Open Access Article
Open Access ArticleFunctionalized epoxy with adjustable fluorescence and UV-shielding enabled by reactive addition of 9-anthracenemethoxyl glycidyl ether†
Wei Xu*,
Matthew D. Eaton ,
Sara Moreno-Da Silva and
Emilio M. Pérez
,
Sara Moreno-Da Silva and
Emilio M. Pérez
IMDEA Nanociencia, Ciudad Universitaria de Cantoblanco, C/Faraday 9, E28049 Madrid, Spain. E-mail: wei.xu@imdea.org
First published on 16th November 2021
Abstract
Functionalized epoxies show unique advantages in some special applications. Herein, a fluorescent and UV-shielding dual-functional epoxy resin, epoxy-EAn, is prepared via synthesizing and incorporating a reactive additive, 9-anthracenemethoxyl glycidyl ether (EAn), into the resin system. The results show that EAn performs an outstanding migration resistance. The addition of a small amount of EAn (0.1%) enables epoxy-EAn to emit bright blue-violet fluorescence under UV light, making epoxy-EAn a feasible fluorescent adhesive for reconstruction and repair. The fluorescence emission intensity is adjustable by the treatment with UV light and heat. Correspondingly, the epoxy-EAn can serve as an information storage material to realize information input and decryption. In addition, epoxy-EAn also possesses the ability to shield UV radiation, and transmittance in the near ultraviolet and middle ultraviolet region (200–400 nm) can decrease to zero when the EAn concentration is 0.8%. Meanwhile, epoxy-EAn also shows excellent mechanical properties similar to that of the pure epoxy. This work paves a facile way to fabricate a multi-functional epoxy suitable for various occasions.
1 Introduction
Given their excellent mechanical strength, adhesion performance, transparency, thermal resistance, and anti-corrosive properties, thermoset epoxy resins have been widely used in various fields, such as adhesives and coatings etc.1–5 Compared to traditional epoxy resins, functionalized epoxies with additional properties have been attracting attention in special applications such as the restoration of art, cultural assets, and odontology where the original appearance should be preserved to the greatest extent.6–9 Thus, the colourlessness and transparency of the epoxy adhesives are required, which, however, makes it inconvenient to detect the repair process and to remove the unexpected and excess adhesive stains and residue.10 Fluorescent epoxy adhesives which are unnoticeable under normal display lighting but fluorescent under UV light can balance this contradiction.6,11–13 Besides, polymers with adjustable fluorescence also have shown the application in the field of anti-counterfeiting, information storage and information encryption etc.14–16 On the other hand, it has been known that UV radiation from sunlight and other environment light source is one factor causing damage to some organic materials, such as wood, paper, and fibers.17,18 This energy-rich radiation usually induces photo-oxidative destruction and further causes material's performance changes.19,20 In this regard, to develop epoxy with UV-shielding properties that can block the harmful high energy UV radiation and diminish UV damage to the coated materials is increasingly of interest.The introduction of additives to epoxy resins to improve UV properties has previously been explored.21 Anthracene, a polycyclic aromatic hydrocarbon, can absorb a wide range of UV radiations and transfer them into harmless fluorescence.22 Meanwhile, the relatively high glass transition temperature of cured epoxy resin benefits limiting the dimerization of the anthracene structures. Therefore, it should be feasible to achieve epoxies with fluorescence and UV-shielding properties simultaneously by adding the anthracene as a functional additive. To our knowledge, there are few studies about employing anthracene or its derivatives to realize the fluorescent or UV-shielding properties in epoxies, and even fewer considering the potential of fluorescent epoxy containing anthracene structure for information encryption and as information storage materials. Moreover, for organic additives, one of the challenges that needs to be considered is the migration of additives from the bulk material towards the surface.19,23 This may lead to the gradual disappearance of the functionality of the materials, and the migrated additives may cause environmental or health problems.20,23 It was reported that employing the reactive additives containing active groups is a feasible strategy to overcome the aforementioned obstacles.24 These reactive additives have the ability of bonding with the polymer chains to prevent the migration of additives.
In this paper, we present a fluorescent and UV-shielding dual-functional epoxy resin (epoxy-EAn) by synthesizing and employing a reactive anthracene derivative, 9-anthracenemethoxyl glycidyl ether (EAn), into the bisphenol-A thermoset epoxy resin. The fluorescence and UV-shielding properties of the resultant epoxy-EAn with different EAn concentrations were systematically studied by the emission spectra and the transmission curves, respectively. The durability of these properties was examined by observing the fluorescence and UV transmission of epoxy-EAn after suffering from UV radiation. The potential of epoxy-EAn as information storage materials was explored by adjusting the fluorescence intensity in the patterned area. Furthermore, the migration resistance of EAn and the mechanical properties of epoxy-EAn were evaluated by leaching experiment and dynamic mechanical analysis (DMA), respectively, for discussing the practicability of epoxy-EAn in various fields.
2 Experimental
2.1. Materials
9-(Hydroxymethyl)anthracene and epichlorohydrin were purchased from TCI and Sigma-Aldrich, respectively. The diglycidyl ether of bisphenol A (DER 332) and the hardener Diethylenetriamine (DETA) were purchased from Sigma-Aldrich. The remaining chemicals and solvents were purchased from Acros Organics. All chemicals were used as received without further purification.2.2. Instrumentation and characterizations
NMR spectra were recorded on a BrukerAvance 400 (1H: 400 MHz) at 298 K, using partially deuterated solvents as internal standards. Chemical shifts (δ) are denoted by ppm. Multiplicities are distinguished as follows: s = singlet, d = doublet, t = triplet, m = multiplet, b = broad, dd = doublet of doublets. Fourier transform infrared (ATR-FTIR) analysis of samples was conducted on the Bruker ALPHA FTIR spectrometer. Fluorescence spectra were taken using HORIBA Fluoromax-4 spectrofluorometer with excitation slit width of 0.3 nm and emission slit width of 1 nm. Transmission curves was measured using Agilent Technologies Cary Series UV-Vis-NIR Spectrophotometer. The used UV light source for functional durability test is VL-4.LC from VILBER LOURMAT. The distance between the light source and samples is about 5 cm. Dynamic mechanical analysis (DMA) was conducted by DMA 850 from TA Instruments in the single cantilever mode with a strain of 50 μm and a frequency of 1 Hz. The sample size is 7 mm × 2 mm × 50 mm and the temperature range is from 0 to 150 °C with a heating rate of 3 °C min−1.2.3. Synthesis
 | ||
| Scheme 1 Synthesis of 9-anthracenemethoxyl glycidyl ether (EAn) from 9-(hydroxymethyl)anthracene (HAn). | ||
1H-NMR (400 MHz, CDCl3): δ (ppm) = 8.48 (s, 1H), 8.41 (d, 2H), 8.02 (d, 2H), 7.58–7.46 (m, 4H), 5.69–5.54 (m, 2H), 3.94–3.91 (m, 1H), 3.64–3.63 (m, 1H), 3.23–3.22 (m, 1H), 2.81–2.78 (m, 1H), 2.65–2.63 (m, 1H).
3 Results and discussion
The epoxy resin represents a class of cured resin that is formed by the crosslinking between diglycidyl ethers and hardeners. Herein, we prepare a bisphenol-A epoxy resin by using epoxy monomer DER 332 and the hardener DETA and take it as the example to demonstrate the feasibility of constructing the fluorescent and UV-shielding dual-functional epoxy resin. To endow the epoxy with aforementioned functions, an anthracene derivative, namely 9-anthracenemethoxyl glycidyl ether (EAn), was synthesized. According to Scheme 1, EAn can be attained by the simple one-step reaction between 9-(hydroxymethyl)anthracene (HAn) and epichlorohydrin, and its structure has been confirmed by NMR (Fig. S1, ESI†). The disappearance of hydroxyl absorptions (3408 cm−1) in FTIR spectrum of EAn compared to HAn also reveals the success of the reaction (Fig. S2, ESI†). The structure of EAn includes an epoxide group and an anthracene part. The epoxide group can react with almost all epoxy hardeners, which enables EAn to covalently bond with epoxy resin molecular network. Meanwhile, the anthracene part in EAn plays the role of generating fluorescence by absorbing UV light. Therefore, after adding EAn for the epoxy preparation, the obtained epoxy resin (epoxy-EAn) is expected to possess the corresponding functions of fluorescence and UV-shielding.To confirm the fluorescence of the prepared epoxy-EAn, the UV-VIS absorption and the photoluminescence spectrum of samples were tested as shown in Fig. 1a. It was found that the epoxy-EAn can absorb the wide waveband range of UV light with three main absorption peaks appearing at around 350 nm, 368 nm, and 389 nm attributed to the existence of anthracene structure in EAn. Correspondingly, under the excitation of UV light (368 nm), epoxy-EAn shows an obvious fluorescence emission spectrum at the visible light region from 400 nm to 500 nm, and the maximum emission peak is located at around 418 nm. In contrast, for the neat epoxy without any EAn addition, there is no obvious UV absorption peaks (Fig. S3†) and fluorescence emission (Fig. 1b) observed at the same test condition. This indicates the contribution of EAn addition in forming fluorescent epoxies. Additionally, the fluorescence emission spectra of samples using different EAn concentrations were also tested. As illustrated in Fig. 1b, epoxy-EAn with EAn concentration of 0.1% performs the noticeably improved fluorescence intensity than that of the sample with low EAn concentration (0.01%). Further increasing the EAn concentration (0.8%) leads to partly decrease in emission intensity and the spectrum redshift, which may be attributed to the aggregation-caused quenching and relaxation of EAn additives. The mechanism about the effect of EAn concentration on fluorescence spectra needs further discussed.
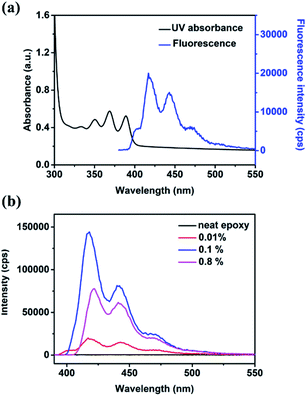 | ||
| Fig. 1 (a) UV-VIS absorption and fluorescence emission spectrum of epoxy-EAn (EAn concentration of 0.01%). (b) Fluorescence emission spectra of epoxy-EAn with different EAn concentrations. | ||
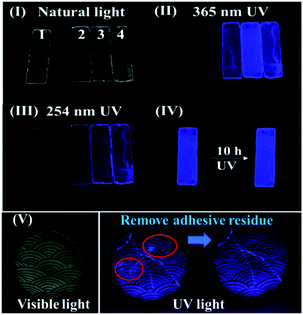
Even though emission spectra of a series of epoxy-EAn samples were detected, its further application for restoration requires (1) the fluorescence should be generated under some common UV light sources, and (2) the generated fluorescence has to be visible to the naked eyes. Herein, a common and commercially available UV light source with wavelength of 365 nm and 254 nm are chosen as the exciting light source. Fig. 2 illustrates the photograph of the fluorescence from epoxy-EAn with different EAn concentration from 0.01 to 0.8%. It is found that under the natural light, all epoxy samples are transparent and colourless (I). After putting these samples under the 365 nm UV light source, the neat epoxy remains the original state and cannot emit the observable light. In contrast, all epoxy-EAn samples show visible and strong blue-violet fluorescence (II). Among them, the sample with EAn concentration of 0.1% shows the relatively brighter fluorescence compared to its counterparts, which is in consistent with the fluorescence emission spectra in Fig. 1b. The fluorescence becomes much weaker under short wave UV light of 254 nm (III), which is confirmed by the decreased fluorescence emission intensity shown in Fig. S4,† either. Therefore, the 365 nm wavelength UV light is recommended as the ideal exciting light source for epoxy-EAn to generate fluorescence. Based on these results, a usually transparent but UV triggered fluorescent epoxy was successfully obtained, which could serve as the adhesives for the restoration of materials. Fig. 2(V) illustrates a repaired ceramic bowl using epoxy-EAn adhesives. The broken pieces of the ceramic bowl were bonded by epoxy-EAn cured in an oven. The repaired cracks and the adhesive residue on the ceramic bowl are not obvious under natural light but clearly observable under 365 nm UV light because of the fluorescence from epoxy-EAn adhesives. The adhesive residue therefore can be easily identified and removed before curing to avoid their negative effect on the appearance of the ceramic bowl.
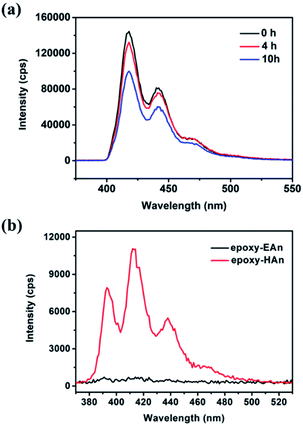
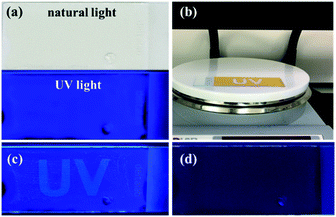
The fluorescence of epoxy-EAn can be maintained for a longer time. As shown in Fig. 2(IV) and Fig. S5,† the epoxy-EAn still can generate bright fluorescence after being exposed under 365 nm UV light for 10 h and under native light for 3 months, which meets the operation requirement of fluorescent adhesives for restoration. We further observe the fluorescent spectra of the UV treated epoxy-EAn samples with different radiation times. The result in Fig. 3a illustrates that the emission intensity of the sample gradually decreases with the extended exposure time. After exposure of 10 h under UV light, the fluorescent intensity of the sample (0.1%) at 418 nm decreases from around 1.45 × 105 cps to 1.01 × 105 cps. This light-triggered adjustable fluorescence may be attributed to the dimerization of anthracene structure in EAn,26 which endows the epoxy-EAn with the potential as information storage materials. Fig. 4 shows the information storage process into an epoxy-EAn layer, including information input and information decryption. Specifically, an epoxy-EAn (0.8% EAn) layer with the 0.1 mm thickness was prepared firstly on a glass slide by curing, which shows fluorescence under UV light (Fig. 4a). Then, the epoxy-EAn layer was heated on the hot plate of 100 °C and meanwhile, exposed under the 365 nm UV radiation. A photomask with patterned holes of the characters “UV” as the stored information was covered on the epoxy-EAn layer for selective UV exposure. After 3 h, the information input was finished as shown in Fig. 4b. Under natural light, the epoxy-EAn layer looks homogeneous without a clue of pattern (Fig. S6†). However, under 254 nm UV light, the character “UV” appears on the epoxy-EAn layer as shown in Fig. 4c, meaning the stored information is decrypted. It is known that the fluorescence intensity of epoxy-EAn reduces accompany with the UV treatment (Fig. 3a). Therefore, after selective UV exposure, the area under the patterned holes of photomask generates weaker fluorescence, while the other area mains the original fluorescence performance. Consequently, the fluorescence pattern is observed to reveal the stored information. Besides, we also found that the operation temperature plays important role during the information input process. This process fails without heating treatment, which may be because the dimerization of EAn is prevented under low temperature. The fluorescence pattern can be erased and destroyed to a certain extent by the overexposure under 254 nm UV and the heat treatment of 100 °C without photomask as shown in Fig. 4d and S7,† which benefits protecting information security and avoiding secondary leak of information.
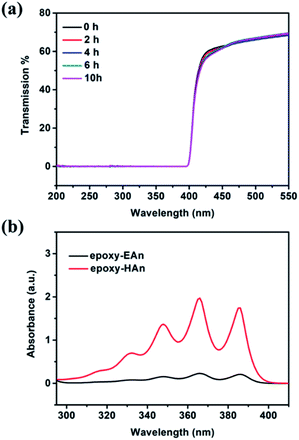
From the perspective of application, the migration resistance of additives is a critical performance metric for the functional epoxies, and it can be evaluated by the leaching experiment. The experimental procedure includes: (1) soaking 450 mg epoxy into 10 ml DMF for leaching of 24 h, and (2) spectral analysis to the leaching solution. As shown in the fluorescence spectra in Fig. 3b, for the leaching solution of epoxy-EAn (0.1%), there is almost no fluorescence emission detected. This indicates that most EAn was reserved in the epoxy-EAn sample after leaching, and the prepared epoxy-EAn therefore shows the excellent migration resistance of EAn. To better highlight this advantage of epoxy-EAn in migration resistance of additives, we also prepared another functional epoxy (epoxy-HAn) by adding the consistent molarity of 9-(hydroxymethyl)anthracene (HAn) as additive for comparison. HAn, as the precursor for synthesizing EAn, is another anthracene derivative, and its structure can be found in Scheme 1. The result in Fig. 3b shows that the leaching solution of epoxy-HAn causes much higher fluorescence intensity than epoxy-EAn leaching solution, which means the HAn easily was extracted into the leaching solution from the epoxy and performs an unsatisfactory migration resistance. This difference in additives migration could be attributed to the different linking way between additives and epoxy network. For epoxy-EAn, the epoxy group in EAn can react directly with the amino groups in the DETA hardener during the curing process, and EAn therefore is covalently bonded to the epoxy network (Scheme 3). This inhibits the migration of EAn from epoxy-EAn into the outside environment. However, for epoxy-HAn, it is hard for HAn to react with both hardener DETA and epoxy monomer DER 332 at the used mild curing condition. Therefore, HAn is mainly physically encapsulated in the epoxy network and prone to migration. The results confirm the excellent migration resistance of EAn as well as the better stability of epoxy-EAn.
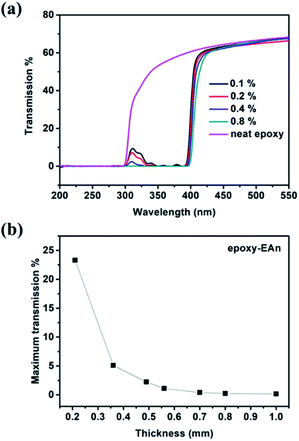
As described in the UV absorbance spectrum in Fig. 1a, epoxy-EAn exhibits an obvious absorbance to a wide waveband range of UV radiation. This endows epoxy-EAn with the potential as UV-shielding materials. The shielding performance can be examined by measuring samples' transmittance to UV light. Fig. 5a demonstrates the transmission curves of epoxy-EAn samples with different EAn concentrations. The results show that the neat epoxy can hardly block the ultraviolet A (315 to 400 nm) and the transmittance to ultraviolet A is as high as 36% to 60%. Even though the blocking performance is relatively enhanced in shorter waveband region, the transmittance remains above 10% until the UV wavelength is lower than 303 nm, implying the lack of the neat epoxy in UV-shielding property. In contrast, the epoxy-EAn shows significantly increased shielding ability to UV radiation. For the sample with EAn concentration of only 0.1%, the maximum transmittance in full test interval (200 to 400 nm) is around 9.7%, which is much lower than that of the neat epoxy (60%). Raising the concentration of EAn contributes to the increment of UV shielding performance. When EAn concentration arrives at 0.8%, the transmittance of epoxy-EAn to UV radiation decreases to almost zero, proving its excellent UV-shielding performance in whole near ultraviolet (300 to 400 nm) and middle ultraviolet (200 to 300 nm) region. Meanwhile, it is observed that epoxy-EAn is as transparent as the neat epoxy in visible light region with the comparable transmittance of around 65% at 500 nm. This further approves the possibility of epoxy-EAn as UV-shielding materials.
The UV-shielding performance of epoxy-EAn is also relevant to their thickness. As shown in Fig. 5b, thinner samples lead to the higher transmittance. Nonetheless, when the thickness decreases to around 0.56 mm, the sample with EAn concentration of 0.8% still shows the adequate blocking performance to UV light and the maximum transmittance in full test interval (200–400 nm) is only 1.1%. By means of adjusting the EAn concentration and the thickness, the epoxy-EAn can serve as a candidate of the UV-shielding coating for conforming different occasions. Besides, it should be mentioned that the UV light may cause the dimerization of EAn and affect the UV-shielding performance of epoxy-EAn. We therefore studied the UV-shielding durability of epoxy-EAn by comparing the transmission curves of samples after UV treatment for different exposure time. As shown in Fig. 6a, for epoxy-EAn with EAn concentration of 0.8% and thickness of 1 mm, no obvious change in transmission curves is observed after exposure of 10 h under UV radiation (365 nm). This can be attributed to the high glass transition temperature of epoxy, which limits the dimerization of EAn. Besides, the adequate addition amount of EAn also contributes the long-term effectiveness of epoxy-EAn in UV-shielding. On the other hand, the advantage of EAn as reactive additives in migration resistance was confirmed again by comparing the UV absorption spectra of the leaching solutions of epoxy-EAn (0.8%) and epoxy-HAn. As shown in Fig. 6b, after immersing 450 mg epoxy samples into 10 ml DMF for leaching of 24 h, the leaching solution of epoxy-HAn generates strong UV absorbance because of HAn's migration into the leaching solution. In contrast, for the leaching solution of epoxy-EAn, the absorption intensity is much weaker due to the covalent bonding of EAn with epoxy network, which is in consistent with the previous leaching experimental results (Fig. 3b). The description above reveals that a functional epoxy resin with adjustable fluorescence and UV-shielding has been obtained by adding EAn, and the resultant epoxy-EAn shows application potential in various occasions.
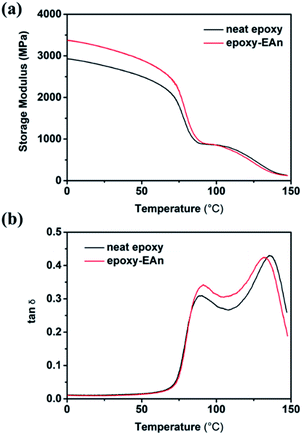
The effect of EAn addition on mechanical properties of epoxies is determined by Dynamic mechanical analysis (DMA). The storage modulus and loss factor (tanδ) curves of both epoxy-EAn (EAn concentration of 0.8%) and the neat epoxy are plotted in Fig. 7a and b, respectively. According to the result in Fig. 7a, epoxy-EAn shows an improved storage modulus compared to the neat epoxy in low temperature region. With the improvement of the temperature, the two samples show the approximate storage modulus with slight difference. The obvious decrement from 70 to 95 °C in curves can be attributed to the glass transition of samples. Besides, it is also noted that there is an additional broad peak from 100 to 140 °C, which is caused by the post-curing of epoxies.27 As aforementioned, the samples were fabricated at 65 °C, which leads to the incomplete curing. The post-curing therefore happens as the temperature increment during the DMA scan and transfers the samples into a new state with improved crosslinking degree, modulus, and glass transition temperature. Consequently, there are also two peaks in the tan(δ) curves (Fig. 7b) at around 90 and 135 °C, which are corresponding to the original glass transition and the glass transition after post-curing, respectively. Even though there are partly difference in the storage modulus and tan(δ) curves between the epoxy-EAn and the neat epoxy, epoxy-EAn performs the comparable mechanical strength and the similar thermal transition to those of the neat epoxy, which indicates the ability of epoxy-EAn to meet the mechanical properties requirement for various application occasions.
4 Conclusions
A functional epoxy with adjustable fluorescence and UV-shielding properties, epoxy-EAn, was successfully prepared via synthesizing and incorporating 9-anthracenemethoxyl glycidyl ether (EAn) into the epoxy network. The addition of a small amount of EAn (0.1%) enables epoxy-EAn to emit bright blue-violet fluorescence under UV light of 365 nm. The resulting epoxy-EAn shows potential as the fluorescent adhesives for restoration. Besides, the fluorescence intensity is light-triggered adjustable to achieve the information storage application. The epoxy-EAn with 0.8% EAn shows not only the fluorescence but also the UV-shielding ability in the whole near ultraviolet and middle ultraviolet region (200–400 nm). Meanwhile, the prepared epoxy-EAn shows the excellent migration resistance of EAn to solvent and the mechanical property similar with that of the original epoxy, revealing its feasibility and advantages conforming various occasions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge funding from Nanocore ApS (Copenhagen, Denmark) the European Union (ERC-PoC-842606), MINECO (CTQ2017-86060-P), and the Comunidad de Madrid and the European Structural Funds for their financial support through FotoArt-CM project (S2018/NMT-4367). IMDEA Nanociencia acknowledges support from the “Severo Ochoa” Programme for Centres of Excellence in R&D (MINECO, grant SEV-2016-0686).Notes and references
- Z. Ahmadi, Prog. Org. Coat., 2019, 135, 449–453 CrossRef CAS.
- C. Verma, L. O. Olasunkanmi, E. D. Akpan, M. A. Quraishi, O. Dagdag, M. El Gouri, E.-S. M. Sherif and E. E. Ebenso, React. Funct. Polym., 2020, 156, 104741 CrossRef CAS.
- X. Shi, T. A. Nguyen, Z. Suo, Y. Liu and R. Avci, Surf. Coat. Technol., 2009, 204, 237–245 CrossRef CAS.
- F.-L. Jin, X. Li and S.-J. Park, J. Ind. Eng. Chem., 2015, 29, 1–11 CrossRef CAS.
- C. Liang, P. Song, H. Gu, C. Ma, Y. Guo, H. Zhang, X. Xu, Q. Zhang and J. Gu, Composites, Part A, 2017, 102, 126–136 CrossRef CAS.
- P. D. McFadden, K. Frederick, L. A. Argüello, Y. Zhang, P. Vandiver, N. Odegaard and D. A. Loy, ACS Appl. Mater. Interfaces, 2017, 9, 10061–10068 CrossRef CAS PubMed.
- S. Malferrari, C. Monaco and R. Scotti, International Journal of Prosthodontics, 2003, 16, 39–44 Search PubMed.
- E. G. Karayannidou, D. S. Achilias and I. D. Sideridou, Eur. Polym. J., 2006, 42, 3311–3323 CrossRef CAS.
- J. Lee and K.-c. Wi, Journal of Conservation Science, 2010, 26, 61–67 Search PubMed.
- Y. Namura, T. Tsuruoka, C. Ryu, M. Kaketani and N. Shimizu, European Journal of Orthodontics, 2010, 32, 620–626 CrossRef.
- Q. Wang, L. He, A. Pan and Y. Wu, Appl. Surf. Sci., 2019, 495, 143570 CrossRef CAS.
- H.-X. Zhang, R.-B. Wei, C.-Z. Chen, X.-L. Tuo and X.-G. Wang, Chin. Chem. Lett., 2015, 26, 39–42 CrossRef CAS.
- O. Korychenska, D. Guzmán, À. Serra, X. Ramis and J. V. Grazulevicius, React. Funct. Polym., 2017, 116, 107–113 CrossRef CAS.
- C. N. Zhu, T. Bai, H. Wang, J. Ling, F. Huang, W. Hong, Q. Zheng and Z. L. Wu, Adv. Mater., 2021, 2102023 CrossRef CAS PubMed.
- X. Le, H. Shang, H. Yan, J. Zhang, W. Lu, M. Liu, L. Wang, G. Lu, Q. Xue and T. Chen, Angew. Chem., 2021, 133, 3684–3690 CrossRef.
- S. Liu, X. Liu, J. Yuan and J. Bao, Research, 2021, 2021, 7897849 CAS.
- W. Shi, Y. Lin, S. Zhang, R. Tian, R. Liang, M. Wei, D. G. Evans and X. Duan, Phys. Chem. Chem. Phys., 2013, 15, 18217–18222 RSC.
- H. Sadeghifar, R. Venditti, J. Jur, R. E. Gorga and J. J. Pawlak, ACS Sustainable Chem. Eng., 2017, 5, 625–631 CrossRef CAS.
- B. Seentrakoon, B. Junhasavasdikul and W. Chavasiri, Polym. Degrad. Stab., 2013, 98, 566–578 CrossRef CAS.
- P. Kotlík, K. Doubravová, J. Horálek, L. Kubáč and J. Akrman, Journal of Cultural Heritage, 2014, 15, 44–48 CrossRef.
- M. Sottile, G. Tomei, S. Borsacchi, F. Martini, M. Geppi, G. Ruggeri and A. Pucci, Eur. Polym. J., 2017, 89, 23–33 CrossRef CAS.
- J. Van Damme and F. Du Prez, Prog. Polym. Sci., 2018, 82, 92–119 CrossRef CAS.
- S. K. Olsson, H. Matsunaga, Y. Kataoka, M. Johansson, J. Matsumura, M. Westin and E. Östmark, Polym. Degrad. Stab., 2015, 113, 40–45 CrossRef CAS.
- S. K. Olsson, M. Johansson, M. Westin and E. Östmark, Polym. Degrad. Stab., 2014, 110, 405–414 CrossRef CAS.
- B. Yu, X. Jiang and J. Yin, Macromolecules, 2012, 45, 7135–7142 CrossRef CAS.
- L. Kan, H. Cheng, B. Li, X. Zhang, Q. Wang, H. Wei and N. Ma, New J. Chem., 2019, 43, 2658–2664 RSC.
- F. M. Margem, S. N. Monteiro, J. Bravo Neto, R. J. S. Rodriguez and B. G. Soares, Matéria, 2010, 15, 164–171 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07433d |
| This journal is © The Royal Society of Chemistry 2021 |