parameters were treated and exported from TRIOS software.
3. Results and discussion
3.1. Sustainable insoluble collagen (IC) extraction
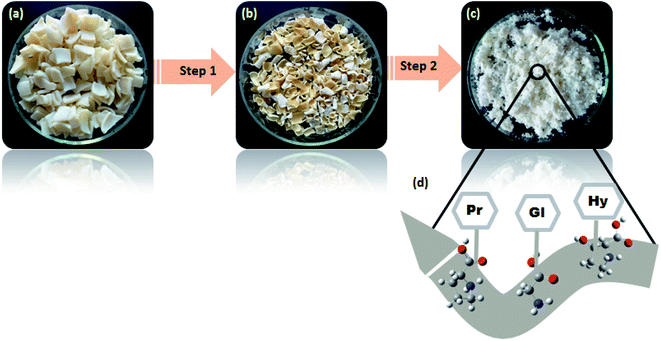
IC extraction has its goal as collagen hide pre-treatment and purification using proper chemicals. Mostly hide powders used to study collagen interactions were prepared from commercially pre-treated sheepskins which are prepared with conventional methods.20,27 Pre-treatment methods used in general required an alkaline environment and a sharpening agent, conventionally lime and sodium sulfide. This method contributes to 60–70% of the entire amount of pollutants generated by beamhouse activities (generation of 50% of COD and 40% of BOD).31 The toxicity of conventional methods prompts researchers to look for ecological alternatives. Pre-treatments reported in this work were employed for this purpose.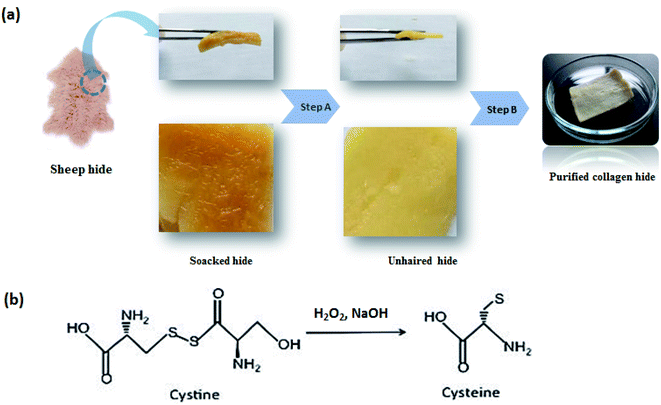 | ||
| Fig. 1 Sheep hide pre-treatment processes. (a): steps from dry sheep skin to a purified hide proper for collagen extraction; (b): unhairing mechanism of the high oxidative process (step A). | ||
As shown in Fig. 1a (step A), hydrogen peroxide (5%), sodium hydroxide (5%) and distilled water (200%) used at the fixed levels exhibited a complete hair disintegration, resulting in clean and swollen skin at the end. The pH of the unhairing bath containing reagents mentioned above was 13 as suggested by Puccini et al.24 Operative pH ranging from 12.5 to 13 is optimal to conduct the oxidative unhairing. The pH was adjusted using sodium hydroxide acting as an alkali, providing OH− ions, which help hydrogen peroxide conduct the process completely. Optimal sodium hydroxide dosage, previously used by Kanagaraj et al.,18 was modified in this experiment from 4% to 5%, because more base is required to increase the pH to an appropriate value; unhairing takes longer if the base is reduced; thus, balancing the suitable base is a crucial factor in the experimentation.32,33 Moreover, experiments were chosen to be under stationary conditions (lab-scale reactor), where it was proved that stationary conditions in the unhairing process are preferable for more availability and contact of hydrogen peroxide with hair keratin.18
It was understood from the literature24 that hydrogen peroxide at a concentration of 9% is sufficient to assure complete unhairing. Instead, Kanagaraj et al.18 experimented sodium percarbonate as an oxidative agent providing 100% unhairing at a dosage of 5%. Sodium percarbonate, existing in the solid-state as the sesquiperhydrate (2Na2CO3, 3H2O), at a concentration of 5%, liberates sufficient initial H2O2 concentration (5%).34 Therefore, hydrogen peroxide is the major key element of skin unhairing and the dominant chemistry of sodium percarbonate in water, so it was evident to use the same efficient dosage in our investigation.
Previous studies31 reported the oxidative unhairing mechanism, due to diffusion reaction of peroxide, suggesting the break-down of some hydrogen bonds from collagen, showing removal of inter-fibrillary and collagen proteins resulting in safer hair removal in the IC pre-treating process, whereas the unhairing mechanism was not only manifested with successive hair detachment but also with hair degradation using oxidative agents.9
As a comparison between unhairing treatments, we incorporate two significant distinctions in relation to the conventional process with lime and sodium sulfide.35 At first, the unhairing products were used. In a traditional unhairing method, sulfide attacked hair as a reductive. Hydrogen peroxide attacks hair acting as an oxidative in the used unhairing process. However, the attack on the hair is the same: breaking-down disulfuric bonds (S–S) of cysteine by an alkali (Fig. 1b) and resulting in hydrolysis of keratin, the main protein found in hair, which facilitates its eventual solubilization.36 In addition, from the traditional liming procedure, there are usually residues of sulfide and sulfydrate on hide and in waste float. On the other hand, hydrogen peroxide interaction with hide promotes its decomposition into the water, so there is no toxicity in the used unhairing method. From this experimental investigation and literature, unhairing by hydrogen peroxide appears effective in avoiding immunization that can occur due to the application of excessive lime prior to applying the sulfide in the conventional process.37 This phenomenon would prevent desired bond breaking of the hair root proteins. Moreover, sodium hydroxide usage instead of lime gives the suitable alkaline medium accompanied by a high swelling degree of the collagen matrix.24
Numerous studies have also described enzymatic unhairing using protease as a sort of eco-friendly unhairing technology. Some of these experiments had positive outcomes as well, although they are more temperature-dependent.38–40 The enzymes used in this method destroy the protein backbone sustaining hair tissues and epidermis components around the follicle,9 inducing their hydrolysis, which also induces the opening of collagen fiber bundles and hair liberation from the hide.41 The enzymatic unhairing process seems to have high potential for recycling and valorization, but it was found to be less efficient, more time demanding and requiring additional costs9 when compared to an oxidative method that is practical, economical and relatively “green and clean”.
Citric acid was used to change skin conditions to acid, leading to good and safe penetration of phenolic compounds during the crosslinking process. As reported by Covington,45 this penetration was explained by another aspect, which is the opening-up reaction of the hide derma. Even if it is slow, this reaction occurs during the pickling process and improves bonding with collagen and its thermostability during the tanning process. Actually, novel pickling processes were investigated as processes significantly acting on hide collagen, i.e., the enzymatic pickling46 and the salt-free pickling.31 These methods produced clean pores and well-dispersed fibers but were generally employed previous to chrome tanning.
The degreasing step of the skin is among the IC purification processes. It is generally applied to remove fat content in sheepskin and other high fatty tissues.47,48 The remaining non-collagenous proteins were removed with an optimal degreasing bath in the degreasing phase26 and visually showed efficient fat removal. Fig. 1a (step B) presents the skin aspect after pickling, and removing residues and all non-collagenous proteins. Purification of collagen was a necessary step and it resulted in a clean white agglomerated powder (Fig. 2).
After each purification step of the IC, the influence of the process on the hide was assessed by determining the amount of removed non-collagenous proteins during unhairing and purification processes. The total amount of residues removed during the collagen extraction process is around 0.65–0.73 kg kg−1 of raw hide. During the unhairing step, hair removed reaches 0.1 kg kg−1 of soaked hide, while in the purification step, residues and non-collagenous proteins vary in the range of 0.55–0.63 kg kg−1 of dried raw hide. This means that the total IC content in the raw hide was determined to be 41 ± 3% w/w.
Compared to previous studies,49 collagen was extracted with a yield of 21% w/w using a lime-sulfide pre-treatment, where usually the amount of collagenous proteins removed during this conventional process varies in the range of 0.2–0.5 g kg−1.50,51
3.2. Crosslinker extract (PE): extraction yield and total phenolic content
Methods used in this study to obtain the appropriate method for extracting phenolic compounds, mainly tannins, from PR were principally based on the literature. The extraction process was undertaken only once, while tannin extraction is improved with repeated extraction,52,53 but this dilutes the solution, necessitating a second concentration step for proper analysis. Yield extraction has shown an efficiency of around 38.2% obtained for 12 h-long extractions, using a solid-to-solvent ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Heated distilled water modified with ethanol was chosen as a solvent for extract preparation because of its frequent use to extract tannins from dry PR.22,29 Microplate quantifications showed that PE crosslinker contains a high amount of phenolic compounds. This content determined by the Folin–Ciocalteu method was quantified in mg GAE/g PE. It seems that in most studies, the phenolic compounds uptaken by the IC were not actually quantified with this method.29 This quantification method showed that phenolic compounds quantified in PE before crosslinking were about 190.88 ± 8.95 mg GAE/g PE (at t = 0). After the crosslinking process (t = 5 h), phenolic compounds were measured to be around 52.08 ± 4.51 mg GAE/g PE. This high amount of phenolic compounds uptaken by IC could highlight the potential of pomegranate byproduct studied in this work as a relevant product for vegetable tanning. However, other indicators have been evaluated to determine its appropriateness.
10. Heated distilled water modified with ethanol was chosen as a solvent for extract preparation because of its frequent use to extract tannins from dry PR.22,29 Microplate quantifications showed that PE crosslinker contains a high amount of phenolic compounds. This content determined by the Folin–Ciocalteu method was quantified in mg GAE/g PE. It seems that in most studies, the phenolic compounds uptaken by the IC were not actually quantified with this method.29 This quantification method showed that phenolic compounds quantified in PE before crosslinking were about 190.88 ± 8.95 mg GAE/g PE (at t = 0). After the crosslinking process (t = 5 h), phenolic compounds were measured to be around 52.08 ± 4.51 mg GAE/g PE. This high amount of phenolic compounds uptaken by IC could highlight the potential of pomegranate byproduct studied in this work as a relevant product for vegetable tanning. However, other indicators have been evaluated to determine its appropriateness.
3.3. Crosslinking ability of extracts
As shown in the illustration (Fig. 3), collagen fibers, unlike most synthetic polymers and other biomasses, contain a richness of functional groups such as –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and NH2, making them ideal for immobilizing phenolic compounds as hydroxyl sources. Vegetable phenolic compound extract can create numerous hydrogen bonds with collagen functional groups, allowing them to crosslink tighter to form an ordered network. After crosslinking, it was seen that the color of IC changes from white to light-yellow, which means that the extract was uptaken by collagen. The quantity of extract uptaken by IC at the end of the process was found to be 138.79 mg g−1 IC (72.7%). From these results, PE crosslinker from PR showed higher crosslinking capacity which would be deeply confirmed with several physico-chemical techniques investigated, to follow collagen's structural, morphological and thermal properties before and after crosslinking (see Section 3.4. Characterization).
O and NH2, making them ideal for immobilizing phenolic compounds as hydroxyl sources. Vegetable phenolic compound extract can create numerous hydrogen bonds with collagen functional groups, allowing them to crosslink tighter to form an ordered network. After crosslinking, it was seen that the color of IC changes from white to light-yellow, which means that the extract was uptaken by collagen. The quantity of extract uptaken by IC at the end of the process was found to be 138.79 mg g−1 IC (72.7%). From these results, PE crosslinker from PR showed higher crosslinking capacity which would be deeply confirmed with several physico-chemical techniques investigated, to follow collagen's structural, morphological and thermal properties before and after crosslinking (see Section 3.4. Characterization).
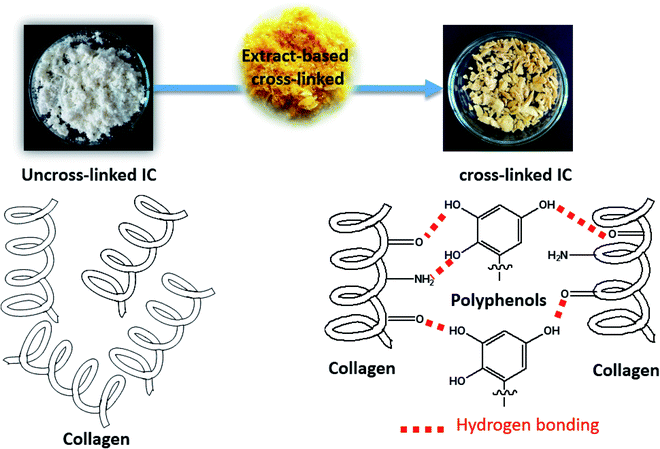 | ||
| Fig. 3 Illustration of the crosslinking process from uncross-linked to cross-linked Insoluble Collagen (IC) using extract-based crosslinking. | ||
3.4. Characterization
3.4.1.1. XRD and FTIR analysis of extracted insoluble collagen (IC). As an important characterization technique, XRD provides precise information about the helix structure of IC after extraction. The XRD outputs lead to the identification of the resulting structure after the extraction process. Fig. 4a illustrates the XRD patterns of IC and that of the commercial tropocollagen type I (TH). As shown in the figure, three main collagen peaks were observed: the peak (1) at around 6° to 8° was associated with the intermolecular lateral packing distance between molecular collagen chains. The peak (2) at around 15° to 23° was attributed to the diffuse scattering. The peak (3) at around 30° originated from the unit height, which was typical of the triple-helical collagen structure.54,55 Since the structure of extracted collagen was found to be similar to commercial tropocollagen type I structure (TH), the extraction process used in this work does not cause any observable effect on the supermolecular structure of IC, which confirms the extraction efficiency.
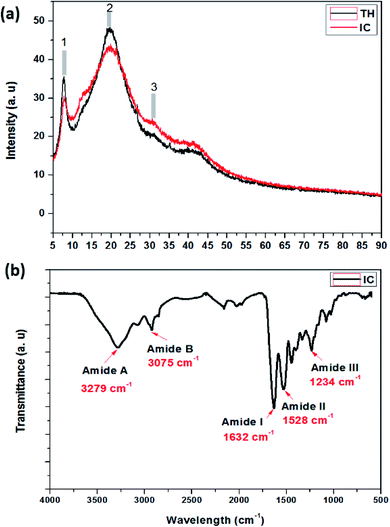 | ||
| Fig. 4 (a) XRD patterns of insoluble collagen (IC) compared to commercial tropocollagen type I triple helix (TH). (b) FTIR vibrational spectrum of insoluble collagen (IC). | ||
FT-IR spectroscopy was conducted to further confirm the collagen structure by visualizing the structure of extracted IC. Fig. 4b shows the IR spectra of IC in the range of 600–3700 cm−1. As shown in the figure, IC exhibits five main peaks; two peaks at 3279 and 3075 cm−1 are attributed to the vibrations of –OH and –NH2 groups of amides A and B.56 The third peak at around 1632 cm−1 is attributed to the stretching vibration of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond for amide I. Hence, the peak at 1528 cm−1 is associated with N–H and C–N bending vibration of amide II. Besides, the last peak is attributed to –C–N stretching, –N–H bending and CH2 wagging vibrations of amide III. FT-IR results showed that the collagen type I structure including helical structure was maintained when the modified extraction was used to promote the process.57–60
O bond for amide I. Hence, the peak at 1528 cm−1 is associated with N–H and C–N bending vibration of amide II. Besides, the last peak is attributed to –C–N stretching, –N–H bending and CH2 wagging vibrations of amide III. FT-IR results showed that the collagen type I structure including helical structure was maintained when the modified extraction was used to promote the process.57–60
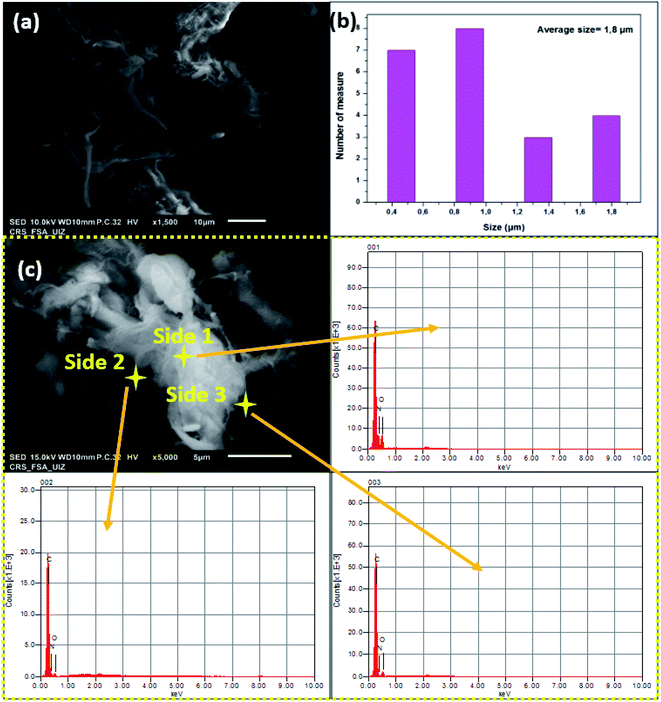 | ||
| Fig. 5 (a) SEM observation, (b) size distribution and (c) EDS analysis of insoluble collagen (IC) at three different sides. | ||
Because of the use of many reagents in the extraction process, it is crucial to verify extracted collagen purity. In this regard, IC was analyzed via EDS analysis on different sides. The image in Fig. 5c was chosen for local analysis. As shown in the figure, it was clear that all sides confirm the existence of the main elements in IC as an organic compound. Mainly, oxygen, carbon and nitrogen were about 17.98, 21.46 and 6.56% atoms, respectively. In conclusion, these results confirm the absence of impurities on the surface of IC fibers which confirms then that extraction was realized with clean and effective washing avoiding any contamination with dissolved chemicals and chlorides used in the IC purification process.
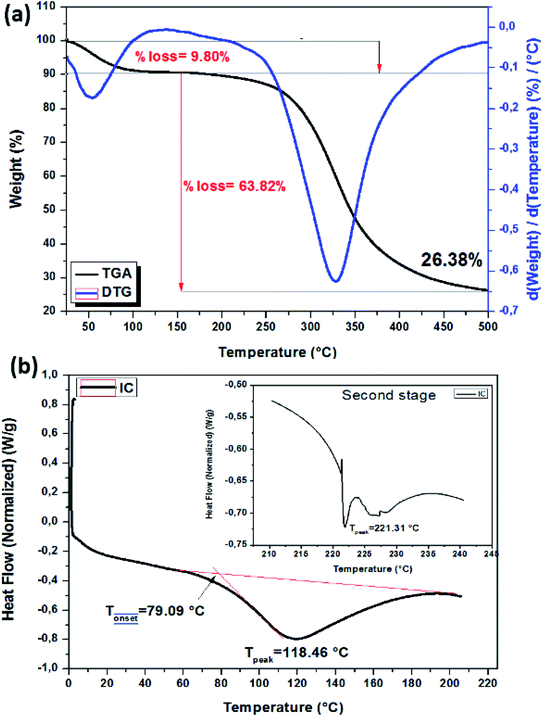
Pure collagen powder thermal stability was also verified by DSC analysis. Fig. 6b shows the DSC diagram. Dehydration temperature or pure collagen denaturation temperature is found at about 118.46 °C. The second endothermic peak at about 221.3 °C is typically attributed to polypeptide chain degradation and strongly bonded water evaporation.
3.4.4.1. Morphological comparison. Crosslinked Insoluble Collagen (CIC) morphology was studied using scanning microscopy after crosslinking with the extract. Fig. 7 shows both images of Non-Crosslinked Insoluble Collagen (NCIC) and Crosslinked Insoluble Collagen (CIC) for comparison. As described before, NCIC was found as a microscopic fiber structure with fluffy white powder (image inset). Hence, in the case of CIC, it was shown clearly that the morphology was changed (collapsed yellow powder). This result confirms that the extract was uptaken by IC. EDS analysis of CIC was further conducted and is presented in the ESI (see Fig. A2).† As a result, nitrogen, carbon and oxygen were found to be at about 8.01, 60.72 and 31.27 %atoms, respectively.
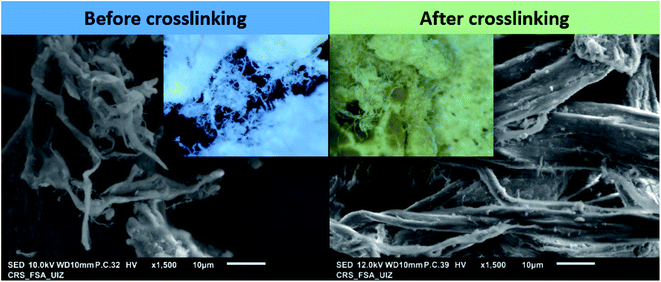 | ||
| Fig. 7 SEM images of non-crosslinked insoluble collagen (NCIC) and crosslinked insoluble collagen (CIC) (inset: optical images): comparison. | ||
3.4.4.2. Structural and thermal investigations. To further explain the mechanistic interaction between NCIC and phenolic compound extract, after crosslinking, XRD and FTIR were carried out. According to the Bragg equation (λ = 2d
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sinθ), the d-spacing collagen molecular chains and the helical rise distance (Fig. 8a) per residue were 1.14 and 1.16 nm, respectively, indicating collagen conformational integrity. Compared to other tanning agents used in the literature similar conclusions were noted with aluminum sulfate, chromium sulfate and P(POSSMAA).61
sinθ), the d-spacing collagen molecular chains and the helical rise distance (Fig. 8a) per residue were 1.14 and 1.16 nm, respectively, indicating collagen conformational integrity. Compared to other tanning agents used in the literature similar conclusions were noted with aluminum sulfate, chromium sulfate and P(POSSMAA).61
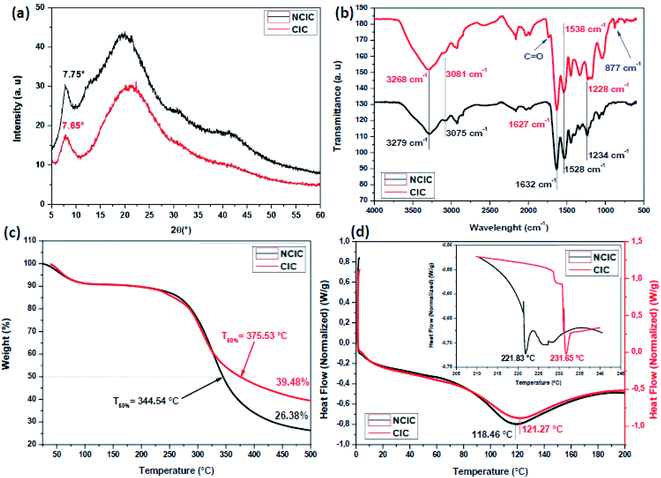 | ||
| Fig. 8 (a) XRD, (b) FTIR, (c) TGA and (d) DSC comparative curves of non-crosslinked insoluble collagen (NCIC) and crosslinked insoluble collagen (CIC). | ||
NCIC and CIC FTIR spectroscopy are presented and compared in Fig. 8b. Typically, the same peaks of NCIC were clearly observed. Hence, two new peaks appear at about 1738 and 788 cm−1, probably attributed to free carbonyl and aromatic groups, respectively58 The same bonds are detected in the FTIR spectrum of PE (see Fig. A3 in the ESI).† To understand the interaction mechanism during the crosslinking process, wavenumber values of different amides were compared. Indeed, absorption peak shift of amide A (7–11 cm−1), amide B (2–9 cm−1), amide I (2–5 cm−1), amide II (8–14 cm−1) and amide III (4–9 cm−1) indicates inter and intramolecular interaction between IC functional groups and PE.62
The thermal stability of CIC powder was evaluated. Fig. 8c shows the thermograms of NCIC and CIC. As shown in the figure, the TGA profiles of uncrosslinked and crosslinked IC are similar. Generally, two weight loss stages were detected. In order to evaluate the influence of crosslinker sources, CIC was compared with a non-crosslinked one. For this, the temperature at 50% weight losses (T°50) and the residues (%) at 500 °C (R%500) were calculated. T°50 increases from 344.54 °C to 375.53 °C and R%500 was 26.35% and 39.48% for NCIC and CIC, respectively.
To verify the crosslinking reactivity of PE into collagen, DSC analysis of NCIC and CIC was carried out. Fig. 8d illustrates the DSC thermographs of NCIC and CIC from 0 °C to 200 °C (the spectra, from 206 °C to 245 °C, are the inset). The endothermic peak corresponding to collagen denaturation at 118 °C increases to 121 °C. This could be explained by the capacity of phenolic compounds to form strong interactions with collagen which maintain more the triple helix structure. This conclusion was also found by Lirong et al63 using procyanidin as a crosslinker. Furthermore, the second peak was shifted at about 10 °C to high temperature. This thermal resistance of collagen degradation is explained by solid interaction between phenolic compounds that existed in the extract and functional groups on collagen fibers. These interactions were formed by hydrogen bonds. In conclusion, these results are important for mechanics of collagen-based materials.61,64
4. Conclusion and perspectives
This study introduces two main aspects. The first focuses on efficient collagen extraction by modifying procedures cited in the literature, while the second is centered on as-extracted collagen crosslinking using phenolic compounds extracted from pomegranate juice processing by-products. Using several techniques such as XRD, FTIR, SEM-EDS, TGA and DSC, the results show that the modified collagen extraction process was efficient, where structure and purity were successfully confirmed. On the other hand, the extracted collagen exhibits good compatibility with phenolic compounds due to inter and intramolecular interaction between the functional groups of each other.Through this study, the authors will envisage these perspectives:
• Explore the effectiveness of using other varieties of pomegranate byproducts. How could phenolic compound loading affect collagen properties?
• Discover more crosslinker agents from other low-cost sources, especially from fruits wastes. Are there any synergetic effects of phenolic loading and other contents?
• The study of phenolic loading distribution unto crosslinking performances. How could phenolic compound concentration affect the thermal, structural and morphological properties?
Funding
The financial assistance of Francophone University Agency (AUF) and Romania Government through “Eugen Ionescu” mobility program towards this research is hereby acknowledged.Conflicts of interest
There is no conflict of interest regarding the publication of this paper.Acknowledgements
The authors are indebted to the University of “Dunarea de Jos” of Galati, Romania. Also we are grateful for the assistance and the support of Sidi Mohamed Ben Abdellah University of Fez, Morocco. S. E. thanks all those who directly or indirectly contributed to this research, in particular the kind reviewer and the editor. The financial assistance of Francophone University Agency (AUF) towards this research is hereby acknowledged.References
- MASCIANÀ Patrizia, Intergovernmental group on meat and diary products sub-group on hides and skins, Food And Agricultural Organization of the United Nations, Rome, 2015, p. 141 Search PubMed.
- Y. Zhang, B. Ingham, J. Leveneur, S. Cheong, Y. Yao, D. J. Clarke, G. Holmes, J. Kennedy and S. Prabakar, RSC Adv., 2017, 7, 11665–11671 RSC.
- S. E. Fendorf and R. J. Zasoski, Environ. Sci. Technol., 1992, 26, 79–85 CrossRef CAS.
- Z. Sebestyén, E. Jakab, E. Badea, E. Barta-Rajnai, C. Şendrea and Zs. Czégény, J. Anal. Appl. Pyrolysis, 2019, 138, 178–187 CrossRef.
- C. R. China, A. Hilonga, S. S. Nyandoro, M. Schroepfer, S. V. Kanth, M. Meyer and K. N. Njau, J. Cleaner Prod., 2020, 251, 119687 CrossRef CAS.
- M. Schropfer, Res. J. Phytochem., 2016, 10, 58–66 CrossRef.
- E. Brown, M. Taylor and C.-K. Liu, J. Am. Leather Chem. Assoc., 2016, 111, 141–147 CAS.
- J. M. Morera, E. Bartolí and C. Singla, J. Cleaner Prod., 2013, 59, 79–85 CrossRef CAS.
- C. Anzani, B. Prandi, S. Buhler, T. Tedeschi, C. Baldinelli, G. Sorlini, A. Dossena and S. Sforza, J. Cleaner Prod., 2017, 164, 1446–1454 CrossRef CAS.
- Z.-R. Guo, G. Zhang, J. Fang and X. Dou, J. Cleaner Prod., 2006, 14, 75–79 CrossRef.
- K. Li, H. Chen, Y. Wang, Z. Shan, J. Yang and P. Brutto, J. Cleaner Prod., 2009, 17, 1603–1606 CrossRef CAS.
- J. M. Morera, E. Bartolí, R. Chico, C. Solé and L. F. Cabeza, J. Cleaner Prod., 2011, 19, 2128–2132 CrossRef CAS.
- V. J. Sundar, J. Raghava Rao and C. Muralidharan, J. Cleaner Prod., 2002, 10, 69–74 CrossRef.
- S. Silambarasan, R. Aravindhan, J. R. Rao and P. Thanikaivelan, RSC Adv., 2015, 5, 66815–66823 RSC.
- J. Hu, Z. Xiao, R. Zhou, W. Deng, M. Wang and S. Ma, J. Cleaner Prod., 2011, 19, 221–228 CrossRef CAS.
- Z. Jiang, M. Gao, J. Remón, W. Ding, C. Hu and B. Shi, Green Chem., 2021, 23, 2640–2651 RSC.
- A. Dettmer, J. Coelho Cavalheiro, E. Cavalli, D. Misturini Rossi, C. de Souza Gusatti, M. A. Zachia Ayub and M. Gutterres, Chem. Eng. Technol., 2012, 35, 803–810 CrossRef CAS.
- J. Kanagaraj, R. C. Panda and T. Senthilvelan, Process Saf. Environ. Prot., 2016, 100, 36–48 CrossRef CAS.
- H. I. Oh, J. E. Hoff, G. S. Armstrong and L. A. Haff, J. Agric. Food Chem., 1980, 28, 394–398 CrossRef CAS.
- H. R. Tang, A. D. Covington and R. A. Hancock, Biopolymers, 2003, 70, 403–413 CrossRef CAS PubMed.
- L. Wu, H. Shao, Z. Fang, Y. Zhao, C. Y. Cao and Q. Li, ACS Biomater. Sci. Eng., 2019, 5, 4272–4284 CrossRef CAS PubMed.
- S. El moujahed, F. O. Chahdi, Y. K. Rodi, L. El ghadraoui, L. Lemjallad and F. Errachidi, Waste Biomass Valorization, 2021, 12, 5383–5399 CrossRef CAS.
- J. M. Morera, E. Bartolí, R. Chico, C. Solé and L. F. Cabeza, J. Cleaner Prod., 2011, 19, 2128–2132 CrossRef CAS.
- M. Puccini, M. Seggiani, D. Castiello and S. Vitolo, Chem. Eng. Trans., 2014, 36, 193–198 Search PubMed.
- J. M. Morera, E. Bartolí and R. M. Gavilanes, J. Cleaner Prod., 2016, 112, 3040–3047 CrossRef CAS.
- S. Noorzai, C. J. R. VERBEEK and LAY, Waste Biomass Valorization, 2020, 11, 5687–5698 CrossRef CAS.
- H. R. Tang, A. D. Covington and R. A. Hancock, J. Agric. Food Chem., 2003, 51, 6652–6656 CrossRef CAS PubMed.
- K. H. Gustavson, Chemistry of Tanning Processes, Academic Press, New York, 1956 Search PubMed.
- I. J. Seabra, R. B. Chim, P. Salgueiro, M. E. Braga and H. C. de Sousa, J. Chem. Technol. Biotechnol., 2018, 93, 1169–1182 CrossRef CAS.
- K. Kruawan and K. Kangsadalampai, Thai J. Pharm. Sci., 2006, 30, 28–35 CAS.
- J. Kanagaraj, R. C. Panda and M. Vinodh Kumar, J. Environ. Chem. Eng., 2020, 8, 104379 CrossRef.
- D. E. Richardson, C. A. Regino, H. Yao and J. V. Johnson, Free Radical Biol. Med., 2003, 35, 1538–1550 CrossRef CAS PubMed.
- W. N. Marmer and R. L. Dudley, J. Am. Leather Chem. Assoc., 2005, 100, 165–173 CAS.
- W. N. Marmer and R. L. Dudley, J. Am. Leather Chem. Assoc., 2004, 99, 386–392 CAS.
- G. D. McLaughlin and E. Raymond, Chemistry of leather manufacture, Reinhold Publishing Corp, 1945 Search PubMed.
- P. Saravanan, T. Shiny Renitha, M. K. Gowthaman and N. R. Kamini, J. Cleaner Prod., 2014, 79, 258–264 CrossRef CAS.
- D. Castiello, M. Puccini, D. Shelly and S. Vitolo, J. Am. Leather Chem. Assoc., 2007, 102, 341–346 CAS.
- N. Kandasamy, P. Velmurugan, A. Sundarvel, R. Jonnalagadda Raghava, C. Bangaru and T. Palanisamy, J. Cleaner Prod., 2012, 25, 27–33 CrossRef CAS.
- A. Verma, H. S. Pal, R. Singh and S. Agarwal, Int. J. Environ. Agric. Biotech., 2011, 4, 173–178 Search PubMed.
- D. Brady, J. R. Duncan and A. E. Russell, J. Am. Leather Chem. Assoc., 1990, 85, 334–343 CAS.
- S. Jian, T. Wenyi and C. Wuyong, Ultrason. Sonochem., 2010, 17, 376–382 CrossRef PubMed.
- V. Sivakumar, V. J. Sundar, T. Rangasamy, C. Muralidharan and G. Swaminathan, J. Cleaner Prod., 2005, 13, 699–703 CrossRef.
- D. Rahmawati, N. M. Setyadewi and S. Sugihartono, IOP Conf. Ser.: Earth Environ. Sci., 2019, 306, 012022 CrossRef.
- M. J. Collins, M. S. Riley, A. M. Child and G. Turner-Walker, J. Archaeol. Sci., 1995, 22, 175–183 CrossRef.
- A. D. Covington, Tanning chemistry: the science of leather, Royal Society of Chemistry, 2009 Search PubMed.
- R. Biškauskaitė, V. Valeikienė and V. Valeika, Materials, 2021, 14, 1480 CrossRef PubMed.
- R. Palop, A. Marsal and J. Cot, J. Soc. Leather Technol. Chem., 2000, 84, 170–176 CAS.
- B. Lyu, K. Cheng, J. Ma, X. Hou, D. Gao, H. Gao, J. Zhang and Y. Qi, J. Cleaner Prod., 2017, 148, 701–708 CrossRef CAS.
- D. Masilamani, B. Madhan, G. Shanmugam, S. Palanivel and B. Narayan, J. Cleaner Prod., 2016, 113, 338–344 CrossRef CAS.
- V. Valeika, J. Balciuniene, K. Beleska and V. Valeikiene, J. Soc. Leather Technol. Chem., 2000, 84, 165–169 CAS.
- S. Jian, T. Wenyi and C. Wuyong, J. Cleaner Prod., 2011, 19, 325–331 CrossRef.
- A. V. A. Resurreccion, Food Chem., 2009, 117, 356–363 CrossRef.
- T. Esatbeyoglu, V. Wray and P. Winterhalter, J. Agric. Food Chem., 2010, 58, 7820–7830 CrossRef CAS PubMed.
- Z. Xu, X. Guan, J. Liu, H. Fan and Y. Chen, J. Appl. Polym. Sci., 2017, 134, 45424 CrossRef.
- R. Socrates, O. Prymak, K. Loza, N. Sakthivel, A. Rajaram, M. Epple and S. Narayana Kalkura, Mater. Sci. Eng. C ., 2019, 99, 357–366 CrossRef CAS PubMed.
- H. Jiang, M. Zheng, X. Liu, S. Zhang, X. Wang, Y. Chen, M. Hou and J. Zhu, ACS Omega, 2019, 4, 12606–12615 CrossRef CAS PubMed.
- T. Douglas and H. J. Haugen, J. Mater. Sci.: Mater. Med., 2008, 19, 2713–2719 CrossRef CAS PubMed.
- T. Riaz, R. Zeeshan, F. Zarif, K. Ilyas, N. Muhammad, S. Z. Safi, A. Rahim, S. A. A. Rizvi and I. U. Rehman, Appl. Spectrosc. Rev., 2018, 53, 703–746 CrossRef CAS.
- D. Masilamani, B. Madhan, G. Shanmugam, S. Palanivel and B. Narayan, J. Cleaner Prod., 2016, 113, 338–344 CrossRef CAS.
- J. Zhang and W. Chen, RSC Adv., 2020, 10, 23503–23509 RSC.
- D. Gao, Y. Cheng, P. Wang, F. Li, Y. Wu, B. Lyu, J. Ma and J. Qin, J. Cleaner Prod., 2020, 257, 120546 CrossRef CAS.
- M. Andonegi, A. Irastorza, A. Izeta, S. Cabezudo, K. de la Caba and P. Guerrero, Polymers, 2020, 12, 1597 CrossRef CAS PubMed.
- L. He, C. Mu, J. Shi, Q. Zhang, B. Shi and W. Lin, Int. J. Biol. Macromol., 2011, 48, 354–359 CrossRef CAS PubMed.
- M. Schroepfer and M. Meyer, Int. J. Biol. Macromol., 2017, 103, 120–128 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08059h |
| This journal is © The Royal Society of Chemistry 2022 |