Open Access Article
Open Access ArticlePreparation of hybrid paper electrode based on hexagonal boron nitride integrated graphene nanocomposite for free-standing flexible supercapacitors†
Jerome Rajendrana,
Anatoly N. Reshetilovb and
Ashok K. Sundramoorthy *a
*a
aDepartment of Chemistry, SRM Institute of Science and Technology, Kattankulathur-603 203, Tamil Nadu, India. E-mail: ashokkus@srmist.edu.in
bG.K. Skryabin Institute of Biochemistry and Physiology of Microorganisms of the Russian Academy of Sciences (IBPM RAS), Subdivision of “Federal Research Center Pushchino Biological Research Center of the Russian Academy of Sciences” (FRC PBRC RAS), 142290, Pushchino, Moscow oblast, Russia
First published on 15th January 2021
Abstract
Flexible energy storage devices have received great interest due to the increasing demand for wearable and flexible electronic devices with high-power energy sources. Herein, a novel hybrid flexible hexagonal boron nitride integrated graphene paper (BN/GrP) is fabricated from 2D hexagonal boron nitride (h-BN) nanosheets integrated with graphene sheets dispersion via a simple vacuum filtration method. FE-SEM indicated that layered graphene nanosheets tightly confined with h-BN nanosheets. Further, the Raman spectroscopy confirmed successful integration of BN with graphene. As-prepared BN/GrP free-standing flexible conductive paper showed high electrical conductivity of 5.36 × 104 S m−1 with the sheet resistance of 8.87 Ω sq−1. However, after 1000 continuous bending cycles, the BN/GrP sheet resistance increased just about 8.7% which indicated good flexibility of the paper. Furthermore, as-prepared BN/GrP showed excellent specific capacitance of 321.95 F g−1 at current density of 0.5 A g−1. In addition, the power and energy densities were obtained as 3588.3 W kg−1, and 44.7 W h kg−1, respectively. The stability of the prepared flexible electrode was tested in galvanostatic charge/discharge cycles, where the results showed the 96.3% retention even after 6000 cycles. These results exhibited that the proposed BN/GrP may be useful to prepare flexible energy-storage systems.
Introduction
Over the past decade, significant research progress has been made to prepare flexible supercapacitors (SCs) for smart and efficient energy-storage devices because of their remarkable applications in wearable and miniaturized electronic devices.1,2 So far, majority of the flexible SCs have been fabricated using two dimensional (2D) layered materials such as graphene,3,4 molybdenum disulfide (MoS2),5 hexagonal boron nitride (h-BN),6 cobalt selenide (CoSe2),7 tin oxide (SnO2)8,9 and tungsten disulfide (WS2).10 These 2D layered materials have offered excellent chemical and anisotropic properties due of their interconnected crystalline arrangements.11–15 Similarly, traditional supercapacitors have also been prepared using carbon-based materials and MoS2.16–20 Although, significant research progress has been made with various carbon-based materials, the emphasis on graphene and h-BN nanosheets based hybrid material is growing due to their high thermal conductivity, mechanical strength, optical transparency, flexibility, thermal stability (up to 1000 °C), chemical tolerance (for protective coatings) and so on.11,14,15,21–30 Therefore, researchers have been exploited these materials in various research fields.2D-nanosheets (examples: graphene and h-BN) could be used to form various morphologies such as nanotubes and layer-by-layer structures. In recent times, various strategies have been reported to prepare nanosheets by adopting either top-down or bottom-up methods.31,32 Epitaxial growth and chemical vapour deposition (CVD) are well known bottom-up techniques,33,34 which suffers from limited scaling up and expensive production cost; nevertheless, it offers high-quality defect-free sheets. Similarly, top-down techniques35,36 such as mechanical cleavage, electrochemical and liquid phase exfoliation could be utilized to prepare the scalable high-quality defect-free sheets at a much lower cost using bulk h-BN and graphite sheet. Furthermore, these 2D-nanosheets (graphene, h-BN, MoS2 and WS2) have been combined to prepare flexible paper,37 foam38 and film39 through several methods such as mechanical pressuring of 2D-nanosheets aerogels40,41 or hydrogels,42 vacuum filtration3,4 and drying on flexible substrates.39 As prepared, free-standing 2D-nanosheets based papers had shown great mechanical strength, low density and high electrical conductivity,43,44 which can be applied in biosensors, actuators, shape memory devices and reliable source of energy storage, such as batteries and supercapacitors (SCs).36,45 However, there are some vital challenges in developing flexible SCs. For examples, (i) requirement of flexible electrode materials with high efficiency, (ii) enhanced interface performance between electrolytes and electrodes, and (iii) simple device preparation process.2 So, the development of highly efficient flexible SCs are required as an alternative energy source at low cost. Recently, graphene-based hybrid films have received more attention in developing flexible electrodes. Liu et al.,46 reported rGO film, which had the capacity of 81.7 F g−1 at 10 mV s−1. Freeze-drying method47 was used to prepare graphene paper as an electrode material with the capacity of 172 F g−1 at 1 A g−1. Sadak et al.,4 have also used the electrochemical deposition method to prepare GrP/MnO2 composite with good super-capacitive performance.
In this work, graphene nanosheet dispersion is prepared by electrochemical exfoliation technique which may depends on the intercalation of ions/molecules with graphite layers in an electrolytic cell where graphite sheets are used as electrodes.48,49 Similarly, the dispersion of h-BN nanosheets is prepared by a liquid phase exfoliation that yielded a significant amount of h-BN nanosheets. Finally, using graphene and h-BN nanosheet co-dispersion, a binary hybrid flexible paper (BN/GrP) is obtained by vacuum filtration (Scheme S1†). Field emission scanning electron microscopy (FE-SEM) and Raman spectroscopy results confirmed that h-BN homogeneously integrated with graphene. Moreover, the BN/GrP paper electrode exhibited high specific capacitance of 321.95 F g−1 at 0.5 A g−1 current density in 1 M H2SO4 and stable charging/discharging cycle stability up to 6000 cycles.
Experimental
Materials
Graphite sheets are obtained from Graphite Shop, Inc. (USA). Hexagonal boron nitride (h-BN) was purchased from Sigma-Aldrich, India. Sodium phosphate dibasic heptahydrate (Na2HPO4·7H2O) and sodium dihydrogen phosphate monohydrate (H2NaPO4·H2O) were purchased from Spectrochem Pvt, Ltd, India. Potassium chloride (KCl) was purchased from Fisher Scientific, India. All chemicals were used without further any purification. Distilled water (18.2 MΩ cm at room temperature) was collected from a Millipore ultrapure water system.Characterizations
The microstructure and morphological features of the BN/GrP were characterized by field emission scanning electron microscope (FESEM-EDS) (Quanta 400 FEG, FEI). Raman spectroscopy was performed at ambient condition using LabRAM HR evolution, (Horiba). X-ray diffraction data was acquired (XRD) (X'Pert Powder XRD system, Malvern Panalytical, United Kingdom) with Cu-Kα radiation (λ = 0.15406 nm) at 45 kV (tension) and 40 mA (current) with a 0.02° per step scan at 1° per min speed. Sheet resistance (Rs) was calculated by a two-point probe station (Keithley 2612B, USA) using the following equation,3
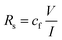 | (1) |
An electrochemical workstation CHI-760E (CH Instrument, USA) was used to perform the electrochemical measurements with platinum wire and Ag/AgCl (3 M KCl) as counter and reference electrodes with a three-electrode system. BN/GrP (freestanding film) was used as a working electrode. The galvanostatic charge/discharge (GCD) and cyclic voltammetry (CV) measurements were carried out with 1 M H2SO4. The working BN/GrP electrode is fabricated as reported elsewhere.3,4 Briefly, peeled BN/graphene paper (BN/GrP) was attached to a polyethylene terephthalate (PET) substrate using double-sided carbon tape to allow optimal electrical contact using an alligator clip with a copper tape (Fig. S1†).
The specific capacitance (Cp, F g−1) of our proposed flexible paper was calculated by the following equation, (galvanostatic charge/discharge curves):50–52
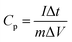 | (2) |
According to the following equations, energy density (E) and power density (P) were determined:50,53–55
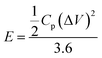 | (3) |
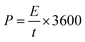 | (4) |
Preparation of h-BN/graphene nanocomposite dispersion
In a typical experiment, the hybrid h-BN/graphene nanosheet dispersion was prepared according to our previously reported procedure.29 Briefly, 500 mg of h-BN powder was dispersed through bath-sonication in 0.1 M of phosphate-buffer solution (PBS) (pH 7), followed by tip sonication for about 2 h. Further, the above prepared h-BN nanosheets dispersion was centrifuged for 40 min at 4000 rpm. Then, top one third portion was collected and washed with water several times to remove PBS. Then, fresh distilled water was added into BN nanosheets to re-disperse it. This step was repeated 3–4 times to remove all sodium salts and anions from the BN. Finally, h-BN nanosheets are re-dispersed in distilled water and the concentration of BN was maintained as 3 mg mL−1.On the other hand, graphene dispersion was synthesized via simple electrochemical exfoliation method.3,4 Initially, the graphite sheets (40 mm × 8 mm) were employed as both cathode and anode electrodes which were placed in 10 mL of 0.1 M PBS (pH 7) and an applied voltage of 10 V was supplied for 30 min. The obtained graphene sheets are collected and washed with water. After that, it was re-dispersed in distilled water using tip sonication. The graphene dispersion was then centrifuged for 40 min at 4000 rpm and the resulted graphene suspension (5 mg mL−1) was collected from the top supernatant (∼70%) solution. Furthermore, BN and graphene dispersions were mixed in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and continuously stirred for 2 h using a magnetic stirrer at RT, followed by tip sonication for 1 h. The resultant BN/graphene dispersion was utilized for the preparation of flexible paper.
9 and continuously stirred for 2 h using a magnetic stirrer at RT, followed by tip sonication for 1 h. The resultant BN/graphene dispersion was utilized for the preparation of flexible paper.
Preparation of BN/graphene paper (BN/GrP)
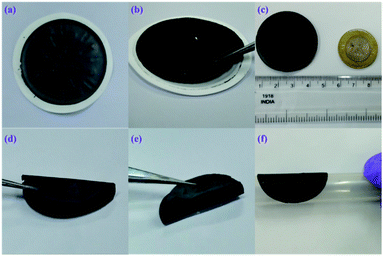
As illustrated in Scheme S1,† BN/GrP was prepared by a vacuum filtration method. Briefly, 3 mL of BN/graphene (4.5 mg mL−1) dispersion was mixed with 75 mL of water and then 1 M of H2SO4 was used to change the pH to ∼3.5. After that, BN/graphene dispersion was filtered through cellulose nitrate membrane filter paper (pore size = 0.45 μm) and naturally dried for overnight as shown in Fig. 1a. Finally, it was possible to peel off the BN/GrP from the filter paper (Fig. 1b).Results and discussion
SEM-EDS analysis
The prepared flexible BN/GrP was mechanically stable and it can be easily bent or/cut into preferred shape as shown in Fig. 1a–f. It is also possible to control the thickness of BN/graphene paper (BN/GrP) by adjusting the concentration and volume of the dispersion. The cross-sectional view of the BN/GrP was recorded by FE-SEM, which showed the thickness of ∼21 μm (4.5 mg mL−1). As observed, BN/GrP is made of tightly well stacked and interconnected layered sheets (Fig. 2a and b). In addition, the energy dispersive X-ray spectroscopy (EDS) mapping pattern (Fig. S2†) supported the elementary composition of BN/GrP, which clearly showed the uniformity of boron and nitrogen along with carbon and oxygen presence over the entire BN/GrP region. EDS has also confirmed that B (21.73%), N (26.55%), C (40.02%) and O (11.70%) were present in the respective atomic percentages in the BN/GrP as shown in Fig. S3.†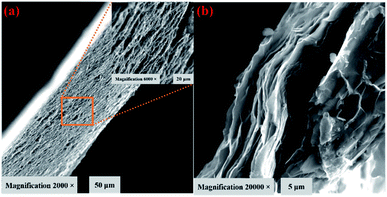 | ||
| Fig. 2 FE-SEM images of (a) cross sectional views of BN/GrP (inset) at low and (b) high magnifications. | ||
Raman, XRD and electrical measurements
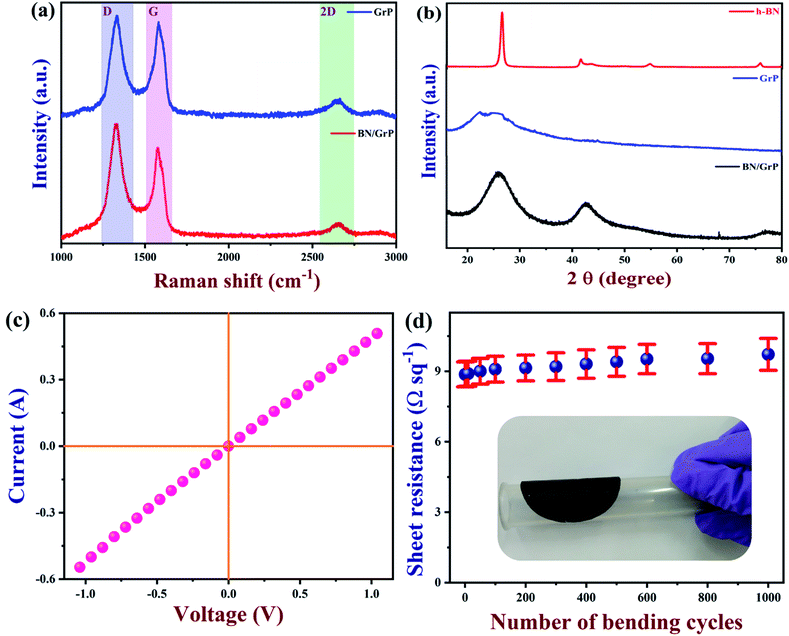
The Raman spectrum of BN/GrP and GrP are shown in Fig. 3a, in which the I2D/IG ratio of GrP and BN/GrP were less than 0.5, indicated the multilayer structure of the graphene with BN. GrP typically has two distinctive peaks at 1330 and 1581.2 cm−1, respectively, displaying D and G bands.56 Further, BN/GrP spectrum is exhibited both strong D (∼1326.3 cm−1) and G (∼1576.6 cm−1) bands which confirmed the presence of graphene. Moreover, strong D band at 1326.3 cm−1, indicated that E2g vibration mode of BN peak which is overlapped with the D band of graphene.57In Fig. 3b, XRD pattern (red curve) of h-BN's revealed a sharp peak at 26.5° due to the (002) plane of h-BN. The weaker peaks at 2θ of 41.5, 54.7 and 75.8° are indexed as (101), (102) and (110) plane's correspond to h-BN (JCPDS file 073-2095).58 As expected, graphene was showed (blue curve) a broad peak around 24.9°. However, after the intercalation of h-BN with graphene, XRD pattern of the BN/GrP showed (black curve) a broad peak around the 2θ of 25.7° as h-BN. Due to the overlap of the XRD peaks of h-BN with graphene, it was hard to distinguish the peaks. But, the XRD peak at 42.3° indicated the (101) plane of h-BN present in BN/GrP, which confirmed the formation of hybrid paper (BN/GrP).
Next, the sheet resistance and electrical conductivity of the BN/GrP were measured using a two-point probe station (Keithley 2612B, USA) on the flexible hybrid paper in different locations (each measurement was repeated three times). Fig. 3c shows the linear current–voltage (I–V) curve of BN/GrP, which indicated the ohmic behaviour of BN/GrP paper. The conductivity and sheet resistance were calculated as 5.36 × 104 S m−1 and 8.87 Ω sq−1, respectively. Furthermore, the mechanical strength and flexibility of the BN/GrP were tested by measuring the sheet resistance after bending the paper up to 1000 cycles (at the bending angle of 60°). The sheet resistance was increased about 8.7%. It was again demonstrated that as-prepared BN/GrP had high mechanical stability due to interconnected network of the film (Fig. 3d).
Electrochemical properties of BN/GrP electrode
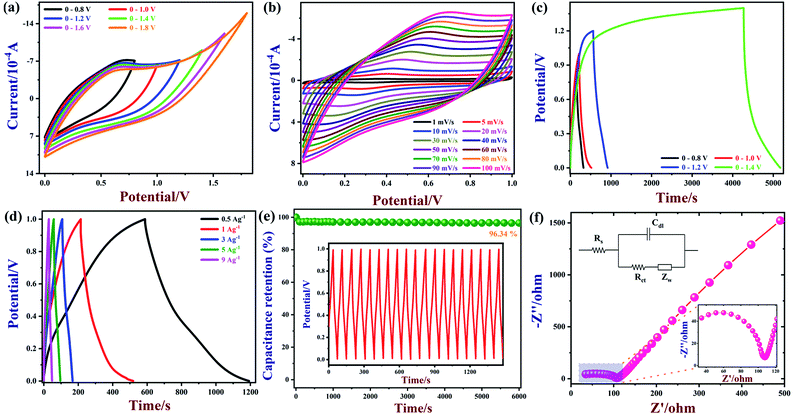
The electrochemical properties of BN/GrP are evaluated by cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), electrochemical impedance spectroscopy (EIS) and potential cycling test using a three-electrode configuration with 1 M H2SO4 as the electrolyte. As shown in Fig. 4a, CV measurements were carried out using BN/GrP at a scan rate of 100 mV s−1 in the potential window of 0 to 1.8 V. The rectangular symmetric shape of the CV curves were maintained; even when the potential window was increased up to 1.8 V without any oxygen evaluation peak, which indicated the good capacitive behaviour of BN/GrP. Fig. 4b depicts CV curves of BN/GrP recorded at different scan rates (1 to 100 mV s−1) between 0 and 1 V. Even at a high scan rate of 100 mV s−1, the rectangular shape of CV curve retained without obvious distortion. It might be due to the desirable fast charging/discharging properties and high capacitive behaviour of BN/GrP. In order to ascertain the detailed charge storage behaviour of the BN/GrP, the kinetics process of electrode was examined by using CV measurements. In general, the relationship between the current (i) and scan rate (ν) is given by,5| i = aνb | (5) |
Next, the specific capacitance of the BN/GrP was calculated using GCD curves as 321.95 F g−1 at a current density of 0.5 A g−1 (Fig. 4d). As noted, the specific capacitance value was decreased with an increasing current density. The energy density (E) and power density (P) of the BN/GrP were calculated and summarized using GCD curves. As prepared BN/GrP exhibited the excellent power density of 44.7 W h kg−1 at 0.5 A g−1 and high energy density of 249.99 W kg−1. In order to examine the electrochemical properties of the as-synthesized GrP and BN/GrP electrodes, the GCD curves were recorded in 1 M H2SO4 in the potential range of 0.0 to 1.0 V at a current density of 1 A g−1 (Fig. S5†). Compared with GrP, the BN/GrP electrode was demonstrated longer charging/discharging time as well as high specific capacitance. It may be due to the charge storage capacity of BN/GrP as a function of the free π electrons and the respective changes in the oxidation state of B and N atoms.60 Furthermore, the cycle life of BN/GrP was tested by repeating the GCD curves between 0 and 1 V at a current density of 4 A g−1 up to 6000 cycles (Fig. 4e inset). After 6000 cycles, 93.6% of capacitance was retained as shown in Fig. 4e. Electrochemical impedance spectroscopy (EIS) spectrum of BN/GrP was recorded in 0.1 M KCl electrolyte containing Fe(CN)64−/3−. The diameter of the semicircle is directly proportional to the charge transfer resistance (Rct).61 In Fig. 4f, the diameter of semicircle was indicated the high Rct (72.1 Ω) with low equivalent series resistance (Rs ∼ 34.75) of BN/GrP. The electrochemical properties of previously reported materials3,4,46,47,62 were also compared with our proposed BN/GrP in Table. S1.† It was found that BN/GrP had showed higher specific capacitance and good cycling stability compared to some of the reported electrodes.
Conclusions
In summary, the hexagonal boron nitride (h-BN) nanosheets were integrated with graphene to prepare a flexible free-standing paper which showed high electrical conductivity of 5.36 × 104 S m−1 and sheet resistance of 8.87 Ω sq−1. Furthermore, Raman spectroscopy and FE-SEM results had confirmed the successful integration of BN within graphene paper. This flexible paper electrode exhibited high specific capacitance of 321.95 F g−1 at 0.5 A g−1. It also showed excellent energy density of 44.7 W h kg−1 and power density of 3588.3 W kg−1 in a three-electrode system. Moreover, the prepared flexible paper showed reasonable stability even after 6000 charging/discharging cycles. From the above results, we believe that our proposed flexible BN/GrP has high potential to be used as wearable/flexible power sources for the next generation flexible electronics.Author contributions
AKS and JR both conceived the idea. JR carried out all the experiments. Both AKS and JR interpreted the results and wrote the manuscript. RA provided useful suggestions and supported our research to improve scientific content of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We sincerely thank Department of Science and Technology (DST) (International Bilateral Cooperation Division), India for financial support through “INDO-RUSSIA Project (File No. INT/RUS/RFBR/385)”. JR thanks SRM IST for PhD student fellowship.References
- B. G. Choi, J. Hong, W. H. Hong, P. T. Hammond and H. Park, ACS Nano, 2011, 5, 7205–7213 CrossRef CAS.
- Z. Wu, A. Winter, L. Chen, Y. Sun, A. Turchanin, X. Feng and K. Müllen, Adv. Mater., 2012, 24, 5130–5135 CrossRef CAS.
- O. Sadak, A. K. Sundramoorthy and S. Gunasekaran, Carbon, 2018, 138, 108–117 CrossRef CAS.
- O. Sadak, W. Wang, J. Guan, A. K. Sundramoorthy and S. Gunasekaran, ACS Appl. Nano Mater., 2019, 2, 4386–4394 CrossRef CAS.
- G. Suo, J. Zhang, D. Li, Q. Yu, M. He, L. Feng, X. Hou, Y. Yang, X. Ye, L. Zhang and W. Wang, J. Colloid Interface Sci., 2020, 566, 427–433 CrossRef CAS.
- T. Li, X. Jiao, T. You, F. Dai, P. Zhang, F. Yu, L. Hu, L. Ding, L. Zhang, Z. Wen and Y. Wu, Ceram. Int., 2019, 45, 4283–4289 CrossRef.
- G. Suo, J. Zhang, D. Li, Q. Yu, W. Wang, M. He, L. Feng, X. Hou, Y. Yang, X. Ye and L. Zhang, Chem. Eng. J., 2020, 388, 124396 CrossRef CAS.
- D. Li, J. Zhang, S. M. Ahmed, G. Suo, W. Wang, L. Feng, X. Hou, Y. Yang, X. Ye and L. Zhang, J. Colloid Interface Sci., 2020, 574, 174–181 CrossRef CAS.
- G. Suo, D. Li, L. Feng, X. Hou, X. Ye, L. Zhang, Q. Yu, Y. Yang and W. Wang, J. Mater. Sci. Technol., 2020, 55, 167–172 CrossRef.
- W. Chen, X. Yu, Z. Zhao, S. Ji and L. Feng, Electrochim. Acta, 2019, 298, 313–320 CrossRef CAS.
- M. Xu, T. Liang, M. Shi and H. Chen, Chem. Rev., 2013, 113, 3766–3798 CrossRef CAS.
- A. Ciesielski and P. Samorì, Chem. Soc. Rev., 2014, 43, 381–398 RSC.
- Y. Sheng, T. Chen, Y. Lu, R.-J. Chang, S. Sinha and J. H. Warner, ACS Nano, 2019, 13, 4530–4537 CrossRef CAS.
- M. A. Yamoah, W. Yang, E. Pop and D. Goldhaber-Gordon, ACS Nano, 2017, 11, 9914–9919 CrossRef CAS.
- R. Jerome and A. K. Sundramoorthy, J. Electrochem. Soc., 2019, 166, B3017–B3024 CrossRef CAS.
- D. Wang, Y. Xiao, X. Luo, Z. Wu, Y.-J. Wang and B. Fang, ACS Sustainable Chem. Eng., 2017, 5, 2509–2515 CrossRef CAS.
- B. Fang, Y.-Z. Wei, K. Suzuki and M. Kumagai, Electrochim. Acta, 2005, 50, 3616–3621 CrossRef CAS.
- B. Fang, J. H. Kim, M.-S. Kim and J.-S. Yu, Acc. Chem. Res., 2013, 46, 1397–1406 CrossRef CAS.
- B. Fang, A. Bonakdarpour, M.-S. Kim, J. H. Kim, D. P. Wilkinson and J.-S. Yu, Microporous Mesoporous Mater., 2013, 182, 1–7 CrossRef CAS.
- B. Fang, Y.-Z. Wei and M. Kumagai, J. Power Sources, 2006, 155, 487–491 CrossRef CAS.
- X. Yang, M. Xu, W. Qiu, X. Chen, M. Deng, J. Zhang, H. Iwai, E. Watanabe and H. Chen, J. Mater. Chem., 2011, 21, 8096–8103 RSC.
- Y. Gao, X. Chen, H. Xu, Y. Zou, R. Gu, M. Xu, A. K.-Y. Jen and H. Chen, Carbon, 2010, 48, 4475–4482 CrossRef CAS.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J.-H. Ahn, P. Kim, J.-Y. Choi and B. H. Hong, Nature, 2009, 457, 706 CrossRef CAS.
- Y. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz, M. Thommes, D. Su, E. A. Stach and R. S. Ruoff, Science, 2011, 332, 1537–1541 CrossRef CAS.
- D. Golberg, Y. Bando, Y. Huang, T. Terao, M. Mitome, C. Tang and C. Zhi, ACS Nano, 2010, 4, 2979–2993 CrossRef CAS.
- M. Deng, X. Yang, M. Silke, W. Qiu, M. Xu, G. Borghs and H. Chen, Sens. Actuators, B, 2011, 158, 176–184 CrossRef CAS.
- S. Garaj, W. Hubbard, A. Reina, J. Kong, D. Branton and J. A. Golovchenko, Nature, 2010, 467, 190 CrossRef CAS.
- R. D. Nagarajan and A. K. Sundramoorthy, Sens. Actuators, B, 2019, 301, 127132 CrossRef CAS.
- R. Jerome and A. K. Sundramoorthy, Anal. Chim. Acta, 2020, 1132, 110–120 CrossRef CAS.
- R. Jerome, P. V. Keerthivasan, N. Murugan, N. R. Devi and A. K. Sundramoorthy, ChemistrySelect, 2020, 5, 9111–9118 CrossRef CAS.
- Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Sun, S. De, I. T. McGovern, B. Holland, M. Byrne, Y. K. Gun'Ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563–568 CrossRef CAS.
- K. R. Paton, E. Varrla, C. Backes, R. J. Smith, U. Khan, A. O'Neill, C. Boland, M. Lotya, O. M. Istrate, P. King, T. Higgins, S. Barwich, P. May, P. Puczkarski, I. Ahmed, M. Moebius, H. Pettersson, E. Long, J. Coelho, S. E. O'Brien, E. K. McGuire, B. M. Sanchez, G. S. Duesberg, N. McEvoy, T. J. Pennycook, C. Downing, A. Crossley, V. Nicolosi and J. N. Coleman, Nat. Mater., 2014, 13, 624 CrossRef CAS.
- Y. Wang, L. Li, L. Yan, X. Gu, P. Dai, D. Liu, J. G. Bell, G. Zhao, X. Zhao and K. M. Thomas, Chem. Mater., 2018, 30, 3048–3059 CrossRef CAS.
- R. Sakamoto, K. Hoshiko, Q. Liu, T. Yagi, T. Nagayama, S. Kusaka, M. Tsuchiya, Y. Kitagawa, W.-Y. Wong and H. Nishihara, Nat. Commun., 2015, 6, 6713 CrossRef CAS.
- H. Ba, L. Truong-Phuoc, C. Pham-Huu, W. Luo, W. Baaziz, T. Romero and I. Janowska, ACS Omega, 2017, 2, 8610–8617 CrossRef CAS.
- P. Garrigue, M.-H. Delville, C. Labrugère, E. Cloutet, P. J. Kulesza, J. P. Morand and A. Kuhn, Chem. Mater., 2004, 16, 2984–2986 CrossRef CAS.
- L. Liu, Z. Niu, L. Zhang, W. Zhou, X. Chen and S. Xie, Adv. Mater., 2014, 26, 4855–4862 CrossRef CAS.
- J. Sha, C. Gao, S.-K. Lee, Y. Li, N. Zhao and J. M. Tour, ACS Nano, 2016, 10, 1411–1416 CrossRef CAS.
- V. Strong, S. Dubin, M. F. El-Kady, A. Lech, Y. Wang, B. H. Weiller and R. B. Kaner, ACS Nano, 2012, 6, 1395–1403 CrossRef CAS.
- Y. Lv, L. Li, Y. Zhou, M. Yu, J. Wang, J. Liu, J. Zhou, Z. Fan and Z. Shao, RSC Adv., 2017, 7, 43512–43520 RSC.
- S. M. Jung, D. L. Mafra, C.-T. Lin, H. Y. Jung and J. Kong, Nanoscale, 2015, 7, 4386–4393 RSC.
- H. Li, Y. Tao, X. Zheng, Z. Li, D. Liu, Z. Xu, C. Luo, J. Luo, F. Kang and Q.-H. Yang, Nanoscale, 2015, 7, 18459–18463 RSC.
- Z. Zhang, F. Xiao, L. Qian, J. Xiao, S. Wang and Y. Liu, Adv. Energy Mater., 2014, 4, 1400064 CrossRef.
- C. Ji, K. Zhang, L. Li, X. Chen, J. Hu, D. Yan, G. Xiao and X. He, J. Mater. Chem. A, 2017, 5, 11263–11270 RSC.
- N. I. Kovtyukhova, N. Perea-López, M. Terrones and T. E. Mallouk, ACS Nano, 2017, 11, 6746–6754 CrossRef CAS.
- W. Liu, X. Yan, J. Lang, C. Peng and Q. Xue, J. Mater. Chem., 2012, 22, 17245–17253 RSC.
- F. Liu, S. Song, D. Xue and H. Zhang, Adv. Mater., 2012, 24, 1089–1094 CrossRef CAS.
- J. M. Munuera, J. I. Paredes, M. Enterría, A. Pagán, S. Villar-Rodil, M. F. R. Pereira, J. I. Martins, J. L. Figueiredo, J. L. Cenis, A. Martínez-Alonso and J. M. D. Tascón, ACS Appl. Mater. Interfaces, 2017, 9, 24085–24099 CrossRef CAS.
- A. K. Sundramoorthy, T. H. Vignesh Kumar and S. Gunasekaran, in Advanced Nanomaterials, ed. A. B. T.-G. B. Tiwari, Elsevier, 2018, pp. 267–306 Search PubMed.
- M. F. Iqbal, M. Ul-Hassan, M. N. Ashiq, S. Iqbal, N. Bibi and B. Parveen, Electrochim. Acta, 2017, 246, 1097–1103 CrossRef CAS.
- X. Liang, G. Long, C. Fu, M. Pang, Y. Xi, J. Li, W. Han, G. Wei and Y. Ji, Chem. Eng. J., 2018, 345, 186–195 CrossRef CAS.
- D. M. El-Gendy, N. A. A. Ghany and N. K. Allam, RSC Adv., 2019, 9, 12555–12566 RSC.
- M. Khairy and S. A. El-Safty, Sens. Actuators, B, 2014, 193, 644–652 CrossRef CAS.
- R. Li, S. Wang, Z. Huang, F. Lu and H. Taobin, J. Power Sources, 2016, 312, 156–164 CrossRef CAS.
- J. Ma, S. Tang, J. A. Syed and X. Meng, RSC Adv., 2016, 6, 82995–83002 RSC.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- M. Wang, T. Zhang, D. Mao, Y. Yao, X. Zeng, L. Ren, Q. Cai, S. Mateti, L. H. Li, X. Zeng, G. Du, R. Sun, Y. Chen, J.-B. Xu and C.-P. Wong, ACS Nano, 2019, 13, 7402–7409 CrossRef CAS.
- V. Guerra, C. Wan, V. Degirmenci, J. Sloan, D. Presvytis and T. McNally, Nanoscale, 2018, 10, 19469–19477 RSC.
- A. Teli, S. Beknalkar, S. Pawar, D. Dubal, T. Dongale, D. Patil, P. Patil and J. C. Shin, Energies, 2020, 13, 6124 CrossRef CAS.
- S. Saha, M. Jana, P. Khanra, P. Samanta, H. Koo, N. C. Murmu and T. Kuila, ACS Appl. Mater. Interfaces, 2015, 7, 14211–14222 CrossRef CAS.
- B. L. Corso, I. Perez, T. Sheps, P. C. Sims, O. T. Gül and P. G. Collins, Nano Lett., 2014, 14, 1329–1336 CrossRef CAS.
- Y. He, W. Chen, X. Li, Z. Zhang, J. Fu, C. Zhao and E. Xie, ACS Nano, 2013, 7, 174–182 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Schematic illustration for the preparation of freestanding BN/GrP flexible paper using BN/graphene dispersion, preparation of BN/GrP electrode for electrochemical measurements of supercapacitor; EDX mapping analysis of BN/GrP; the peak current was plotted against the scan rate to determine the “b” value of the BN/GrP anodic curve; galvanostatic charge/discharge (GCD) curves for GrP and BN/GrP electrodes at a current density of 1 A g−1; comparison of specific capacitances, retention and power density of the reported supercapacitors were provided. See DOI: 10.1039/d0ra10735b |
| This journal is © The Royal Society of Chemistry 2021 |