Open Access Article
Open Access ArticleSuccession of the microbial community during the process of mechanical and biological pretreatment coupled with a bio-filter for removal of VOCs derived from domestic waste: a field study
Jiaqi Houab,
Chengze Yuab,
Fanhua Mengab,
Xiaosong Heab,
Yong Wangab,
Wangmi Chenab and
Mingxiao Li *ab
*ab
aState Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing 100012, China. E-mail: limingxiao8122@163.com
bState Environmental Protection Key Laboratory of Simulation and Control of Groundwater Pollution, Beijing 100012, China
First published on 14th December 2021
Abstract
Changes in the microbial community can not only reflect the efficiency of waste disposal, but also reveal the effect of odor control during the treatment process. This study aimed to evaluate the removal efficiency of volatile organic compounds (VOCs) by the process of mechanical and biological pretreatment (MBP) coupled with a bio-filter (BF). An interesting phenomenon was found that the VOCs were effectively reduced through the MBP process. To understand the removal mechanism of VOCs, the abundance and diversity of microbial bacteria and fungi in the biological dehydration (BD) process, biological fermentation process, and BF process were explored. The abundance and diversity of microbes in the BF were relatively high, of which the bacteria such as Lactobacillus, Bacillus and Candida were the dominant species for VOCs treatment. The proposed technical process and the positive effects observed in this study indicate that it could be applied to the control of VOCs in the treatment of domestic waste.
1. Introduction
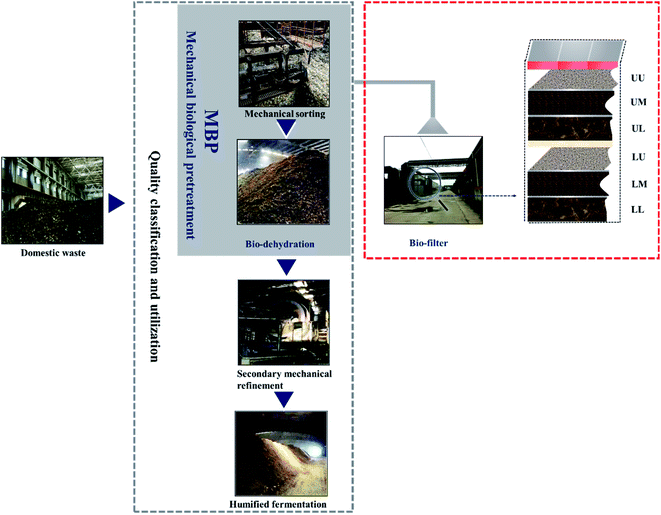
The rapid growth of domestic waste with high moisture content and large fluctuations in composition has brought tremendous pressure and challenges to its treatment. Disposal methods for domestic waste include stacking, landfilling, composting, incineration and so forth.1,2 Since landfill will occupy a large amount of land and incineration will cause pollution such as dioxins, composting has become the main technical method for domestic waste treatment in recent years. Numerous studies have shown that domestic waste is separated before composting to ensure the quality of the composting products.3–5 Therefore, mechanical biological treatment (MBT), which is composed of mechanical screening and biological fermentation, has become an emerging technological hotspot. For example, single-stream or separated-stream MBT has been applied in Italy for waste treatment.6 Also, in the domestic waste plants in Phitsanulok, Thailand, MBT is used as a process to turn household waste into fertilizers or fuels.7 However, the high moisture content of domestic waste in China increases the length of the composting time. Simultaneously, domestic waste has also suffered from dehydration, which ultimately slows down the humification rate. Furthermore, if the front-end mechanical screening effect is not good, it will seriously restrict the back-end composting effect.To overcome this difficulty, a quality classification and utilization technology (QCU) that adds the mechanical biological pretreatment (MBP) link on MBT has become an improved new technology adopted by Chinese factories in accordance with the characteristics of Chinese waste. MBP is mainly composed of mechanical sorting and biological dehydration (BD). Mechanical sorting is very necessary due to its advantages of being relatively harmless, showing an obvious reduction effect and having low processing costs.8 The water content of domestic waste can be reduced by the BD process, making the final humic acid content derived from humified fermentation higher and fertilizer efficiency better. Then, domestic waste pretreated by MBP is further treated by secondary mechanical refinement and humified fermentation to improve its classification and utilization, and ensure the effective control of secondary pollutants such as volatile organic compounds (VOCs).
Various toxic and harmful gases (such as ammonia, hydrogen sulfide, and toluene) and a large amount of VOCs (e.g., dimethyl disulfide, dimethyl sulfide, benzene, 2-butanone, limonene, and methylene chloride) can be generated during domestic waste treatment,9,10 causing environmental pollution in and around the domestic waste plant. Removing inorganic odors and VOCs is important in the quality classification and utilization of domestic waste and secondary pollution control.11–13 In the research on the removal of VOCs under the bio-filter (BF) treatment, there are numerous reports about the removal rate, as well as the influence of process parameters such as inlet, gas flow, microbes and pressure drop.14–16 However, in the process of domestic waste treatment, the types and quantities of VOCs produced at different processing stages have a great influence on the treatment effect of the BF, but there are few relevant studies.
The specific microbes in the BF can mineralize VOCs into carbon dioxide and water,17–20 or convert target pollutants into intermediate products and final products that are less harmful.21 Some reports have examined the microbial action mechanism in the treatment of VOCs by evaluating microbial population dynamics.22,23 Zhang et al. used a BF to treat the gases produced by domestic sewage and found that overall or functional microorganisms can explain the difference in fine purification performance.24 Zheng et al. used a BF to remove ammonia and sulfurated hydrogen from landfills and found that the microenvironment within the packing materials is an important factor in the removal performance of the BF.25 Therefore, the microbial changes in the processing stage and the BF are critical to the evaluation of the removal potential of VOCs.
This study was performed in a domestic waste treatment plant in Shanghai. The types and quantities of VOCs produced in gases from the MBP process were analysed. The succession of the microbial community was also studied to evaluate the performance of the technology of QCU for the removal of VOCs.
2. Materials and methods
2.1 Sample collection
The QCU used in the domestic waste treatment plant included the following processes: mechanical sorting–biological dehydration–secondary mechanical refinement–humified fermentation (Fig. 1). The cycle of the QCU of domestic waste takes approximately two months. Initial domestic waste underwent BD for 20 days after mechanical sorting, and then the dehydrated waste was further processed by a secondary mechanical refinement to ensure the proportion of organic materials. Finally, the high organic waste was humified fermented for 35 days to produce organic fertilizers. The difference between mechanical sorting and secondary mechanical refinement was the sieve diameter of the trommel screen. The VOCs in the gases from MBP were analysed in this study. Also, the VOCs in the gases produced in the process were collected by the BF. The BF included an upper compartment and a lower compartment, and each compartment had three layers. In each layer, 3 points were randomly selected and mixed into one sample of 300 g. The samples were immediately taken to the laboratory and stored in a refrigerator at −80 °C.26 The collected samples for the process of the QCU and BF are shown in Table 1.| Sample ID | Sampling |
|---|---|
| FW | Fresh waste |
| BD1 | Biological dehydration for 1 day |
| BD7 | Biological dehydration for 7 days |
| BD20 | Biological dehydration for 20 days |
| HF1 | Humified fermentation for 1 day |
| HF20 | Humified fermentation for 20 days |
| HF35 | Humified fermentation for 35 days |
| UU | Up layer from the upper compartment of the BF |
| UM | Middle layer from the upper compartment of the BF |
| UL | Low layer from the upper compartment of the BF |
| LU | Up layer from the lower compartment of the BF |
| LM | Middle layer from the lower compartment of the BF |
| LL | Low layer from the lower compartment of the BF |
2.2 Analysis method
The desorption of the VOCs was performed on an Agilent 6890 gas chromatograph system coupled to a mass spectrometric detector (Agilent Technologies, CA, USA). The oven temperature was programmed from 40 °C (5 min) to 270 °C (20 min) by increasing the temperature at the rate of 5 °C per minute. The transfer line was heated at 280 °C. The carrier gas was helium at a constant flow of 1 mL min−1 (mean velocity 36 cm s−1). The mass spectrometer was operated in the scan mode (35–550 amu). The VOC concentration was calculated according to the regression equation of the identified pollutants.28 The emission concentration lower than 0.0005 mg m−3 is regarded as undetected and no statistics were performed. This value is the detection limit. The detected VOCs were classified into six categories: saturated hydrocarbon, unsaturated hydrocarbon, aromatic hydrocarbon, sulfide, ketones, and ester. The classification details are shown in Table 2.
| Category | Volatile organic compounds |
|---|---|
| Saturated hydrocarbon | Trichloromethane, 1,1-dichloroethane, 1,2-dichloropropane, dichloromethane, cyclohexane, N-heptane, N-hexane |
| Unsaturated hydrocarbon | cis-1,3-Dichloropropene, hexachloro-1,3-butadiene, styrene, perchloroethylene, trans-1,3-dichloropropene, trichloroethylene |
| Aromatic hydrocarbon | Benzene, chlorobenzene, M-dichlorobenzene, P-dichlorobenzene, ethylbenzene, toluene, trimethylbenzene, xylene, 1-ethyl-4-methylbenzene |
| Sulfide | Carbon disulfide |
| Ketones | Acetone, methyl ethyl ketone, methyl isobutyl ketone, methyl n-butyl ketone |
| Ester | Ethyl acetate |
The amplifying condition followed the touchdown PCR, and different amplification conditions were used for bacteria and fungi. The reaction conditions for bacterial amplification were as follows: an initial denaturation of 5 min at 94 °C, 10 cycles of 30 s at 94 °C, and annealing at 60 °C for 30 s (where each cycle drops 1 °C), and the annealing temperature dropped to 50 °C after 10 cycles, and then extended at 72 °C for 1 min, denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, extension at 72 °C for 1 min after 20 cycles, extension at 72 °C for 10 min. The reaction conditions for fungal amplification were as follows: an initial denaturation of 5 min at 94 °C, denaturation at 94 °C for 30 s, and annealing at 60 °C for 30 s (where each cycle drops 1 °C), the annealing temperature dropped to 50 °C after 10 cycles, and then extended at 72 °C for 1 min, denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, extension at 72 °C for 1 min after 20 cycles, and finally at 72 °C for 10 min. All PCR products were stored at 4 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and stained with SYBR Green I for 30 min, and the images were taken using a Gel Doc-It TS imaging system (UVP, USA).
000 and stained with SYBR Green I for 30 min, and the images were taken using a Gel Doc-It TS imaging system (UVP, USA).Bacterial sequencing results were compared with the compiled results of 16S rDNA genes in the Genbank database using BLAST, and the fungus sequencing results were compared using the compiled results of 18S rDNA genes in the Genbank database.33 PCR was conducted using an Omn-E programmable thermal cycler (Hybaid Ltd., Middlesex, UK).
2.3 Data analysis
SPSS 17.0 (SPSS International) and Origin 8.0 (IBM, USA) were used to analyze and process the data. Gene Quest and Quantity One 4.6.2 were used to analyze microbial data.3. Results and discussion
3.1 Removal of VOCs by QCU
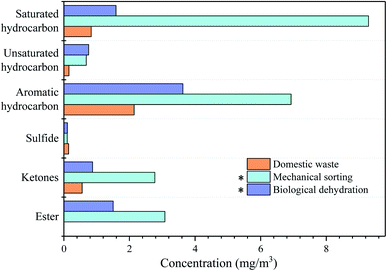
The total content of VOCs in the gases from the initial domestic waste was 8.46 mg m−3 (Fig. 2). The VOCs in the gases released during domestic waste mechanical sorting mainly consisted of saturated hydrocarbons and aromatic hydrocarbons.34 The content of aromatic hydrocarbons is a key issue in VOCs. The concentration of aromatic hydrocarbon was 3.63 mg m−3, accounting for 42.9% of the total VOCs. Zhang et al.35 showed that the content of aromatic compounds was higher than that of other VOCs in the municipal waste treatment. Compared with initial domestic waste, the contents of saturated hydrocarbons, aromatic hydrocarbons, ketones, and esters increased with mechanical sorting. For the mechanical motion, the content of saturated hydrocarbons after the mechanical sorting of domestic waste was 5.3 times that of the initial waste.36 The increase in the content of some VOCs in the gases might also be attributable to the photodegradation caused by the operation of the drum screening machine for mechanical sorting.37,38The domestic waste was further subjected to BD to achieve the purpose of waste volume reduction. The content of total VOCs in the gases detected after BD was 3.81 mg m−3, which was 2.2 times lower than that of the initial waste. As shown in Fig. 2, aromatic hydrocarbons, saturated hydrocarbons, and ketones were the key pollutants that should be controlled during this process. After BD, the content of saturated hydrocarbons decreased to one sixth of its initial value and the content of aromatic hydrocarbon was reduced to half of that of mechanical sorting. However, BD did not contribute much to sulfide degradation but caused a slight increase in its content. The volatile sulfur compounds were the most important aromatic components produced by biodegradation.39 Aromatic hydrocarbons are usually observed in transfer stations, landfills, and aerobic fermentation plants. Studies have shown that aromatic hydrocarbons were not produced through biodegradation but result from the chemically synthesized materials and household solvents contained in wastes.40 BD had a better effect in controlling the release of VOCs compared with mechanical sorting. The results showed that in the MBP process, the content of target pollutants such as saturated hydrocarbons, aromatic hydrocarbons, ketones and esters had been reduced. However, the removal of VOCs in the gases from the MBP process was a synergistic BD and BF, which is the key aspect that needs to be explored through microbial changes.
3.2 Denatured gradient gel electrophoresis profile analysis
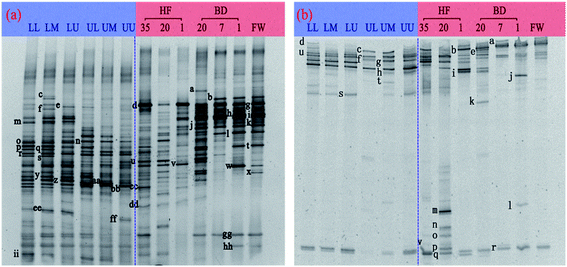
The 16S rDNA and 18S rDNA gene fragments of bacteria and fungi were revealed by denatured gradient gel electrophoresis (DGGE) patterns (Fig. 3). The distribution of microbes corresponding to different samples can be judged according to the position, number and brightness of the bands. As illustrated in Fig. 3, bands r and cc were detected among all padding filters of the BF, while band d was detected throughout the BD and humified fermentation. In addition, bands m and o appeared only in the lower compartment of the BF, while band n was detected only in the upper compartment. The results also revealed that the bacterial diversity decreased with the increase in the height of the padding filter, which was consistent with previous findings.41 Regarding BD and humified fermentation, band v and bands j, l, and gg appeared separately in humified fermentation and BD. The dehydration and fermentation were important for organic matter degradation, thereby accelerating rotting.33,42,43 Significant differences between classification, utilization, and biofiltration were found in the bacterial profiles. It can be seen from the bacterial DGGE map that compared to BD, the BF got a better biodiversity.As shown in Fig. 3b, bands l–r were located in the lower part of the DGGE profile, signifying that these fungi had a higher G + C content33 and better deodorization ability. The numbers of bands were in the order of resource utilization > biofiltration, which was consistent with the bacterial DGGE profile. The bands of the fungal DGGE maps of BD and BF were similar, and there was a small difference in the number of bands. The results showed that resource utilization could increase the amounts and types of the dominant fungi in the humified fermentation and BD. Regarding bands a–k from the top of the DGGE profile, band f appeared in both biofiltration and resource utilization, while band h occurred only in biofiltration. The number of bands in BD20 and HF20 was significantly higher than that in the other lanes. Among these, band k in BD20 and bands m–q in HF20 were the dominant species in their lane. Further analysis of the bands in the BF showed that bands u and s were typical in the lower compartment, and the types and numbers of these bands were in the order of lower compartment > upper compartment. The VOCs discharged by the QCU of the four-stage compartment preferentially passed through the lower compartment of the BF, where their air inflows were higher than those in the upper compartment of the BF. This is because VOCs are introduced into the BF, which has an inner effect on the microbes in the filler.44
3.3 Dominant microbial communities of biofiltration
The number of bands and Shannon index of bacteria were more than those of fungi (Table 3). The number of bands in BD20 of bacteria was 26, which was 3.71 times that of fungi. The diversity (H) of bacteria was 3.19, which was 1.72 times that of fungi. The greater the diversity, the more abundant the species.45 The diversity and abundance of the species decreased with the period of humified fermentation. Regarding biofiltration, the numbers of bands of lower padding filters were much more than those of upper padding filters, but the Pielou index of each padding layer did not show much difference, suggesting that the more abundant the species diversity, the more stable the biological community, which was consistent with the data in the PCR-DGGE analysis. The Shannon index of bacteria in the lowest layer was 0.35 times higher than that in the highest layer. The filter material with the strongest bacterial diversity was the LL layer with a value of 3.48; however, for fungi, the filter material with the strongest fungal diversity was the LU layer with a value of 2.27. From the perspective of microbial abundance and diversity, bacteria in the BF and fungi in BD20 were more abundant.| Sample | Bacteria | Fungi | ||||
|---|---|---|---|---|---|---|
| Band number | Shannon (H) | Pielou (E) | Band number | Shannon (H) | Pielou (E) | |
| FW | 19 | 2.85 | 0.97 | 5 | 1.57 | 0.98 |
| BD1 | 11 | 2.35 | 0.98 | 7 | 1.87 | 0.96 |
| BD7 | 10 | 2.22 | 0.97 | 5 | 1.47 | 0.92 |
| BD20 | 26 | 3.19 | 0.98 | 7 | 1.85 | 0.95 |
| HF1 | 7 | 1.87 | 0.96 | 4 | 1.35 | 0.98 |
| HF20 | 17 | 2.81 | 0.99 | 11 | 2.37 | 0.99 |
| HF35 | 8 | 2.02 | 0.97 | 5 | 1.52 | 0.95 |
| UU | 24 | 3.13 | 0.98 | 4 | 1.34 | 0.96 |
| UM | 19 | 2.90 | 0.99 | 7 | 1.84 | 0.95 |
| UL | 26 | 3.19 | 0.98 | 9 | 2.14 | 0.97 |
| LU | 30 | 3.35 | 0.99 | 11 | 2.27 | 0.95 |
| LM | 34 | 3.47 | 0.98 | 7 | 1.83 | 0.94 |
| LL | 34 | 3.48 | 0.99 | 5 | 1.56 | 0.97 |
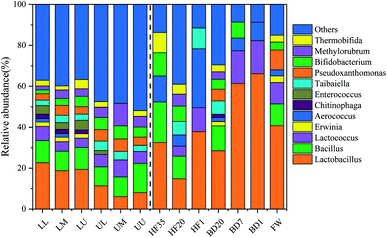
Table 4 presents the sequencing results of the dominant bands of bacteria, which were obtained after comparing the sequence of the bands with the National Center for Biotechnology Information (NCBI) database. The results showed that Lactobacillus and Bacillus were the dominant species. The main difference was that some dominant bacteria in upper-compartment samples had a lower abundance compared with that in the lower compartment such as Lactobacillus (Fig. 4). For the original domestic waste, Lactobacillus, Bacillus, and Lactococcus were dominant with the respective abundances of 40.71%, 10.65%, and 10.52%. After mechanical sorting, BD, secondary mechanical refinement, and 35 day humified fermentation, the proportion of Lactobacillus was 14.85%, which was quite different from that of the original domestic waste. During the deodorization process of the BF, Bacillus could effectively degrade sulfurated hydrogen and ammonia in gases.16 Lactobacillus is anaerobic, facultative anaerobic, or micro aerobic bacteria, which have good removal efficiency for ammonia and other odors.46 Thermobifida, Methylorubrum, Pseudoxanthomonas, Taibaiella, Enterococcus, Aerococcus, and Lactococcus were also detected besides Lactobacillus and Bacillus. Pseudoxanthomonas and Thermobifida secreted xylanase and glucanase, which were involved in the degradation of cellulose and hemicellulose.47 Taibaiella has an excellent effect on the removal of aniline, and it usually appears in the landfill leachate.48 Therefore, during the classification and utilization of FW to HF35, they had always been dominant species. Enterococcus and Lactococcus were pathogenic bacteria, which only existed in the initial stage of composting and disappeared as the temperature rises.49 Enterococcus and Lactococcus were not detected in HF35, which meant that the QCU used in the domestic waste treatment effectively removed pathogenic bacteria. Therefore, the dominant bacterial genera of BD and BF differ in composition. The dominant bacterial genera of BF are Lactobacillus, Taibaiella, etc. These dominant bacterial genera play a positive role in the removal of target pollutants.
| Sequences | Accession number | Species with the greatest similarity | Similarity, % |
|---|---|---|---|
| a | NR104932.1 | Erwinia billingiae strain billing E63 | 100 |
| b | NR113334.1 | Lactobacillus curvatus strain NBRC 15884 | 99 |
| c | NR109520.1 | Flavobacterium marinum strain SW105 | 96.48 |
| d | NR104708.1 | Aerococcus viridans strain NBRC 12219 | 100 |
| e | NR165710.1 | Mucibacter soli strain R1-15 | 91.37 |
| f | NR109509.1 | Chitinophaga cymbidii strain R156-2 | 97.44 |
| g | NR104573.1 | Lactobacillus plantarum strain CIP | 100 |
| h | NR042438.1 | Lactobacillus graminis strain G90 (1) | 98.49 |
| i | NR044702.2 | Lactobacillus amylophilus strain DSM | 98.47 |
| j | NR115765.1 | Enterococcus faecalis strain ATCC 19433 | 99.50 |
| k | NR104926.1 | Lactobacillus bifermentans strain CIP 102811 | 99.49 |
| l | NR113925.1 | Lactococcus lactis subsp. cremoris strain NBRC 100676 | 100 |
| m | NR109732.1 | Fabibacter pacificus strain DY53 | 91.62 |
| n | NR108906.1 | Bacillus eiseniae strain A1-2 | 96.02 |
| o | NR133876.1 | Taibaiella koreensis strain THG-DT86 | 98.45 |
| p | NR074291.1 | Bordetella petrii strain DSM 12804 | 95.98 |
| q | NR043110.1 | Pseudoxanthomonas kalamensis strain JA40 | 98.49 |
| r | NR114009.1 | Galbibacter mesophilus strain NBRC 101624 | 92.31 |
| s | NR029151.1 | Bacillus thermoamylovorans strain DKP | 98.99 |
| t | NR029261.2 | Lactobacillus sanfranciscensis strain L-12 | 99.49 |
| u | NR159904.1 | Bacillus lacus strain AK74 | 97.99 |
| v | NR133975.1 | Bacillus kokeshiiformis strain MO-04 | 95.98 |
| w | NR112108.1 | Chroococcidiopsis thermalis PCC 7203 | 89.83 |
| x | NR117506.1 | Bifidobacterium longum strain KCTC 3128 | 99.43 |
| y | NR114899.1 | Methylorubrum populi BJ001 | 98.28 |
| z | NR044309.1 | Steroidobacter denitrificans strain FS | 96.98 |
| aa | NR148571.2 | Chloroflexus islandicus strain isl-2 | 88.44 |
| bb | NR074876.1 | Dictyoglomus thermophilum H-6-12 | 89.66 |
| cc | NR112015.1 | Thermobifida fusca strain IFO 14071 | 98.95 |
| dd | NR137360.1 | Bacillus campisalis strain SA2-6 | 98.47 |
| ee | NR145530.1 | Actinoallomurus bryophytorum strain NEAU-TX1-15 sequence | 88.33 |
| ff | NR042035.1 | Actinomadura umbrina strain IMSNU 22165 | 89.27 |
| gg | NR043148.1 | Lactobacillus oligofermentans strain AMKR18 | 99.49 |
| hh | NR113958.1 | Lactococcus lactis subsp. hordniae strain NBRC 100931 | 100 |
| ii | NR156918.1 | Luteitalea pratensis strain HEG-639 | 88.83 |
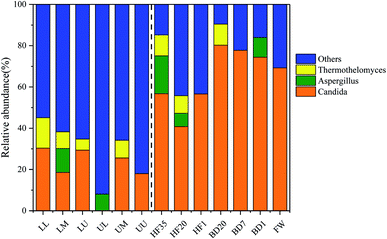
The similar values of sequences a, b, j, s, and u in Table 5 were all more than 99%. The 13 dominant fungal bands were classified as Candida, Aspergillus, and Thermothelomyces. Candida has a significant impact on the production and changes of VOCs.50 As illustrated in Fig. 5, Candida has always been the dominant genus during the QCU process of domestic waste. It had the highest proportion of 80.27% in BD20, but it was not detected in the UL, indicating that VOCs were not conducive to the survival of Candida. Aspergillus can promote the removal rate of ammoniacal nitrogen and chemical oxygen demand in the landfill leachate and the activity of cellulolytic enzymes.51 The concentration of Thermothelomyces began to increase on the 20th day of biological dehydration, and it mainly existed in the padding filter of the BF for VOC degradation. Thermothelomyces was a common thermophilic genus, which has a significant catalytic oxidation effect on polycyclic molecules.52 Thermothelomyces also appeared in BD20, and the diversity of fungi in BD20 was high, indicating that BD also made a certain contribution to the removal of target pollutants.
| No | Accession![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) number number |
Species with the greatest similarity | Similarity, % |
|---|---|---|---|
| a | NG063407.1 | Candida sojae JCM 1644 | 99.14 |
| b | NG063416.1 | Candida quercitrusa JCM 9832 | 99.43 |
| c | NG064977.1 | Tritirachium cinnamomeum IHEM 3714 | 89.82 |
| d | NG062812.1 | Acremonium flavum CBS 596.70 | 96.78 |
| e | NG062131.1 | [Candida] fukazawae JCM 1641 | 97.93 |
| f | NG063411.1 | Candida austromarina JCM 8894 | 95.42 |
| g | NG064994.1 | Thermothelomyces hinnuleus ATCC 52474 | 93.86 |
| h | NG067668.1 | Megasporia hengduanensis BJFC Cui 8076 | 97.20 |
| i | NG063422.1 | Candida edax JCM 9470 | 93.33 |
| j | NG064802.1 | Aspergillus proliferans CBS 121.45 | 99.14 |
| k | NG065474.1 | [Candida] intermedia JCM 1607 | 97.33 |
| l | NG065472.1 | [Candida] pseudointermedia JCM 1592 | 97.04 |
| m | NG063232.1 | Aspergillus cervinus NRRL 5025 | 80.5 |
| n | NG063232.1 | Aspergillus cervinus NRRL 5025 | 80.28 |
| o | NG064802.1 | Aspergillus proliferans CBS 121.45 | 98.56 |
| p | NG063232.1 | Aspergillus cervinus NRRL 5025 | 80.5 |
| q | NG063419.1 | Candida viswanathii JCM 9567 | 92.84 |
| r | NG063242.1 | [Candida] glabrata NRRL Y-65 | 98.57 |
| s | NG064994.1 | Thermothelomyces hinnuleus ATCC 52474 | 99.12 |
| t | NG064938.1 | Aspergillus caninus UAMH 10337 | 80.5 |
| u | NG063242.1 | [Candida] glabrata NRRL Y-65 | 99.43 |
| v | NG064994.1 | Thermothelomyces hinnuleus ATCC 52474 | 92.75 |
4. Conclusions
This study compared the types and contents of VOCs in the gases from the original domestic waste, and illustrated that VOCs can be removed during the MBP process. It was recommended that VOCs produced in 20 days before BD should be monitored. Lactobacillus and Bacillus among bacteria, and Candida, Aspergillus, and Thermothelomyces among fungi were dominant with a positive role in the disposal of VOCs. In addition, the bacteria in the BF are the key step to remove VOCs in the gases, and the fungi in BD have also made a small contribution in the removal of VOCs. Moreover, inoculating Lactobacillus, Bacillus, Candida, Aspergillus, and Thermothelomyces into BF is expected to improve the removal efficiency of VOCs.Author contributions
Jiaqi Hou: Data curation, Writing – original draft preparation, and Funding acquisition; Chengze Yu: Software and Formal analysis; Fanhua Meng: Validation and Software; Xiaosong He: Validation and Software; Yong Wang: Conceptualization and Methodology; Wangmi Chen: Reviewing and Editing; Mingxiao Li: Ideas and Supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2019YFD1100304) and the National Natural Science Foundation of China (51908524).References
- P. Kjeldsen, M. A. Barlaz, A. P. Rooker, A. Baun, A. Ledin and T. H. Christensen, Present and Long-Term Composition of MSW Landfill Leachate: A Review, Crit. Rev. Environ. Sci. Technol., 2002, 32(4), 297–336 CrossRef CAS.
- H. Ma, Y. Cao, X. Lu, Z. Ding and W. Zhou, Review of Typical Municipal Solid Waste Disposal Status and Energy Technology, Energy Procedia, 2016, 88, 589–594 CrossRef CAS.
- M. Fuss, M. Vergara-Araya, R. T. V. Barros and W.-R. Poganietz, Implementing mechanical biological treatment in an emerging waste management system predominated by waste pickers: a Brazilian case study, Resour. Conserv. Recycl., 2020, 162 Search PubMed.
- M. X. Paes, G. A. de Medeiros, S. D. Mancini, C. Gasol, J. R. Pons and X. G. Durany, Transition towards eco-efficiency in municipal solid waste management to reduce GHG emissions: the case of Brazil, J. Clean. Prod., 2020, 263 Search PubMed.
- V. S. N. S. Goli, D. N. Singh and T. Baser, A critical review on thermal treatment technologies of combustible fractions from mechanical biological treatment plants, J. Environ. Chem. Eng., 2021, 9(4), 105643 CrossRef CAS.
- L. Rigamonti, G. Borghi, G. Martignon and M. Grosso, Life cycle costing of energy recovery from solid recovered fuel produced in MBT plants in Italy, Waste Manag., 2019, 99, 154–162 CrossRef PubMed.
- W. Punin, S. Maneewan and C. Punlek, The feasibility of converting solid waste into refuse-derived fuel 5 via mechanical biological treatment process, J. Mater. Cycles Waste Manag., 2013, 16(4), 753–762 CrossRef.
- M. López, M. Soliva, F. X. Martínez-Farré, M. Fernández and O. Huerta-Pujol, Evaluation of MSW organic fraction for composting: separate collection or mechanical sorting, Resour. Conserv. Recycl., 2010, 54(4), 222–228 CrossRef.
- P. He, J. Tang, D. Zhang, Y. Zeng and L. Shao, Release of volatile organic compounds during bio-drying of municipal solid waste, J. Environ. Sci., 2010, 22(5), 752–759 CrossRef CAS.
- I. H. Suffet, V. Decottignies, E. Senante and A. Bruchet, Sensory assessment and characterization of odor nuisance emissions during the composting of wastewater biosolids, Water Environ. Res., 2009, 81(7), 670–679 CrossRef CAS PubMed.
- R. Lopez, I. O. Cabeza, I. Giraldez and M. J. Diaz, Biofiltration of composting gases using different municipal solid waste-pruning residue composts: monitoring by using an electronic nose, Bioresour. Technol., 2011, 102(17), 7984–7993 CrossRef CAS PubMed.
- D. P. Komilis, R. K. Ham and J. K. Park, Emission of volatile organic compounds during composting of municipal solid wastes, Water Res., 2004, 38(7), 1707–1714 CrossRef CAS PubMed.
- J. Hu, X. Gao, Q. Fan and X. Gao, Facial controlled synthesis of Pt/MnO2 catalysts with high efficiency for VOCs combustion, RSC Adv., 2021, 11(27), 16547–16556 RSC.
- G. Á. Mhuireach, L. Dietz, W. Griffiths, P. F. Horve, A. Laguerre, D. Northcutt, R. Vandegrift, E. Gall and K. Van Den Wymelenberg, Differing effects of four building materials on viable bacterial communities and VOCs, Developments in the Built Environment, 2021, 7, 100055 CrossRef.
- Y. Zhang, J. Liu, J. Li and T. Yue, Effects of filler voidage on pressure drop and microbial community evolution in fungal bio-trickling filters, Chemosphere, 2021, 273, 129710 CrossRef CAS PubMed.
- Z. Yang, J. Li, J. Liu, J. Cao, D. Sheng and T. Cai, Evaluation of a pilot-scale bio-trickling filter as a VOCs control technology for the chemical fibre wastewater treatment plant, J. Environ. Manag., 2019, 246, 71–76 CrossRef CAS PubMed.
- Y. Zhang, J. Liu, H. Xing and J. Li, Performance and fungal diversity of bio-trickling filters packed with composite media of polydimethylsiloxane and foam ceramics for hydrophobic VOC removal, Chemosphere, 2020, 256, 127093 CrossRef CAS PubMed.
- S. K. Padhi and S. Gokhale, Treatment of gaseous volatile organic compounds using a rotating biological filter, Bioresour. Technol., 2017, 244(Pt 1), 270–280 CrossRef CAS PubMed.
- H. Wu, H. Yan, Y. Quan, H. Zhao, N. Jiang and C. Yin, Recent progress and perspectives in biotrickling filters for VOCs and odorous gases treatment, J. Environ. Manag., 2018, 222, 409–419 CrossRef CAS PubMed.
- E. R. Rene, B. T. Mohammad, M. C. Veiga and C. Kennes, Biodegradation of BTEX in a fungal biofilter: influence of operational parameters, effect of shock-loads and substrate stratification, Bioresour. Technol., 2012, 116, 204–213 CrossRef CAS PubMed.
- J. Hu, L. Zhang, J. Chen, Y. Luo, B. Sun and G. Chu, Performance and microbial analysis of a biotrickling filter inoculated by a specific bacteria consortium for removal of a simulated mixture of pharmaceutical volatile organic compounds, Chem. Eng. J., 2016, 304, 757–765 CrossRef CAS.
- M. C. Perez, F. J. Alvarez-Hornos, K. H. Engesser, D. Dobslaw and C. Gabaldon, Removal of 2-butoxyethanol gaseous emissions by biotrickling filtration packed with polyurethane foam, N. Biotechnol., 2016, 33(2), 263–272 CrossRef CAS PubMed.
- F. J. Álvarez-Hornos, C. Lafita, V. Martínez-Soria, J. M. Penya-Roja, M. C. Pérez and C. Gabaldón, Evaluation of a pilot-scale biotrickling filter as a VOC control technology for the plastic coating sector, Biochem. Eng. J., 2011, 58–59, 154–161 CrossRef.
- S. Zhang, S. Xia, W. Sang, J. Yuan and W. Guo, Evaluation of functional microbes in explaining biofilter performance during start-up1, J. Clean. Prod., 2020, 277 Search PubMed.
- T. Zheng, L. Li, F. Chai and Y. Wang, Factors impacting the performance and microbial populations of three biofilters for co-treatment of H2S and NH3 in a domestic waste landfill site, Process Saf. Environ. Prot., 2021, 149, 410–421 CrossRef CAS.
- Y. Yin, J. Gu, X. Wang, Y. Zhang, W. Zheng, R. Chen and X. Wang, Effects of rhamnolipid and Tween-80 on cellulase activities and metabolic functions of the bacterial community during chicken manure composting, Bioresour. Technol., 2019, 288, 121507 CrossRef CAS PubMed.
- M. Delgado-Rodríguez, M. Ruiz-Montoya, I. Giraldez, R. López, E. Madejón and M. J. Díaz, Use of electronic nose and GC-MS in detection and monitoring some VOC, Atmos. Environ., 2012, 51, 278–285 CrossRef.
- Y. Yan, M. Wang, B. Jin, J. Yang and S. Li, Performance evaluation and microbial community analysis of the biofilter for removing grease and volatile organic compounds in the kitchen exhaust fume, Bioresour. Technol., 2021, 319, 124132 CrossRef CAS PubMed.
- A. Sumi, K. Fukushi, R. Hon Da and K. M. Hassan, Sustainable Water Resource Management in China: A Great Challenge, Springer, Netherlands, 2010, ch. 1, 10.1007/978-90-481-9914-3, pp. 3–10 Search PubMed.
- H. Liu, W. Lei, L. Mei, B. Wang, K. Jiang, S. Ma and Q. Li, The intestinal microbial diversity in Chinese shrimp (Fenneropenaeus chinensis) as determined by PCR–DGGE and clone library analyses, Aquaculture, 2011, 317(1–4), 32–36 CrossRef CAS.
- S. Saijai, A. Ando, R. Inukai, M. Shinohara and J. Ogawa, Analysis of microbial community and nitrogen transition with enriched nitrifying soil microbes for organic hydroponics, Biosci. Biotechnol. Biochem., 2016, 80(11), 2247–2254 CrossRef CAS PubMed.
- M. Das, T. V. Royer and L. G. Leff, Diversity of fungi, bacteria, and actinomycetes on leaves decomposing in a stream, Appl. Environ. Microbiol., 2007, 73(3), 756–767 CrossRef CAS PubMed.
- B. Xi, X. He, Q. Dang, T. Yang, M. Li, X. Wang, D. Li and J. Tang, Effect of multi-stage inoculation on the bacterial and fungal community structure during organic municipal solid wastes composting, Bioresour. Technol., 2015, 196, 399–405 CrossRef CAS PubMed.
- C. Wu, J. Liu, S. Liu, W. Li, L. Yan, M. Shu, P. Zhao, P. Zhou and W. Cao, Assessment of the health risks and odor concentration of volatile compounds from a municipal solid waste landfill in China, Chemosphere, 2018, 202, 1–8 CrossRef CAS PubMed.
- L. Zhang, X. Su, Z. Zhang, S. Liu, Y. Xiao, M. Sun and J. Su, Characterization of fly ash from a circulating fluidized bed incinerator of municipal solid waste, Environ. Sci. Pollut. Res. Int., 2014, 21(22), 12767–12779 CrossRef CAS PubMed.
- J. F. Saldarriaga, R. Aguado and G. E. Morales, Assessment of VOC Emissions from Municipal Solid Waste Composting, Environ. Eng. Sci., 2014, 31(6), 300–307 CrossRef CAS.
- M. R. Ras, R. M. Marce and F. Borrull, Volatile organic compounds in air at urban and industrial areas in the Tarragona region by thermal desorption and gas chromatography-mass spectrometry, Environ. Monit. Assess., 2010, 161(1–4), 389–402 CrossRef PubMed.
- E. Nie, G. Zheng, Z. Shao, J. Yang and T. Chen, Emission characteristics and health risk assessment of volatile organic compounds produced during municipal solid waste composting, Waste Manag., 2018, 79, 188–195 CrossRef CAS PubMed.
- L. Wenjing, D. Zhenhan, L. Dong, L. M. Jimenez, L. Yanjun, G. Hanwen and W. Hongtao, Characterization of odor emission on the working face of landfill and establishing of odorous compounds index, Waste Manag., 2015, 42, 74–81 CrossRef PubMed.
- J. Liu, G. Zheng, J. Liu and G. Zheng, Emission of volatile organic compounds from a small-scale municipal solid waste transfer station: ozone-formation potential and health risk assessment, Waste Manag., 2020, 106, 193–202 CrossRef CAS PubMed.
- H. Benbelkacem, R. Bayard, A. Abdelhay, Y. Zhang and R. Gourdon, Effect of leachate injection modes on municipal solid waste degradation in anaerobic bioreactor, Bioresour. Technol., 2010, 101(14), 5206–5212 CrossRef CAS PubMed.
- B. Xi, W. Meng, H. Liu, G. Huang, G. Zeng and Q. Wang, The variation of inoculation complex microbial community in three stages MSW composting process controlled by temperature, Huanjing Kexue, 2003, 24(2), 152–155 Search PubMed.
- B. Xi, G. Zhang and H. Liu, Process kinetics of inoculation composting of municipal solid waste, J. Hazard Mater., 2005, 124(1–3), 165–172 CrossRef CAS PubMed.
- Z. Liang, J. Wang, Y. Zhang, C. Han, S. Ma, J. Chen, G. Li and T. An, Removal of volatile organic compounds (VOCs) emitted from a textile dyeing wastewater treatment plant and the attenuation of respiratory health risks using a pilot-scale biofilter, J. Clean. Prod., 2020, 253 Search PubMed.
- W. Zhang, Y. Mo, J. Yang, J. Zhou, Y. Lin, A. Isabwe, J. Zhang, X. Gao and Z. Yu, Genetic diversity pattern of microeukaryotic communities and its relationship with the environment based on PCR-DGGE and T-RFLP techniques in Dongshan Bay, southeast China, Continent. Shelf Res., 2018, 164, 1–9 CrossRef.
- C. Franke, M. Hilgarth, R. F. Vogel, H. Petermeier and H.-C. Langowski, Characterization of the dynamics of volatile organic compounds released by lactic acid bacteria on modified atmosphere packed beef by PTR-MS, Food Packag. Shelf Life, 2019, 22, 100400 CrossRef.
- X. He, L. Han, B. Fu, S. Du, Y. Liu and G. Huang, Effect and microbial reaction mechanism of rice straw biochar on pore methane production during mainstream large-scale aerobic composting in China, J. Clean. Prod., 2019, 215, 1223–1232 CrossRef CAS.
- M. El-Fadel, F. Sleem, J. Hashisho, P. E. Saikaly, I. Alameddine and S. Ghanimeh, Impact of SRT on the performance of MBRs for the treatment of high strength landfill leachate, Waste Manag., 2018, 73, 165–180 CrossRef CAS PubMed.
- K. Tsigkou, D. Zagklis, P. Tsafrakidou, C. Zafiri and M. Kornaros, Composting of anaerobic sludge from the co-digestion of used disposable nappies and expired food products, Waste Manag., 2020, 118, 655–666 CrossRef CAS PubMed.
- P. Buzzini, A. Martini, U. M. Pagnoni and P. Davoli, Production of flavoured volatile organic compounds (VOCs) by Candida oleophila GK10, Enzyme Microb. Technol., 2003, 33(5), 668–675 CrossRef CAS.
- M. Gopinath, C. Mohanapriya, K. Sivakumar, G. Baskar, C. Muthukumaran and R. Dhanasekar, Microbial abatement of toluene using Aspergillus niger in upflow bioreactor, Ecotoxicol. Environ. Saf., 2016, 134(Pt 2), 370–376 CrossRef CAS PubMed.
- M. J. Furst, S. Savino, H. M. Dudek, J. R. Gomez Castellanos, C. Gutierrez de Souza, S. Rovida, M. W. Fraaije and A. Mattevi, Polycyclic Ketone Monooxygenase from the Thermophilic Fungus Thermothelomyces thermophila: A Structurally Distinct Biocatalyst for Bulky Substrates, J. Am. Chem. Soc., 2017, 139(2), 627–630 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2021 |