Open Access Article
Open Access ArticleEndowment of high buoyancy and antifouling properties upon a simple superamphiphobic cotton fabric†
Jixin
Ai
ab,
Deke
Li
c,
Chenggong
Xu
bd,
Xing
Tang
ab,
Jinxia
Huang
*b and
Zhiguang
Guo
 *ab
*ab
aMinistry of Education Key Laboratory for the Green Preparation and Application of Functional Materials, Hubei University, Wuhan 430062, People's Republic of China. E-mail: zguo@licp.cas.cn; Fax: +86-931-8277088; Tel: +86-931-4968105
bState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou730000, People's Republic of China. E-mail: huangjx@licp.cas.cn
cSchool of materials engineering, Lanzhou Institute of Technology, Lanzhou 730050, People's Republic of China
dUniversity of Chinese Academy of Sciences, Beijing 100049, People's Republic of China
First published on 26th April 2022
Abstract
Functional superamphiphobic fabrics have attracted considerable attention for application thanks to their anti-contamination and increased-buoyancy features. A robust, antifouling superamphiphobic fabric was obtained though a convenient one-pot method involving simultaneous reactions of dopamine (DA), 3-aminopropyltriethoxysilane (APTMS) and 1H,1H,2H,2H-perfluorodedecyl-triethoxysilane (PFDTMS). Under suitable alkaline conditions, DA could be oxidized to benzoquinone, and APTMS molecules could undergo hydrolysis and bind together. Due to the steric hindrance of the benzene ring and long alkyl chain, benzoquinone could react with APTMS by a Schiff base reaction and nanospheres can be formed on the fabric surface. The lyophobicity of the fabric was maintained even after undergoing various harsh tests (sand impingement, ultrasonic, ultraviolet irradiation, acid and alkali corrosion), showing significant durability and stability. The superamphiphobic fabric could load 26.38-times and 22.69-times its own weight in water and oil, respectively, displaying strong buoyancy. Therefore, the obtained fabric has promising commercial applications for swim suits and life jackets.
Nowadays, superamphiphobic surfaces featuring excellent repellence to water and oil have a critical footprint on academic research and industry due to their potential applications in fuel transportation,1 antifouling,2 anti-icing,3 drag reduction,4 anticorrosion5 and increase in buoyancy.6,7 Cotton fibers, as a natural renewable resource, exhibit good hydrophilicity because they have many hydrophilic hydroxyl groups. Thanks to their advantages of softness and scalability, cotton fibers has been applied widely in protective clothing,8,9 conductive devices,10 fire-warning sensors11 and other fields.12,13 However, water/oil droplets or dirt can penetrate cotton fabrics readily and make them contaminated. Therefore, endowing cotton fabric with superamphiphobicity to broaden their applications and prolong their service life is important.
As a strong reducing agent, dopamine (DA), which contains a catechol structure, tends to undergo an oxidation reaction to produce benzoquinone in an oxygen-containing environment. DA contains many reactive groups, such as phenolic hydroxyl, quinone and amino groups.14,15 These active groups can react further with many substances to graft other functional materials on their surfaces. Therefore, DA can be used as a strong adhesive bonding agent and is suitable for various substrates. DA shows great potential in the superamphiphobic functional modification of cotton fabrics.16,17,18,19 Another important effect of DA is that it can greatly enhance the durability of functional fabrics. To satisfy the harsh demand of superamphipohobic properties, superamphiphobic surfaces that can repel liquids with a wide range of surface tensions require ultralow surface-energy components (usually fluorinated compounds) and special surface topography, such as re-entrant, T-shaped overhang, mushroom-like and matchstick-like structures.20–22
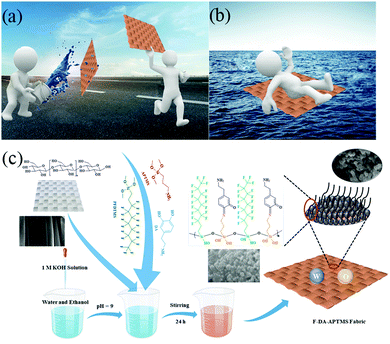
In this work, a facile one-pot method was used to grow silicon nanospheres with fluorinated compounds on the surfaces of cotton fabrics to achieve superamphiphobicity. First, 3-aminopropyltrimethoxysilane (APTMS) and DA can be oxidized to produce benzoquinone in a certain pH environment and oxygen-containing air. Meanwhile, APTMS and 1H,1H,2H,2H-perfluorodecyltrimethoxyslaine (PFDTMS) undergo a hydrolysis reaction and bond together. In addition, the quinone groups in benzoquinone can have a Schiff base reaction with APTMS and, because of the steric hindrance effect of the benzene ring and long-chain alkanes, silicon nanospheres with low surface energy can be obtained. In addition, the surface of cotton fabric has abundant hydroxyl groups that can bond with nanospheres so that the resulting cotton fabric has excellent lyophobic stability. Various stability tests have shown that even under harsh conditions (including sand-impingement cycles, ultrasonic cleaning, strong ultraviolet irradiation, and acid and alkali corrosion), the F-DA-APTMS fabric can maintain good liquid repellency. Especially with regard to application of increased buoyancy and antifouling, it can carry many times its own weight in water and oil, which could be used widely in water transportation-facilities and other fields.
Fig. 1c describes the procedure to synthesize F-DA-APTMS fabric. It is simple and convenient to grow intensive nanospheres in situ on the surface of cotton fabrics by a one-pot method, which creates a productive roughness and is vital for superamphiphobicity.23 DA, PFDTMS and APTMS were added to a reaction system of equal volume of water and ethanol. The reaction mechanism of the whole process is shown in Fig. S1 (ESI†). DA is oxidized to dopamine benzoquinone under aerobic conditions. PFDTMS and APTMS can undergo a hydrolysis reaction under alkaline conditions and be connected together. A Schiff base reaction is carried out between the carbonyl group of dopamine benzoquinone and the amino group hydrolyzed by APTMS, which results in formation of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond. On the one hand, formation of the Si–O–Si bond inhibits the horizontal diffusion of silane so that a long fluorine chain with low surface energy can be deposited smoothly on the surface of the cotton fabric. PFDTMS is chosen as the surface modifier because it can facilitate self-assembly into nanospheres on the surface of cotton fabrics to reduce the surface free energy but not change the surface morphology. On the other hand, due to the steric hindrance of the long fluorosilane and alkyl-containing benzene ring, not all Si–OH bonds can bind to the many hydroxyl groups on the fabric, which is why more complete spherical nanostructures can be grown on the surface of the fabric.24,25 Endowing low surface energy and the establishment of nanostructures are indispensable conditions for preparation of superamphiphobic surfaces.
N double bond. On the one hand, formation of the Si–O–Si bond inhibits the horizontal diffusion of silane so that a long fluorine chain with low surface energy can be deposited smoothly on the surface of the cotton fabric. PFDTMS is chosen as the surface modifier because it can facilitate self-assembly into nanospheres on the surface of cotton fabrics to reduce the surface free energy but not change the surface morphology. On the other hand, due to the steric hindrance of the long fluorosilane and alkyl-containing benzene ring, not all Si–OH bonds can bind to the many hydroxyl groups on the fabric, which is why more complete spherical nanostructures can be grown on the surface of the fabric.24,25 Endowing low surface energy and the establishment of nanostructures are indispensable conditions for preparation of superamphiphobic surfaces.
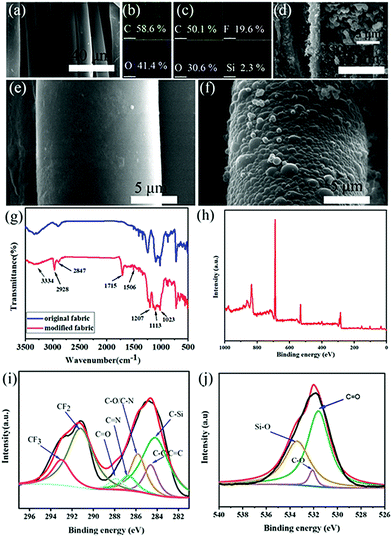
Compared with the original fabric, many interrelated nanospheres with a diameter of about 400–500 nm grow on the surface of the modified fabric (Fig. 2a and d–f). It can be clearly observed that these nanospheres are distributed on each cotton fiber to form hierarchical structures, which can capture a large amount of air and form an “air cushion” on the surface of the cotton fabric. Si and F elements appeared in the spectrum after the original fabric had been modified with PFDTMS (Fig. 2c). The content of F element on the modified fabric was ≤19.7%, and the content of Si element was ≤2.3%, which demonstrates introduction of PFDTMS and APTMS. Compared with the original fabric, the C content of the modified fabric decreased from 58.5% to 50.1% (Fig. 2b), indicating that DA, APTMS, PFDTMS were involved in the reaction system and could generate appropriate micro–nano structures. To explore the variation of functional groups on the characteristic surface samples, original and modified fabrics underwent Fourier transform-infrared (FTIR) spectroscopy and X-ray photoelectron spectroscopy (XPS) (Fig. 2g–j). For details, see the supporting information.
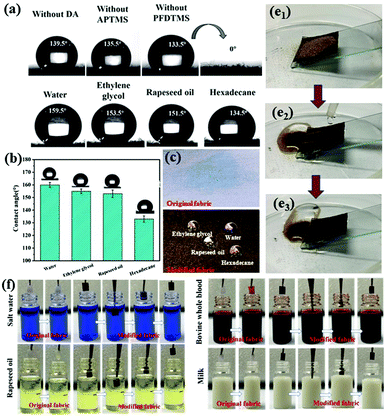
FTIR spectroscopy and XPS revealed that realization of superamphiphobic properties of cotton fabric was closely related to the Schiff base reaction between benzoquinone and APTMS and the low-surface-energy modifier PFDTMS. Thus, control experiments without DA, APTMS or PFDTMS were carried out to show that these three components played an important part in the superamphiphobicity of modified fabrics. The wettability of the sample under different conditions is shown in Fig. 3a. The surface roughness of the F-DA fabric was not constructed only if DA and PFDTMS were added to the reaction system without APTMS (Fig. S2a, ESI†). When DA was not in the reaction system and only APTMS and PFDTMS were added, the surface of F-APTMS fabric was relatively flat and nanospheres were not formed (Fig. S2b, ESI†). Only APTMS and PFDTMS or DA and PFDTMS could not react to obtain “ideal” nanospheres because the Schiff base reaction did not occur due to a lack of DA or APTMS. When PFDTMS was not added (Fig. S2c, ESI†), the water droplets wetted the fabric surface in a few seconds, and the contact angle was almost 0. The lack of a long fluorine-containing alkane made the surface of the modified fabric lose its hydrophobic properties. Therefore, the synergistic effect of the three components (DA, APTMS and PFDTMS) was greatly beneficial to the superamphiphobicity of the modified fabric. As is shown in Fig. 3b, water could wet the original fabric rapidly and exhibit hydrophilic properties because of the abundance of hydroxyl groups on the surfaces. Conversely, water and oil droplets rolled off the relatively horizontal F-DA-APTMS fabric surfaces readily and could maintain a spherical shape (Fig. 3c). The contact angle of water, ethylene glycol, rapeseed and hexadecane droplets was measured to be 159.5 ± 1.2°, 153.6 ± 0.9°, 152.3 ± 0.6° and 137 ± 1.4°, respectively (Fig. 3d). Thus, F-DA-APTMS fabrics displayed excellent liquid repellency.
Superamphiphobic coatings are often expected to express good antifouling, liquid repellence and self-cleaning properties to reflect their feasibility in practical applications. Here, fine sand was chosen as the pollutant and placed on the coated surface. When water droplets were dripped on the F-DA-APTMS fabric, they could roll down the surface smoothly and take away the deposited fine sand simultaneously, thereby resulting in a clean surface (Fig. 3e1, e2 and e3). For the antifouling test, once the original cotton fabric had been immersed in common contaminated liquids, it was soaked, and left many stains on the fabric surface. The coated fabrics were also immersed in various test liquids (water, rapeseed oil, whole blood of cows, or milk), and then removed from the liquid environment. No residue remained on the coated fabrics, thereby displaying excellent antifouling ability (Fig. 3f). This excellent antifouling capacity benefited substantially from the superamphiphobic F-DA-APTMS layer, which consisted mainly of many nano-silicon spheres and fluorinated long chains. Besides, the “silver mirror” phenomenon was attributed to formation of a stable air-protection layer between the liquid and the fabric surface. Therefore, various common droplets were in the Cassie–Baxter state and difficult to immerse in F-DA-APTMS fabric, thereby endowing the fabrics with excellent antifouling ability.
The mechanical stability for superamphiphobic surfaces had been a critical factor restricting large-scale application because of the brittleness of the hierarchical micro-nanostructure constructed on the functional surface. Compared with superhydrophobic surfaces, superamphiphobic surfaces with a hierarchical re-entrant structure display higher accuracy, which is why superamphiphobic coatings can be damaged so readily. We undertook a series of experiments to evaluate the stability of the fabric. In the sand-impingement test, sand granules (20 g) in a funnel fell down to impinge on F-DA-APTMS fabrics, which was defined as one cycle.
The vertical height between the funnel mouth and fabric was 20 cm. For each cycle, the sand granules on the membrane surface were cleared away. After 10 cycles, the water-contact angle of treated fabrics was measured. As shown in Fig. S3a (ESI†), the water-contact angle of F-DA-APTMS cotton fabric to ethylene glycol and rapeseed oil changed only slightly after 10 sand-impingement cycles, indicating that a certain amount of sand impact did not damage the micro- and nanostructures on the modified fabric. We also carried out ultrasonic treatment. Ultrasonic treatment had a significant effect on the oleophobic performance (Fig. S3b, ESI†). A possible reason is that the microporous and mesoporous structures of the surface coating of the F-DA-APTMS fabric were destroyed if the duration of ultrasonic treatment was too long, which reduced its oleophobic performance. However, the contact angle of water and ethylene glycol changed only slightly. In addition, the UV-resistance test was undertaken to evaluate the effect of outdoor light on the wettability of the F-DA-APTMS fabric. The modified fabric was irradiated continuously under a UV lamp and the wettability of the fabric was measured every 6 h. After 24 h of UV irradiation, the contact angle of water, ethylene glycol, rapeseed oil and hexadecane was 156.4°, 151.7°, 145.3° and 136.4°, respectively, which indicates that F-DA-APTMS maintained excellent superhydrophobicity and olephobicity (Fig. S3c, ESI†). The contact angle of water and rapeseed oil decreased slightly from 158.7° to 156.4° and from 152.5° to 145.3°, illustrating that the modified fabric showed good ultraviolet resistance. For the acid- and alkali-resistance test, the modified fabric was immersed in a solution of pH 1–13 for 1 h. A bright cushion on the surface of the F-DA-APTMS fabric could be observed through a transparent corrosive solution. Meanwhile, the variation in contact angle of water and oil in various corrosive media is shown in Fig. S3d (ESI†): the values for the water contact angle remained relatively constant.
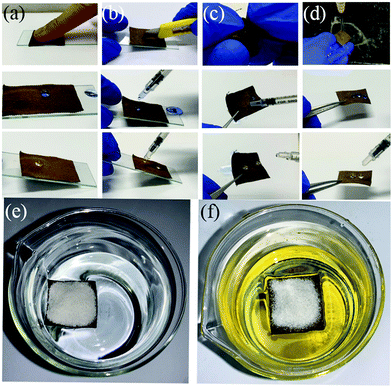
The mechanical stability of the F-DA-APTMS fabric was evaluated qualitatively through finger-touching (Fig. 4a), knife-scratching (Fig. 4b), hand-twisting (Fig. 4c), and turbulent water-flow impact (Fig. 4d). Knife-scratching and hand-twisting could cause partial removal of the fluorinated hierarchical structure, but there was no obvious influence on liquid repellence. The finger-touching test was done to assess the superamphiphobic durability due to the presence of grease on fingers. The F-DA-APTMS fabric retained remarkable superamphiphobicity after finger-touching. After 10 min of turbulent water flow, the surface of the F-DA-APTMS fabric could not be wetted by turbulent water; water droplets and rapeseed-oil droplets can leave the surface of the F-DA-APTMS fabric effortlessly.
In summary, a novel superamphiphobic fabric with excellent antifouling and strong buoyancy abilities was developed by a facile one-pot method. In the whole reaction system, benzoquinone oxidized by DA under appropriate alkaline conditions can react with hydrolyzed APTMS through a Schiff base reaction, resulting in the growth of nanospheres on the fabric surface. Steric hindrance caused by the benzene ring and the long alkyl chain of the low-surface-energy modifier PFDTMS led to formation of nanospheres with low surface energy, which endowed the cotton fabric with superamphiphobicity. The water, ethylene glycol, rapeseed oil and hexadecane contact angle of the F-DA-APTMS fabric could reach up to 159°, 154°, 151° and 137°, respectively. Moreover, the fabric maintained excellent liquid repellence and remarkable durability and stability after 10 sand-impingement cycles, 2 h of ultrasonic treatment, 24 h exposure to UV light and 1 h exposure to strong acids or strong alkalis. In addition, the F-DA-APTMS fabric displayed strong bearing capacity on water and oil surfaces. Therefore, the obtained fabric has promising commercial applications for swim suits and life jackets.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Nature Science Foundation of China (1735013 and 51905520).Notes and references
- Y. Wu, M. Zhao and Z. Guo, Chem. Eng. J., 2018, 334, 1584–1593 CrossRef CAS.
- X. Ou, J. Cai, J. Tian, B. Luo and M. Liu, ACS Sustainable Chem. Eng., 2020, 8, 6690–6699 CrossRef CAS.
- N. Zhai, L. Fan, L. Li and J. Zhang, J. Colloid Interface Sci., 2017, 505, 622–630 CrossRef CAS PubMed.
- G. Luo, L. Wen, K. Yang, X. Li, S. Xu, P. Pi and X. Wen, Chem. Eng. J., 2020, 383, 123125 CrossRef CAS.
- D. Zhang, G. Wu, H. Li, Y. Cui and Y. Zhang, Chem. Eng. J., 2021, 406, 126753 CrossRef CAS.
- G. B. Hwang, A. Patir, K. Page, Y. Lu, E. Allan and I. P. Parkin, Nanoscale, 2017, 9, 7588–7594 RSC.
- X. Han and X. Gong, ACS Appl. Mater. Interfaces, 2021, 13, 31298–31309 CrossRef CAS PubMed.
- T. Yeerken, W. Yu, J. Feng, Q. Xia and H. Liu, Prog. Org. Coat., 2019, 135, 41–50 CrossRef CAS.
- S. Bhattacharjee, C. R. Macintyre, X. Wen, P. Bahl, U. Kumar, A. A. Chughtai and R. Joshi, Carbon, 2020, 166, 148–163 CrossRef CAS.
- X. Li, Y. Li, T. Guan, F. Xu and J. Sun, ACS Appl. Mater. Interfaces, 2018, 10, 12042–12050 CrossRef CAS PubMed.
- J. Wang, J. He, L. Ma, Y. Zhang, L. Shen, S. Xiong, K. Li and M. Qu, Chem. Eng. J., 2020, 390, 124508 CrossRef.
- H. Yu, M. Wu, G. Duan and X. Gong, Nanoscale, 2022, 14, 1296–1309 RSC.
- D. W. Wei, H. Wei, A. C. Gauthier, J. Song, Y. Jin and H. Xiao, J. Bioresour. Bioprod., 2020, 5, 1–15 CrossRef CAS.
- Y. Liu, K. Ai and L. Lu, Chem. Rev., 2014, 114, 5057–5115 CrossRef CAS PubMed.
- M. Liu, G. Zeng, K. Wang, Q. Wan, L. Tao, X. Zhang and Y. Wei, Nanoscale, 2016, 8, 16819–16840 RSC.
- W. Wang, R. Liu, H. Chi, T. Zhang, Z. Xu and Y. Zhao, ACS Appl. Mater. Interfaces, 2019, 11, 35327–35332 CrossRef CAS PubMed.
- X.-J. Guo, C.-H. Xue, S.-T. Jia and J.-Z. Ma, Chem. Eng. J., 2017, 320, 330–341 CrossRef CAS.
- S. Liu, H. Zhou, H. Wang, W. Yang, H. Shao, S. Fu, Y. Zhao, D. Liu, Z. Feng and T. Lin, Small, 2017, 13 Search PubMed.
- F. Li, M. Du and Q. Zheng, ACS Nano, 2016, 10, 2910–2921 CrossRef CAS PubMed.
- A. Tuteja, W. Choi, M. Ma, J. M. Mabry, S. A. Mazzella, G. C. Rutledge, G. H. McKinley and R. E. Cohen, Science, 2007, 318, 1618–1622 CrossRef CAS PubMed.
- J. Choi, W. Jo, S. Y. Lee, Y. S. Jung, S. H. Kim and H. T. Kim, ACS Nano, 2017, 11, 7821–7828 CrossRef CAS PubMed.
- H. Liu, Y. Wang, J. Huang, Z. Chen, G. Chen and Y. Lai, Adv. Funct. Mater., 2018, 28, 1707415 CrossRef.
- H. Liu, Y. Wang, J. Huang, Z. Chen, G. Chen and Y. Lai, Adv. Funct. Mater., 2018, 28, 1707415 CrossRef.
- H. S. Khoo and F. G. Tseng, Nanotechnology, 2008, 19, 345603 CrossRef PubMed.
- Y. Xi, Y. Sun, W. Li and Z. Li, Polymer, 2021, 213, 123317 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00274d |
| This journal is © The Royal Society of Chemistry 2022 |