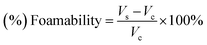Open Access Article
Open Access ArticleDevelopment of highly porous calcium phosphate bone cements applying nonionic surface active agents†
Ewelina Cichoń *,
Bartosz Mielan
*,
Bartosz Mielan ,
Elżbieta Pamuła
,
Elżbieta Pamuła ,
Anna Ślósarczyk
,
Anna Ślósarczyk and
Aneta Zima
and
Aneta Zima *
*
Faculty of Materials Science and Ceramics, AGH University of Science and Technology, Mickiewicza Av. 30, 30-059, Krakow, Poland. E-mail: ecichon@agh.edu.pl; azima@agh.edu.pl
First published on 6th July 2021
Abstract
A novel way of obtaining highly porous cements is foaming them with the use of nonionic surface active agents (surfactants). In this study, foamed calcium phosphate cements (fCPCs) intended for in situ use were fabricated by a surfactant-assisted foaming process. Three different surface active agents, Tween 20, Tween 80 and Tetronic 90R4, were used. The amount of surfactant, based on its critical micelle concentration and cytotoxicity as well as foaming method, was determined. It has been established that in order to avoid cytotoxic effects the concentration of all applied surfactants in the cement liquid phases should not exceed 1.25 g L−1. It was found that Tetronic 90R4 had the lowest cytotoxicity whereas Tween 20 had the highest. The influence of the type of surfactant used in the fabrication process of bioactive macroporous cement on the physicochemical and biological properties of fCPCs was studied. The obtained materials reached higher than 50 vol% open porosity and possessed compressive strength which corresponds to the values for cancellous bone. The highest porosity and compressive strength was found for the material with the addition of Tween 80. In vitro investigations proved the chemical stability and high bioactive potential of the examined materials.
1. Introduction
Calcium phosphates (CaPs) are excellent candidates for bone repair and regeneration due to their chemical and mineralogical composition being similar to the inorganic component of bone.1,2 An ideal tissue engineering scaffold should provide initial support for osteoprogenitor cells to deposit a mineralised bone matrix. At the same time, it should be also porous and resorbable which as a consequence leads to the ingrowth of newly formed bone tissue inside the scaffold.3Micropores facilitate the free flow of ions and nutrients whereas macropores present in the scaffold allow osteoblast migration and neovascularisation.
Different techniques are used to obtain highly porous CaPs scaffolds. The method of replication of porous organic matrix4–7 is a well-known fabrication technique for the ceramic bone scaffolds. During this process, a polymer sponge (usually polyurethane) is soaked in a ceramic slurry, dried and finally submitted to thermal treatment and sintering. The polymeric sponge is burned whereas the ceramics fixes its shape and microstructure.
To achieve high porosity in chemically bonded calcium phosphate bone cements (CPCs), where heat treatment is generally not applied, other methods are used.8,9 In CPCs porosity can be created before as well as after setting reaction – by their degradation. Porogens addition is a common way to obtain porosity in bone cements.10,11 Smith et al.12 have applied glucose as a porogen which led to the increased porosity and faster degradation of the resulting cement. Sariibrahimoglu et al.13 tried to introduce the macroporosity to CPCs by the addition of porogens such as PLGA and glucono-delta-lactone (GDL). The disadvantages of using porogens are that the pores are not present in the scaffold early enough to allow the formation of biological junction with surrounding tissue. What is more, leaching of the porogen may have a negative impact on the regeneration process. Also, the size of porogen particles must be large enough to form macropores, and might thus have negative impact on cement injectability.14 Highly porous CPCs can be obtained also by the presence of effervescent additives. Hesaraki et al.15,16 employed this technique and reached interconnected macropores in CPCs using sodium carbonate and citric acid monohydrate or acetic acid. The main drawback of effervescent additives is a risk associated with sudden gas release in body fluids.17
The new approach to obtain macroporous CPCs is the surfactant-assisted foaming. Following this procedure, no potentially toxic gases as well as acidic degradation products are liberated after cement implantation. Surfactants tend to adsorb on the surfaces due to their amphiphilic character. A fundamental characteristic of surfactants is their specific behaviour in the water environment. In aqueous solutions, surfactant molecules self-organize to form aggregates called micelles. The concentration at which micelles begin to emerge is termed the critical micelle concentration (CMC). CMC is a characteristic parameter for each surfactant.18 Addition of surfactant to the suspending medium allows the modification of surface tension.19 Mainly, these kinds of chemicals are applied as emulsifiers, detergents and foaming or wetting agents.20
Surfactants, in general, stabilize foams at the water/air interface, by lowering the surface free energy required to maintain the larger interfacial area which is often associated with the formation of air bubbles.21 This process can be used in the fabrication of porous foamed cement scaffolds. To date, the strategy of using surfactants as air-entraining agents in CPCs has received little attention in literature. Only a few surfactants have been applied as CPCs macroporosity enhancers.22 In the latest research, Vásquez et al. attempted to find out the relationship between the amount of added nonionic surfactant, namely Lutensol® ON 110, and the porosity of the final cement.23 Zhang et al.24 used liquid with silanized-hydroxypropyl methylcellulose (Si-HPMC) as a foaming agent of CPCs and mixed it with powder using a domestic food mixer. Unosson et al.25 also employed the same technique as Zhang and introduced the macroporosity into brushite cements using Tween 80 and Pluronic F127. Highly porous brushite foams were obtained with total porosity of up to 75% and a macroporosity above 55%. Vorndran et al.26 mixed CPCs with oil-based suspension. The oil phase contained two surface-active agents – castor oil ethoxylate 35 and hexadecyl-phosphate. The addition of surfactants improved the porosity of cements. Pastorino et al.14 applied Tween 80 as a foaming agent for enhancement of antibiotic release in porous CPCs. It should be highlighted that surfactants do not only affect the porosity of cements, but also their other properties.27
Our previous study showed that Tween 20, Tween 80 and Tetronic 90R4 are interesting modifiers of non-foamed calcium phosphate cements.28 The present work focuses on the new concept which is to determine the appropriate concentration of each surfactant in the liquid phase of the highly porous foamed cements. To the best of our knowledge, no studies showed the minimal required concentration of a surfactant in cement liquid phase based on its critical micelle concentration as well as a non-cytotoxic surfactant concentration in foamed cement based on materials' cytotoxicity tests. In addition, the foaming procedure was chosen for each of the surfactants used. In the published literature, we have not found any attempts in choosing the cement foaming procedure for surfactant depending on its type and cement foamability.
The aims of this study were surfactant-assisted fabrication and evaluation of the highly porous chemically bonded alpha tricalcium phosphate (α-TCP) based cements designed for bone tissue engineering. The influence of three types of nonionic surfactants (Tween 20, Tween 80 and Tetronic 90R4) on the physicochemical and biological properties of foamed calcium phosphate cements (fCPCs) has been investigated. Firstly, surfactants concentrations over their critical micelle concentration (CMC) values in the presence of setting reaction accelerator – Na2HPO4, were specified. As produced fCPCs are intended for use in situ the non-cytotoxic concentration of surfactants was determined by indirect cellular cytotoxicity test performed with the use of osteoblast-like MG-63 cells. The next step was the evaluation of the surfactant's foamability depending on the foaming procedure applied. Based on the cytotoxicity and foamability results, the non-cytotoxic surfactant concentration and foaming procedure were optimised to produce fCPCs. Obtained fCPCs were then subjected to the physicochemical studies.
2. Experimental
2.1. Materials
The solid phase of obtained bone cements consisted of the highly reactive α-tricalcium phosphate (α-TCP). The initial α-TCP powder was synthesized by the wet chemical method, described previously.29 Briefly, for synthesis, Ca(OH)2 (MERCK, Germany) and 85% phosphate(V) acid (POCH, Poland), both with chemical purity, were used. The obtained powder was sieved to grains below 100 μm, sintered for 5 hours at 1300 °C, then milled in the attrition mill and sieved using #230 mesh (ASTM-E11). The specific surface area (SSA) of the initial α-TCP powder was 1.73 ± 0.01 m2 g−1. The liquid phase of the cements was aqueous 2% (w/v) Na2HPO4 solutions with addition of selected surfactants: Tween 20, Tween 80 and Tetronic 90R4 (all purchased from Sigma Aldrich, Germany) at a concentration of 10 g L−1 for the samples dedicated for cytotoxicity and foamability studies or 1.25 g L−1 for foamability and the rest of the physicochemical studies. Molecular weight of surfactants and molarity of the liquid phases used during the preparation of the cements are shown in Table 1.| Surfactant | Molecular weight [g mol−1] | Molarity [mol dm−3] |
|---|---|---|
| Tween 20 | 1228 | 0.0814 |
| Tween 80 | 1310 | 0.0763 |
| Tetronic 90R4 | 7200 | 0.0139 |
2.2. Preparation of foamed cements
Cements were obtained via two different procedures described in Section 2.3.3. The liquid to powder ratio (L/P) was 0.7 g g−1. The foaming step was performed with the use of domestic food mixer (BOMANN, Germany). In the same way, the control samples – without any surfactant, were obtained. Set and hardened cements were used for further research. The obtained materials are listed in Table 2.2.3. Methods
Extracts from cements produced with the use of different surfactants (fCTRL, fTW20_10, fTW80_10 and f90R4_10) were prepared according to ISO 10993-5 with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 sample to medium ratio (g mL1). The series of extract dilutions were made: 1 (undiluted), and diluted by a factor of 2, 4, 8, 16, 32 times.
10 sample to medium ratio (g mL1). The series of extract dilutions were made: 1 (undiluted), and diluted by a factor of 2, 4, 8, 16, 32 times.
MG-63 cells were seeded in 48 well plates at a concentration of 1 × 104 cells per well and after 24 hours medium was aspired and extracts (1 mL) were added (day 0); in such conditions cells were cultured up to 7 days.
Alamar Blue reagent was prepared by dissolving resazurin sodium salt in phosphate buffered saline (PBS, 0.1 mg mL−1, Sigma Aldrich). At predetermined time intervals (1, 3 and 7 days) 10% Alamar Blue reagent was added to the wells with MEM. After 3 h incubation, 100 μL aliquots were transferred into black 96 well plate. The fluorescence was measured at λex = 530 nm, λem = 590 nm using a microplate reader (FluoroSTAR Omega, BMG Labtech). The percentage of resazurin reduction was calculated according to the formula:31
| [(Fx − F0%)/(F100% − F0%)] × 100% |
The experiment was performed in six replicates for each type of cement. The statistical analyses of obtained data were done using a one-way analysis of variance (one-way ANOVA) followed by Tuckey's post-hoc test. The assumptions of normal distribution and equal variance were verified using the Shapiro–Wilk and Levene median test, respectively (p-value < 0.01). The analyses were performed using OriginPro 2021 software (OriginLab, Corp.). The results are presented as mean ± standard deviation. Test materials were considered as non-cytotoxic if the percent viability was ≥70% of the control medium MEM, following the ISO 10993-5 standard.32
For live/dead staining cells on days 1, 3 and 7 MEM was removed from wells and they were rinsed with PBS. Then cells were incubated in 0.1% calcein and 0.1% propidium iodide solution in PBS for 20 min in the dark and fluorescence microscopy (Axiovert 40 with HXP 120C Metal Halide Illuminator, Zeiss) pictures were taken. For hematoxylin/eosin staining cells on day 7 were stained with 200 μL per well of hematoxylin (3 minutes), rinsed with tap water, then the cells were stained with 200 μL per well of eosin (30 seconds) and rinsed again with tap water. To capture pictures of cells after hematoxylin-eosin staining on day 7 of culture digital optical microscope (Keyence VHX-900F) was used.
Foamability is often defined as the volume increase of a solution after the foaming process.23 In our study a comparative method, where the control cement paste possessed 100% foamability, was adopted.
Materials were foamed using two different procedures:
• Foaming of the liquid phase, adding the powder phase to the foamed liquid phase and mixing (method I)
• Mixing liquid phase with a powder phase and then foaming of the premixed cement paste (method II)
Studies were performed in polypropylene containers and foamability was measured according to the equation:
All measurements were done in triplicate and the results were rounded to the nearest multiple of 5%.
2.4. Statistics
Statistical analysis was done using one-way analysis of variance (ANOVA) and post hoc Tukey HSD multiple comparisons. Where n < 5, results were expressed as means ± standard deviation.3. Results
3.1. Critical micelle concentration
Surfactants concentrations over their CMC values in the presence of cement setting reaction accelerator – Na2HPO4, were specified. Surface tension values concerning studied surfactants' concentration are shown in Fig. 1. Critical micelle concentrations for Tween 20, Tween 80 and Tetronic 90R4 were approximately 250 mg L−1, 50 mg L−1 and 25 mg L−1, respectively. In this study, as a liquid phase, solutions with surfactant in higher concentrations than 1000 mg L−1 were applied.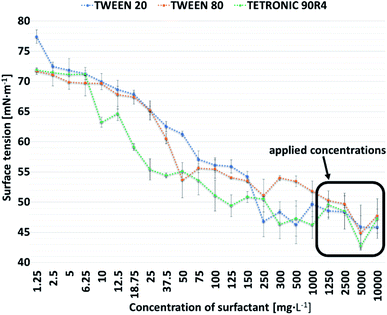 | ||
| Fig. 1 Dependence of surface tension on the concentration of tested surfactants in Na2HPO4 solution. | ||
3.2. Cytotoxicity studies
The non-cytotoxic concentration of surfactants was specified based on indirect cellular cytotoxicity tests with MG-63 cells. Viability of MG-63 cells determined by Alamar Blue reduction on days 1, 3 and 7 is shown in Fig. 2. Individual bars in the plot indicate the degree of dilution of the extracts (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 1
8, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16, 1
16, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32) prepared from materials fCTRL, fTW20_10, fTW80_10 and f90R4_10.
32) prepared from materials fCTRL, fTW20_10, fTW80_10 and f90R4_10.
On day one (Fig. 2A) cell viability for all investigated samples starting from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 dilution was at a similar level if compared with the control (cells cultured in medium MEM). For all these samples Alamar Blue reduction was about 20%. For undiluted extracts from fCTRL and f90R4 cell viability was rather high (58 and 59% of MEM, respectively), however for fTW20 and fTW80 it was close to zero, suggesting that probably all cells died. Even in 1
8 dilution was at a similar level if compared with the control (cells cultured in medium MEM). For all these samples Alamar Blue reduction was about 20%. For undiluted extracts from fCTRL and f90R4 cell viability was rather high (58 and 59% of MEM, respectively), however for fTW20 and fTW80 it was close to zero, suggesting that probably all cells died. Even in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dilution of extract from fTW20 no significant improvement was observed. The extract from fCTRL diluted by a factor of 1
2 dilution of extract from fTW20 no significant improvement was observed. The extract from fCTRL diluted by a factor of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 had no statistically significant effect on cell viability if compared with control medium MEM (p ≤ 0.01). Extracts from materials f90R4 and fTW80 were not cytotoxic at 1
2 had no statistically significant effect on cell viability if compared with control medium MEM (p ≤ 0.01). Extracts from materials f90R4 and fTW80 were not cytotoxic at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 dilution, while those from fTW20 at 1
4 dilution, while those from fTW20 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.
8.
On day 3 (Fig. 2B) Alamar Blue reduction increased for control MEM up to 45%, suggesting that the cells were in a good condition and were well proliferating. As regards the extracts from the cements similar trends as on day 1 were observed; again, undiluted extracts (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) from all examined samples were lethal for MG-63 cells. The lowest cytotoxicity similar to control MEM exhibited fCTRL diluted at 1
1) from all examined samples were lethal for MG-63 cells. The lowest cytotoxicity similar to control MEM exhibited fCTRL diluted at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 factor and all samples from fCTRL with higher dilution i.e.: 1
2 factor and all samples from fCTRL with higher dilution i.e.: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 1
8, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 and 1
16 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32. Lack of cytotoxicity for fTW20, fTW80 and f90R4 was observed if the extracts were diluted by a factor of 1
32. Lack of cytotoxicity for fTW20, fTW80 and f90R4 was observed if the extracts were diluted by a factor of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.
8.
On day 7 (Fig. 2C) viability of cells cultured in control conditions (MEM) and in all extracts diluted by a factor of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 excided 60%. It means that cells still proliferated and their number increased as compared to day 3, for which Alamar Blue reduction was 45%. Interestingly, viability of the cells cultured in extracts diluted by a factor of 1
32 excided 60%. It means that cells still proliferated and their number increased as compared to day 3, for which Alamar Blue reduction was 45%. Interestingly, viability of the cells cultured in extracts diluted by a factor of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and 1
8 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 was significantly lower, as compared to control.
16 was significantly lower, as compared to control.
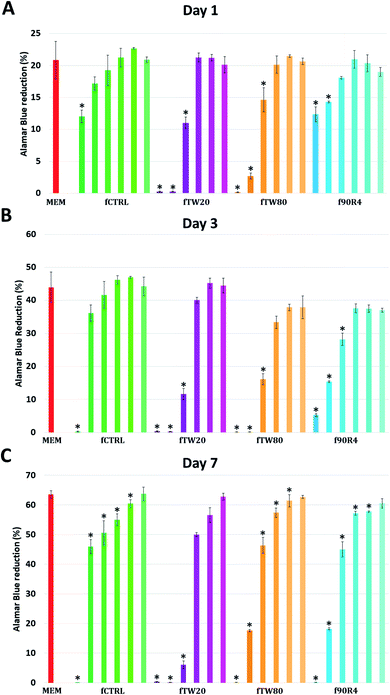
Live/dead staining was performed to provide additional information about cell viability and morphology. On day 1 trends observed in AlamarBlue® were confirmed; cells were growing well in fCTRL extracts, especially at dilution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and higher. For fTW80 and f90R4 good cell growth was observed starting from dilution 1
2 and higher. For fTW80 and f90R4 good cell growth was observed starting from dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, while for fTW20 starting from 1
4, while for fTW20 starting from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 dilution (ESI, Fig. S1†). Interestingly all cells were stained green, thus alive.
8 dilution (ESI, Fig. S1†). Interestingly all cells were stained green, thus alive.
On day 3 the same extract concentrations exhibited no harmful effect on cells; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for fCTRL, 1
2 for fCTRL, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for fTW80 and f90R4 and 1
4 for fTW80 and f90R4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 for fTW20. It is worth noting, that number of cells growing in pointed dilutions was comparatively high and most of the area was covered by a cell monolayer (Fig. 3).
8 for fTW20. It is worth noting, that number of cells growing in pointed dilutions was comparatively high and most of the area was covered by a cell monolayer (Fig. 3).
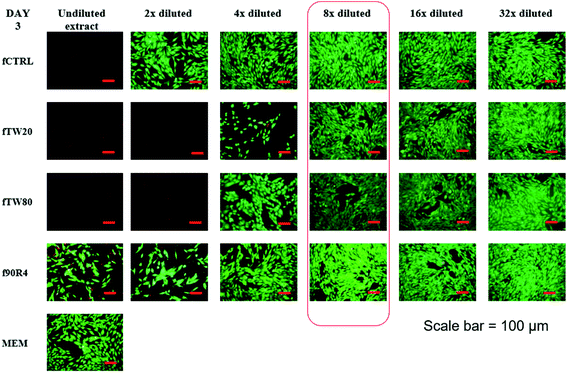
The same effect was observed on day 7 and the same dilutions seem to do not negatively impact on cell growth and proliferation (ESI, Fig. S2†). Results were confirmed also by hematoxylin/eosin staining of the cells on day 7 (Fig. 4), which show that for extracts from all the cements diluted by the factor of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, a dense cell monolayer was observed – the same as for cells growing in control MEM.
8, a dense cell monolayer was observed – the same as for cells growing in control MEM.
3.3. Foamability
Through foamability assessment, it was verified whether the lower concentration of surfactants in the liquid phase of cements affects the deterioration of their foaming properties. The foamabilities of cements in two different surfactant concentrations (10 g L−1 and 1.25 g L−1) were compared. What is more, two different foaming procedures (named method I and method II) were also compared. Foamability values of the cements are shown in Fig. 6A.It was noted, that the lowering of surfactant concentrations in liquid phases of the cements from 10 g L−1 to 1.25 g L−1 did not reduce their foaming potential. However, the foamability of the cements depended on the foaming procedure which was used during their preparation. The first procedure led to higher foamability of the obtained cements, apart from the samples with addition of Tetronic 90R4. From this point, the method I was used for fabrication of fCTRL, fTW20, fTW80, while method II was applied for f90R4. Taking into account the cytotoxicity studies outcome combined with the above results the physicochemical characterization was done for materials with 1.25 g L−1 concentration of surfactant in liquid phases of the cements.
3.4. Specific surface area
The SSA of pulverized studied cements were investigated. It can be noticed that in all cases SSAs were higher than in the case of the initial powder (1.73 ± 0.01 m2 g−1). The specified surface areas were 6.56 ± 0.13 and 7.74 ± 0.07 m2 g−1 for fTW80 and fTW20, respectively. The highest values were obtained for the control cement – fCTRL (11.66 ± 0.04 m2 g−1) and f90R4 (11.00 ± 0.04 m2 g−1).3.5. Phase composition
The powder X-ray diffractograms of the powdered samples after 7 days in air and 7 days in SBF are shown in Fig. 5. Phase composition (wt%) are collected in Table 3.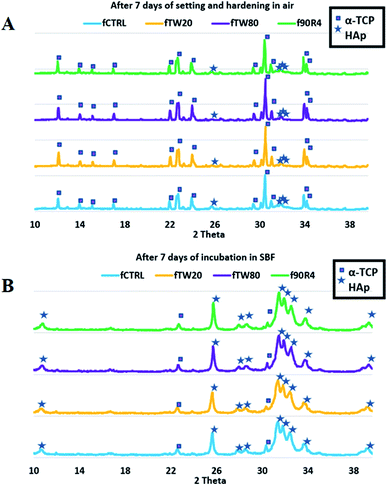 | ||
| Fig. 5 The powder X-ray diffractograms of the powdered cements fCTRL, fTW20, fTW80, f90R4 after 7 days in air (A) and 7 day incubation in SBF (B). | ||
| Sample | Conditions | α-TCP [wt%] | HAp [wt%] |
|---|---|---|---|
| fCTRL | After 7 days in air | 68 | 32 |
| After 7 days of incubation in SBF | 7 | 93 | |
| fTW20 | After 7 days in air | 87 | 13 |
| After 7 days of incubation in SBF | 7 | 93 | |
| fTW80 | After 7 days in air | 85 | 15 |
| After 7 days of incubation in SBF | 6 | 94 | |
| f90R4 | After 7 days in air | 73 | 27 |
| After 7 days of incubation in SBF | 5 | 95 |
The diffractograms of all prepared cements revealed the presence of reflections from two crystalline phases α-TCP and hydroxyapatite (HAp). After 7 days in air, cements fTW20 and fTW80 revealed approximately twofold lower amount of HAp phase than cement without any surfactant – fCTRL, meanwhile results for cement f90R4 was comparable to it. Polysorbates (Tween 20 and Tween 80) have influenced the process of α-TCP hydrolysis, decelerating it. After incubation in the simulated body fluid, the amount of HAp phase in all materials reached almost 100%, which confirms almost full hydrolysis of α-TCP.
3.6. Porosity
The porosity of the control cement without any surfactant addition reached 54.8 ± 0.5 vol%. For the f90R4 material, this value was slightly higher and equaled to 57.5 ± 0.8 vol%. Cements with the addition of polysorbates had porosity 72.9 ± 0.8 and 78.1 ± 2.3 vol%, for fTW20 and fTW80, respectively. Also, in the plot of the pore size diameter distribution differences were noticeable – sample f90R4 was similar to fCTRL and fTW20 was comparable to fTW80 (Fig. 6B). Pore size distributions for fCTRL (max at 0.22 and 1.45 μm) and f90R4 (max at 0.22 and 1.56 μm) were bimodal, while for fTW20 (max at 0.11, 1.68 and 91.52 μm) and fTW80 (max at 0.11, 1.67 and 141.17 μm) possessed trimodal behaviour.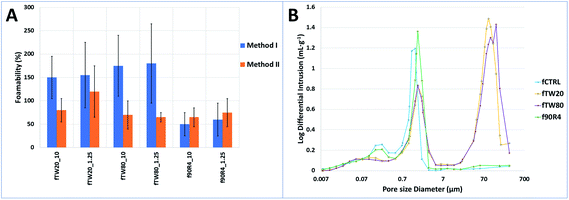 | ||
| Fig. 6 (A) Foamability of obtained cements in comparison with control cement (results were rounded to the nearest multiple of 5%). (B) Pore size diameter distribution of the studied materials. | ||
3.7. Compressive strength
Compressive strength of the foamed cements was relatively low due to their high porosity (>50 vol%) connected to the high L/P ratio of 0.7 g g−1 and foaming procedure. This parameter for the control material – fCTRL cement without surfactant was equal to 2.37 ± 0.50 MPa. The addition of surfactant further affected the compressive strength of the studied cements, which were 1.13 ± 0.35 MPa, 1.79 ± 0.48 MPa, and 1.43 ± 0.43 MPa for fTW20, fTW80 and f90R4, respectively (Fig. 7). Among cements with surfactants, the fTW80 material had the highest compressive strength.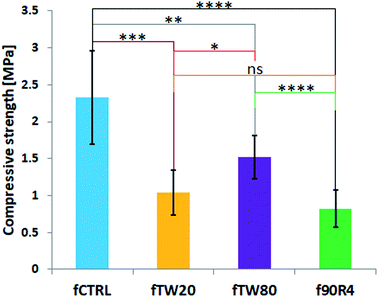 | ||
| Fig. 7 Compressive strength of the investigated materials. Asterisks indicate the statistical differences: ns – not significant, *p < 0.05, **p < 0.01, ***p < 0.001. | ||
The apparent densities of the examined materials were consistent with the compressive strength results and equaled to 1.06 ± 0.05, 0.90 ± 0.05, 1.00 ± 0.02, and 0.95 ± 0.06 g cm−3 for fCTRL, fTW20, fTW80 and f90R4, correspondingly.
3.8. Microstructure
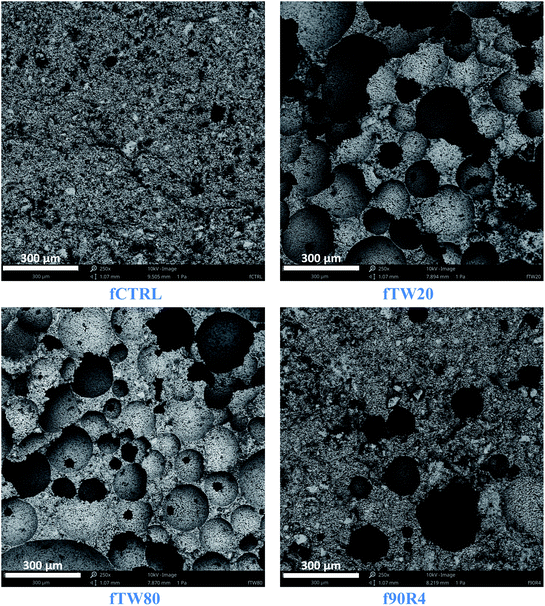
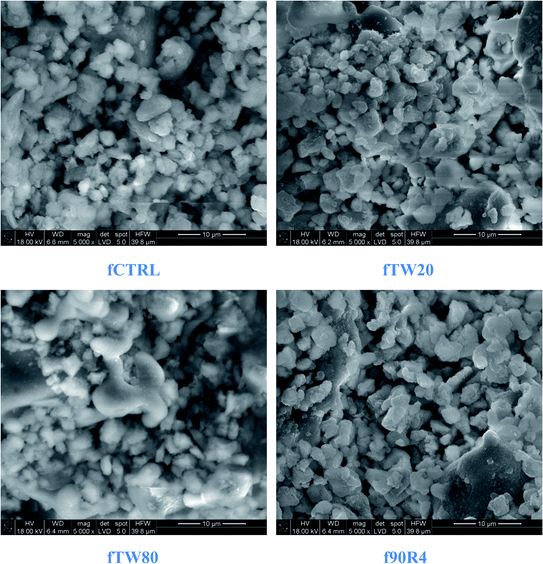
Observations of cement surfaces at low magnification performed by SEM showed the presence of multiple pores on the cements obtained by surfactant assisted process (Fig. 8). The numerous pores were visible on the surface of fTW20 and fTW80 materials. On the other hand, the pores in the f90R4 material were less frequent.The microstructure observations revealed that at higher magnifications the obtained cements did not differ significantly from each other. Their common feature was the blurred grain boundaries (Fig. 9).
3.9. Chemical stability and bioactivity studies in vitro
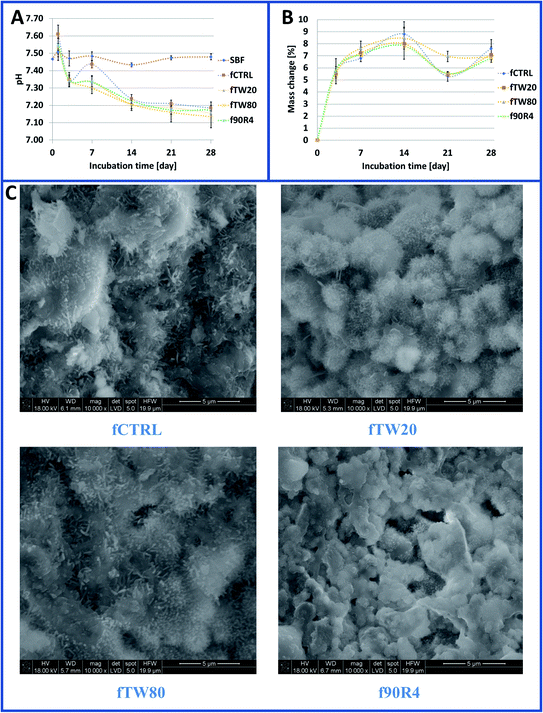
Investigated foamed cements were chemically stable in vitro. The tested materials during their incubation caused a slight decrease in pH of SBF (Fig. 10A). After 28 days pH of SBF where materials were incubated ranged from 7.09 to 7.19 (for the control at this point pH equaled to 7.48 ± 0.02). After the fourteenth day of incubation, the changes in pH were already negligible.After incubation in SBF, numerous apatite-like forms were visible (Fig. 10C). Surprisingly, apatite precipitates on the surface of SBF-treated samples that contained surfactant were less visible than on the control cement (fCTRL). However, at the cross-sections of the specimens, this difference was no longer visible. The high bioactivity of the obtained cements has also been confirmed by mass changes of the samples subjected to the immersion in SBF. After 28 days, the increase of weight of samples in SBF was about 7% (Fig. 10B).
4. Discussion
The bioactive, highly porous calcium phosphate bone cements obtained by the surfactant-assisted foaming process are a relatively new group of bone tissue substitutes.36 Such materials can mimic the architecture of cancellous bone tissue and due to their porosity, fCPCs are expected to be prosperous scaffolds for new regenerating bone tissue. They can be used as pre-set apatitic scaffolds with the α-TCP → CDHA (calcium deficient hydroxyapatite) transformation already completed,37 as bioceramic sinters with higher compressive strength38 or as non-injectable or injectable foamed cement pastes introduced into the cavity set in situ along with the release of the incorporated surfactant.The use of surfactants for bone cement preparation is a very difficult issue because it is necessary to focus on many properties of the surfactant itself such as: chemical character (ionic, nonionic, etc.), hydrophilic-lipophilic balance value, form (liquid, powder), solubility in water. Surfactant properties have a direct impact on the final material. The experiment should be planned concerning many aspects of the material application, for example, the method of its implantation. If the cement scaffold is placed in a defect, ready-made, sintered or previously incubated (even if in SBF), it is not necessary to worry about the influence of the foaming agent on cytotoxicity. In the case of a direct application of freshly-prepared cement paste to damaged bone tissue (by spatula or by injection), it is necessary to take into account the effects of each of its components, and thus also the surfactant, more precisely its physicochemical and biological properties.
In this study, as a liquid phase of the CPCs, 2% (w/v) aqueous solutions of Na2HPO4 with or without surfactant addition were used. The three different nonionic surfactants (Tween 20, Tween 80 or Tetronic 90R4) were added to the cement liquid phases in order to obtain highly porous, foamed CPCs. Surfactant-assisted foaming during the cement preparation leads to the pore formation in the material so it is extremely important to select a suitable surfactant with foaming activity.
The surfactant foaming activity depends on surface tension and its critical micelle concentration.39 Thus, it is important to indicate these values when working with surface active agents. According to the literature, the surface tension of the water/air interface is equal to 72.86 ± 0.05 mN m−1.40 Any substance added to the water may affect this property. Surface tension of 2% (w/v) aqueous solution of Na2HPO4, used as control liquid phase of the obtained CPCs, was 72.64 ± 0.40 mN m−1. It can be stated that the addition of this salt does not change the surface tension of pure water. The foamability of a surfactant solution is maximum at concentrations around and above its critical micelle concentration, therefore this value should be determined.41 Foam generation generally increases with surfactant concentration up to the CMC value above which surfactant concentration has a minor impact.42 As specified by a manufacturer CMC of Tween 20 is 60 mg L−1. In our study, when Tween 20 was added to Na2HPO4 solution, no major surface tension fluctuations, after reaching a Tween 20 concentration over 250 mg L−1, were noted. A similar trend was observed for Tween 80 which according to the manufacturer possess CMC equal to 13–15 mg L−1, while in Na2HPO4 solution, the plot of the correlation between surface tension and concentration began to flatten after the surfactant concentration exceeded 50 mg L−1. Tetronic 90R4 behaved no differently in Na2HPO4 solution in the same manner and had CMC over 25 mg L−1, whereas in distilled water according to Chen et al. 5 mg L−1.43 Taking into account the aforementioned results it can be stated that CMCs of the surfactants used in our study in Na2HPO4 solution are about 4 to 5 fold higher than in the distilled water. These results indicate that the determination of the surface active agent critical micelle concentration in the solution in which we intend to use it during the study (in our case in 2% (w/v) aqueous solution of Na2HPO4), should be carried out. The surface tension of all studied surfactants solutions did not change significantly if their concentration reached 250 mg L−1. In order to exceed the CMC of applied surfactants, as a liquid phase of the cements, solutions with surfactant concentrations higher than this value should be used.
The non-cytotoxic concentration of surfactants in the cements liquid phases was determined based on indirect cellular (MG-63) cytotoxicity tests. Alamar Blue test based on resazurin reduction is an important redox indicator that is used to evaluate metabolic function and cellular health.44 Our results have shown that even the control sample extract (α-TCP cement without any surfactant addition) was cytotoxic. It is suggested, that the cytotoxicity of the CPCs constituent – α-TCP results from the pH decrease of medium due to an increase of the phosphoric acid ion arising from α-TCP hydrolysis to CDHA.45,46 Decreasing of pH by α-TCP based cements was confirmed also in chemical stability studies in SBF. The pH drop results in a less favourable environment for cells.47 This phenomenon can be also caused by capturing of Ca2+ ions by α-TCP, described previously.48 The mechanism of “false cytotoxicity” of highly reactive calcium phosphates has been already explained49 – the decreased osteoblast viability tested in standard cytotoxicity assays may be the consequence not of toxin release but of a high capacity to ion-adsorption and removal of essential compounds from the medium. Twice diluted extract from fCTRL sample was already cytocompatible and it should serve as the correct reference sample (in addition to the MEM sample). The results of cell viability obtained on days 1 and 3 of culture suggest that “safe dilution” for all cements containing surfactants is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. This dilution corresponds to the surfactant concentration in the liquid phase of 1.25 g L−1. Although on day 7 cell viability was significantly lower for this surfactant dilution as compared to control, one should keep in mind that in tissue environment there is a constant exchange of fluids, so even harmful leached molecules can be neutralized by the cells and the concentration of the toxic substance locally decreases over time.50,51 It is also in line with standard recommendations, which consider materials cytocompatible if percent viability is ≥70% of the control.31 In our case cell viability already exceeded 70% of the control at 1
8. This dilution corresponds to the surfactant concentration in the liquid phase of 1.25 g L−1. Although on day 7 cell viability was significantly lower for this surfactant dilution as compared to control, one should keep in mind that in tissue environment there is a constant exchange of fluids, so even harmful leached molecules can be neutralized by the cells and the concentration of the toxic substance locally decreases over time.50,51 It is also in line with standard recommendations, which consider materials cytocompatible if percent viability is ≥70% of the control.31 In our case cell viability already exceeded 70% of the control at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dilution for fCTRL, at 1
2 dilution for fCTRL, at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 dilution for f90R4 and fTW80 whereas at 1
4 dilution for f90R4 and fTW80 whereas at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 for fTW20. Eight times diluted materials extracts with the addition of surfactants were already nearly cytocompatible if compared with fCTRL or MEM. This dilution corresponded to 1.25 g L−1 of each surfactant in 2% (w/v) Na2HPO4 solution which can be used as liquid phases for preparation of non-cytotoxic cements. It has been noticed that cytotoxicity depends not so much on the surfactant content in the liquid phase as on its molarity (each of the surfactants used in the study is characterized by a different molecular weight – Table 1). The higher molarity and thus the increased number of surfactant molecules, the higher cytotoxicity of the cements containing it. Moreover, often cell studies of foamed cements were performed on samples that have already been incubated, i.e., with a complete α-TCP → CDHA transformation.52 In our case, to highlight the effect of surfactant on cytotoxicity this step was omitted.
8 for fTW20. Eight times diluted materials extracts with the addition of surfactants were already nearly cytocompatible if compared with fCTRL or MEM. This dilution corresponded to 1.25 g L−1 of each surfactant in 2% (w/v) Na2HPO4 solution which can be used as liquid phases for preparation of non-cytotoxic cements. It has been noticed that cytotoxicity depends not so much on the surfactant content in the liquid phase as on its molarity (each of the surfactants used in the study is characterized by a different molecular weight – Table 1). The higher molarity and thus the increased number of surfactant molecules, the higher cytotoxicity of the cements containing it. Moreover, often cell studies of foamed cements were performed on samples that have already been incubated, i.e., with a complete α-TCP → CDHA transformation.52 In our case, to highlight the effect of surfactant on cytotoxicity this step was omitted.
Through cements' foamability assessment, it was verified whether the lower concentration of surfactants in the cements liquid phases selected according to the cytotoxicity studies results affects the deterioration of their foaming properties. The foamability studies have shown that the surfactant concentration (10 g L−1 or 1.25 g L−1) was irrelevant. The alterations between foamed cements with different surfactant concentrations were caused rather by a particular example of produced material – in principle, the amount of air that was enclosed in the sample during mixing, as evidenced by high standard deviations. Looking at the results of cement foamability, it is difficult to design it without controlling the number of air bubbles produced by the mixing surfactant solution. One of the possible approaches already described by Zhang et al. is syringe-foaming, where the previously measured amount of air in one syringe is introduced into the cement paste in the second one.24 As cement foamability and the porosity of the final material can be linked, the foaming procedure was chosen on the basis of the foamability highest values. The method I was used for fabrication of fCTRL, fTW20, fTW80, while method II was applied for f90R4 cement. To conclude, the foaming procedure should be matched individually for each surfactant.
Considering the cytotoxicity studies outcomes combined with the foamability assessment the physicochemical characterization was done for materials where surfactant concentration in cements liquid phases equaled to 1.25 g L−1.
The diffractograms of all prepared cements revealed the presence of reflections from two crystalline phases α-TCP and hydroxyapatite (HAp). What is more, the level of α-TCP hydrolysis can be correlated with SSA results – a higher amount of HAp phase implies larger SSA. It should be pointed out that Tweens have influenced the process of α-TCP hydrolysis, decelerating it. This can be caused by the presence of numerous air bubbles in fTW20 and fTW80. The amount of HAp phase in all materials reached almost 100% after 7 day incubation in SBF, confirming almost full hydrolysis of α-TCP.
An extremely important feature of bone substitutes is its open porosity, which enables the migration of nutrients and cells. Highly porous materials for bone tissue defects treatment and bone tissue engineering are assumed to have an open porosity of over 50 vol% (ref. 53) what corresponds to the porosity of spongy bone (50–90 vol%).54 In this study, the objective of the high porosity was achieved, especially for the cements with the addition of polysorbates which possessed porosity over 70 vol%. The porosity of the control cement without any surfactant addition reached about 55 vol% whereas the f90R4 material near to 58 vol%.
The mechanical strength of bone varies depending on its type. Compressive strength of cortical bone is in the range of 100–200 MPa, whereas for spongy bone 2–20 MPa (ref. 55) (in some sources 0.1–16 MPa (ref. 56 and 57)). The compressive strength of the materials with surfactant addition ranged from 0.80 to 2.30 MPa (fTW80 material possessed the highest one) whereas for the control cement reached up to 2.90 MPa. Low values were caused by the high porosity of the materials which synergistically resulted from the high L/P ratio, foaming step as well as the presence of surfactants. Nevertheless, the obtained results correspond to the compressive strength of the cancellous bone. In order to achieve the wider application potential of the foamed cements, it would be necessary to increase their compressive strength, for example by modifying the foaming method or adding other components to the mixture such as polymers (e.g. polysaccharides) that could strengthen their final microstructure. The L/P ratio can also be reduced up to a certain value as an excessively low L/P ratio will at the same time prevent the cement from foaming.
According to SEM observations, the presence of numerous pores on the cements surfaces was noticed. Pores on the f90R4 surface appeared less frequently. This would explain the relatively low open porosity obtained in the mercury intrusion porosimetry method for this material. At high magnification, no differences in the microstructure of the materials after setting and hardening were visualised.
The tested materials were chemically stable. Their incubation in SBF caused a slight decrease in its pH. The measured pH values were close to the physiological one. What is more, obtained fCPCs possessed high bioactive potential which was confirmed by about a 7% mass increase of samples after 28 days of incubation in SBF. Conventional CPCs usually experience a mass increase as a result of α-TCP hydrolysis and the formation of apatite on the surface of the samples.58 Other confirmation of high bioactive potential was the presence of numerous apatite forms on the materials surfaces after 7 day incubation in SBF. However, their occurrence and morphology are different for surfactant-containing cements against the fCTRL material. The presence of surfactants releasing from cements into SBF affects the apatite forms on the samples' surfaces. On the surfaces of cements with surfactants oval-shaped crystals over plate- or needle-like morphologies were dominant.
In order to have a total view on the behaviour of the obtained fCPCs in aqueous media, degradation process of materials (based on changes of ionic concentration during the incubation of the cements in SBF or MEM), occurring along with precipitation of apatite, should be studied. This query may be the subject of future research regarding fCPCs.
Our results indicate that type and surfactant concentration as well as the foaming procedure are crucial in the fabrication of macroporous calcium phosphate cements.
5. Conclusions
In this study, highly porous CPCs have been successfully obtained by surfactant-assisted process and their properties were investigated. We focused on the determination of surfactants (Tween 20, Tween 80 and Tetronic 90R4) critical micelle concentration, cytotoxicity depending on its amount in a liquid phase of the cement and the influence of the foaming procedure on the cement foamability, i.e. indirectly the porosity of the final materials.According to the CMC results we established that solutions with surfactant concentrations at least higher than 250 mg L−1 should be used as a liquid phase of the cements. Cytotoxicity studies showed that among used surfactants, Tween 20 was the most cytotoxic whereas the least Tetronic 90R4. We agreed that 1.25 g L−1 addition of studied surfactants into the liquid phase of cement is a “safe” concentration for MG-63 cells. What is more, applied surface active agents in such concentration in a liquid phase provide sufficient foaming ability which is extremely important for the preparation of fCPCs. The foamability of the cements depends on the foaming procedure which is used during their preparation. We established that the foaming procedure should be matched individually for each surfactant. Phase composition of tested materials revealed the presence of two crystalline phases α-TCP and hydroxyapatite (HAp). If polysorbates were added to the liquid phase the hydrolysis slowed down. The obtained cements after a week of incubation in SBF were almost completely hydrolysed. Cements with the addition of Tween 20 or Tween 80 possessed open porosity over 70 vol%. The material with an addition of Tetronic 90R4 had open porosity near to 58 vol%. Compressive strength of the materials with surfactant addition ranged from 0.80 to 2.30 MPa (the highest one possessed fTW80 material). All studied materials were chemically stable in vitro and show only a subtle pH decrease during 28 day incubation in SBF. After 7 days of incubation in SBF, numerous apatitic forms on the materials' surfaces can be observed, indicating their bioactive potential.
In conclusion, a holistic glance not only at the properties of the material itself but also at the characteristics of the applied surfactant is essential in order to achieve the best possible outcomes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the National Science Centre, Poland (Project No. 2017/27/N/ST8/00913), by the subvention from the Ministry of Science and Higher Education (Poland) for Faculty of Materials Science and Ceramics AGH UST – University of Science and Technology, Krakow, Poland, Project No. 16.16.160.557 (2021) and partially by the Program “Excellent Initiative – Research University” for the AGH University of Science and Technology. E. C. acknowledges financial support from the National Science Centre, Poland under Doctoral Scholarship No. 2019/32/T/ST5/00207.References
- L. L. Hench and J. Wilson, Science, 1984, 226, 630–636 CrossRef CAS PubMed.
- W. Wang and K. W. K. Yeung, Bioact. Mater., 2017, 2, 224–247 CrossRef.
- A. Sugawara, K. Asaoka and S.-J. Ding, J. Mater. Chem. B, 2013, 1, 1081–1089 RSC.
- A. Scarano, F. Lorusso, P. Santos de Oliveira, S. Kunjalukkal Padmanabhan and A. Licciulli, Materials, 2019, 12, 3079 CrossRef CAS.
- W. T. Barbosa, K. V. de Almeida, G. G. de Lima, M. A. Rodriguez, M. V. Lia Fook, R. García-Carrodeguas, V. Amaro da Silva Junior, F. A. de Sousa Segundo and M. J. C. de Sá, J. Biomed. Mater. Res., Part B, 2020, 108, 1107–1116 CrossRef CAS.
- E. Cichoń, K. Haraźna, S. Skibiński, T. Witko, A. Zima, A. Ślósarczyk, M. Zimowska, M. Witko, B. Leszczyński, A. Wróbel and M. Guzik, J. Mech. Behav. Biomed. Mater., 2019, 98, 235–245 CrossRef.
- S. Skibiński, E. Cichoń, K. Haraźna, E. Marcello, I. Roy, M. Witko, A. Ślósarczyk, J. Czechowska, M. Guzik and A. Zima, Ceram. Int., 2021, 47, 3876–3883 CrossRef.
- I. Lodoso-Torrecilla, F. Stumpel, J. A. Jansen and J. J. J. P. van den Beucken, Mater. Today Commun., 2020, 23, 100901 CrossRef CAS.
- M. Sladkova, M. Palmer, C. Öhman, J. Cheng, S. Al-Ansari, M. Saad, H. Engqvist and G. M. de Peppo, J. Tissue Eng. Regener. Med., 2018, 12, 715–726 CrossRef CAS.
- H. H. K. Xu, M. D. Weir, E. F. Burguera and A. M. Fraser, Biomaterials, 2006, 27, 4279–4287 CrossRef CAS PubMed.
- E. C. Grosfeld, B. T. Smith, M. Santoro, I. Lodoso-Torrecilla, J. A. Jansen, D. J. Ulrich, A. J. Melchiorri, D. W. Scott, A. G. Mikos and J. J. J. P. van den Beucken, Biomed. Mater., 2020, 15, 025002 CrossRef CAS PubMed.
- B. T. Smith, M. Santoro, E. C. Grosfeld, S. R. Shah, J. J. J. P. van den Beucken, J. A. Jansen and A. G. Mikos, Acta Biomater., 2017, 50, 68–77 CrossRef CAS PubMed.
- K. Sariibrahimoglu, J. An, B. A. J. A. van Oirschot, A. W. G. Nijhuis, R. M. Eman, J. Alblas, J. G. C. Wolke, J. J. J. P. van den Beucken, S. C. G. Leeuwenburgh and J. A. Jansen, Tissue Eng., Part A, 2014, 20, 2870–2882 CrossRef CAS PubMed.
- D. Pastorino, C. Canal and M.-P. Ginebra, Acta Biomater., 2015, 12, 250–259 CrossRef CAS PubMed.
- S. Hesaraki, F. Moztarzadeh and M. Solati-Hashjin, J. Biomed. Mater. Res., Part B, 2006, 79, 203–209 CrossRef.
- S. Hesaraki, A. Zamanian and F. Moztarzadeh, J. Biomed. Mater. Res., Part B, 2008, 86, 208–216 CrossRef.
- A. Bercier, S. Gonçalves, O. Lignon and J. Fitremann, Materials, 2010, 3, 4695–4709 CrossRef PubMed.
- M. Colilla, in Bio-Ceramics with Clinical Applications, John Wiley & Sons, Ltd, Chichester, UK, 2014, pp. 109–151 Search PubMed.
- R. G. Horn, J. Am. Ceram. Soc., 1990, 73, 1117–1135 CrossRef CAS.
- M. Mishra, P. Muthuprasanna, K. S. Prabha, P. S. Rani, A. S. Babu, I. S. Chandiran, G. Arunachalam and S. Shalini, Int. J. PharmTech Res., 2009, 1, 1354 CAS.
- L. L. Schramm, Emulsions, foams, and suspensions: Fundamentals and applications, Wiley-VCH Verlag, Weinheim, Germany, 2006 Search PubMed.
- S. Heinemann, S. Rössler, M. Lemm, M. Ruhnow and B. Nies, Acta Biomater., 2013, 9, 6199–6207 CrossRef CAS PubMed.
- A. F. Vásquez, S. Domínguez and L. A. Loureiro Dos Santos, Mater. Sci. Eng., C, 2017, 81, 148–155 CrossRef PubMed.
- J. Zhang, W. Liu, O. Gauthier, S. Sourice, P. Pilet, G. Rethore, K. Khairoun, J.-M. Bouler, F. Tancret and P. Weiss, Acta Biomater., 2016, 31, 326–338 CrossRef CAS.
- J. Unosson, E. B. Montufar, H. Engqvist, M.-P. Ginebra and C. Persson, J. Biomed. Mater. Res., Part B, 2016, 104, 67–77 CrossRef CAS.
- E. Vorndran, M. Geffers, A. Ewald, M. Lemm, B. Nies and U. Gbureck, Acta Biomater., 2013, 9, 9558–9567 CrossRef CAS.
- S. Hesaraki and R. Nemati, J. Biomed. Mater. Res., Part B, 2009, 89, 342–352 CrossRef.
- E. Cichoń, A. Ślósarczyk and A. Zima, Langmuir, 2019, 35, 13656–13662 CrossRef PubMed.
- J. Czechowska, A. Zima, D. Siek and A. Ślósarczyk, Ceram. Int., 2018, 44, 6533–6540 CrossRef CAS.
- J. D. Berry, M. J. Neeson, R. R. Dagastine, D. Y. C. Chan and R. F. Tabor, J. Colloid Interface Sci., 2015, 454, 226–237 CrossRef CAS.
- A. E. Markaki, AlamarBlue Assay for Assessment of Cell Proliferation using the FLUOstar OPTIMA, BMG Lab Tech., Offenburg, 2009 Search PubMed.
- ISO 10993-5:2009(en) biological evaluation of medical devices — Part 5: tests for in vitro cytotoxicity.
- N. Doebelin and R. Kleeberg, J. Appl. Crystallogr., 2015, 48, 1573–1580 CrossRef CAS PubMed.
- J. Bergmann, P. Friedel and R. Kleeberg, CPD Newsletter, 1998, 20, 5 Search PubMed.
- T. Kokubo and H. Takadama, Biomaterials, 2006, 27, 2907–2915 CrossRef CAS.
- M. P. Ginebra, M. Espanol, E. B. Montufar, R. A. Perez and G. Mestres, Acta Biomater., 2010, 6, 2863–2873 CrossRef CAS PubMed.
- A. Barba, A. Diez-Escudero, Y. Maazouz, K. Rappe, M. Espanol, E. B. Montufar, M. Bonany, J. M. Sadowska, J. Guillem-Marti, C. Öhman-Mägi, C. Persson, M.-C. Manzanares, J. Franch and M.-P. Ginebra, ACS Appl. Mater. Interfaces, 2017, 9, 41722–41736 CrossRef CAS PubMed.
- E. B. Montufar, C. Gil, T. Traykova, M. P. Ginebra and J. A. Planell, Key Eng. Mater., 2008, 361, 323–326 Search PubMed.
- M. H. Amaral, J. das Neves, Â. Z. Oliveira and M. F. Bahia, J. Surfactants Deterg., 2008, 11, 275–278 CrossRef CAS.
- N. R. Pallas and Y. Harrison, Colloids Surf., 1990, 43, 169–194 CrossRef CAS.
- K. Shinoda, T. Yamaguchi and R. Hori, Bull. Chem. Soc. Jpn., 1961, 34, 237–241 CrossRef CAS.
- J. C. Chiang, S. K. Sawyal, L. M. Castanier, W. E. Brigham and A. Sufi, SPE California Regional Meeting, Society of Petroleum Engineers, 1980 Search PubMed.
- Y. Chen, T. Liu, G. Xu, J. Zhang, X. Zhai, J. Yuan and Y. Tan, Colloid Polym. Sci., 2015, 293, 97–107 CrossRef CAS.
- S. N. Rampersad, Sensors, 2012, 12, 12347–12360 CrossRef CAS.
- M. Tamai, R. Nakaoka and T. Tsuchiya, Key Eng. Mater., 2006, 309–311, 263–266 CAS.
- E. Fernandez, F. J. Gil, M. P. Ginebra, F. C. M. Driessens, J. A. Planell and S. M. Best, J. Mater. Sci.: Mater. Med., 1999, 10, 223–230 CrossRef CAS PubMed.
- A. M. Galow, A. Rebl, D. Koczan, S. M. Bonk, W. Baumann and J. Gimsa, Biochem. Biophys. Rep., 2017, 10, 17–25 Search PubMed.
- A. Zima, J. Czechowska, T. Szponder and A. Ślósarczyk, J. Biomed. Mater. Res., Part A, 2020, 108, 1243–1255 CrossRef CAS PubMed.
- K. Klimek, A. Belcarz, R. Pazik, P. Sobierajska, T. Han, R. J. Wiglusz and G. Ginalska, Mater. Sci. Eng., C, 2016, 65, 70–79 CrossRef CAS PubMed.
- A. Sahlin-Platt, Doctoral dissertation, Umeå universitet, 2011.
- A. Barradas, H. Yuan, C. van Blitterswijk and P. Habibovic, Eur. Cells Mater., 2011, 21, 407–429 CrossRef CAS PubMed.
- E. B. Montufar, T. Traykova, C. Gil, I. Harr, A. Almirall, A. Aguirre, E. Engel, J. A. Planell and M. P. Ginebra, Acta Biomater., 2010, 6, 876–885 CrossRef CAS PubMed.
- V. Karageorgiou and D. Kaplan, Biomaterials, 2005, 26, 5474–5491 CrossRef CAS PubMed.
- L. C. Gerhardt and A. R. Boccaccini, Materials, 2010, 3, 3867–3910 CrossRef CAS PubMed.
- M. J. Olszta, X. Cheng, S. S. Jee, R. Kumar, Y.-Y. Kim, M. J. Kaufman, E. P. Douglas and L. B. Gower, Mater. Sci. Eng., R, 2007, 58, 77–116 CrossRef.
- C. J. Hernandez, G. S. Beaupré, T. S. Keller and D. R. Carter, Bone, 2001, 29, 74–78 CrossRef CAS.
- T. M. Keaveny, E. F. Morgan, G. L. Niebur and O. C. Yeh, Annu. Rev. Biomed. Eng., 2001, 3, 307–333 CrossRef CAS PubMed.
- K. Ishikawa, S. Takagi, L. C. Chow, Y. Ishikawa, E. D. Eanes and K. Asaoka, Dent. Mater., 1994, 10, 26–32 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra04266a |
| This journal is © The Royal Society of Chemistry 2021 |