Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile route to synthesize Fe3O4@acacia–SO3H nanocomposite as a heterogeneous magnetic system for catalytic applications†
Reza Taheri-Ledari a,
Mir Saeed Esmaeilia,
Zahra Varzia,
Reza Eivazzadeh-Keihana,
Ali Maleki
a,
Mir Saeed Esmaeilia,
Zahra Varzia,
Reza Eivazzadeh-Keihana,
Ali Maleki *a and
Ahmed Esmail Shalan
*a and
Ahmed Esmail Shalan *bc
*bc
aCatalysts and Organic Synthesis Research Laboratory, Department of Chemistry, Iran University of Science and Technology (IUST), Tehran 16846-13114, Iran. E-mail: maleki@iust.ac.ir; Fax: +98 21 73021584; Tel: +98 21 77240640-50
bCentral Metallurgical Research and Development Institute (CMRDI), P. O. Box 87, Helwan, Cairo 11421, Egypt. E-mail: a.shalan133@gmail.com
cBCMaterials, Basque Center for Materials, Applications and Nanostructures, Martina Casiano, UPV/EHU Science Park, Barrio Sarriena s/n, Leioa 48940, Spain
First published on 4th November 2020
Abstract
In this work, a novel catalytic system for facilitating the organic multicomponent synthesis of 9-phenyl hexahydroacridine pharmaceutical derivatives is reported. Concisely, this catalyst was constructed from acacia gum (gum arabic) as a natural polymeric base, iron oxide magnetic nanoparticles (Fe3O4 NPs), and sulfone functional groups on the surface as the main active catalytic sites. Herein, a convenient preparation method for this nanoscale composite is introduced. Then, essential characterization methods such as various spectroscopic analyses and electron microscopy (EM) were performed on the fabricated nano-powder. The thermal stability and magnetic properties were also precisely monitored via thermogravimetric analysis (TGA) and vibrating-sample magnetometry (VSM) methods. Then, the performance of the presented catalytic system (Fe3O4@acacia–SO3H) was further investigated in the referred organic reaction by using various derivatives of the components involved in the reaction. Optimization, mechanistic studies, and reusability screening were carried out for this efficient catalyst as well. Overall, remarkable reaction yields (94%) were obtained for the various produced derivatives of 9-phenyl hexahydroacridine in the indicated optimal conditions.
1. Introduction
Recently, powder technology has received much attention in heterogeneous catalytic systems; powders have great potential to be applied in complex organic synthesis reactions and are conveniently separated during the purification processes through their heterogeneity.1,2 One of the best-known members of this family of materials is magnetic nanoscale powder, which has been widely used for various scientific purposes such as drug delivery,3 disease diagnosis,4 water desalination,5 environment cleaning,6 and chemical catalysis.7 In our previous work, we have reported several different catalytic systems constructed with the individual iron oxide (Fe3O4) powder (in nanoscale) and applied them in various catalytic processes.8–15 These nanoparticles could also be composed of other fibrous materials and could be immobilized into polymeric matrices. Through this method, the general properties of the catalytic systems such as the physicomechanical features of the individual Fe3O4 powder are improved. In this regard, numerous studies have been performed, and it has been revealed that the efficiency of the Fe3O4 powder can be significantly modified through its composition with other materials.16–20 For instance, a composition of graphene oxide, Fe3O4 and silver nanoparticles was prepared and applied for enhanced photocatalytic degradation of phenols in the past year.21 Moreover, Javanbakht et al. composited magnetic nanoparticles with a chitosan matrix for the efficient removal of lead(II) from water resources.22 Here, we attempted to prepare a suitable composite of Fe3O4 nanoparticles and “acacia gum” powder, and we applied this composition to facilitate the organic synthesis of 9-phenyl hexahydroacridine (HHA) pharmaceuticals.Acacia gum, also called “gum arabic”, is obtained from wild trees, and its main origin is Somalia. From the organic chemical aspect, there are several hydroxyl groups in the structure of this polymer that can be used as appropriate sites for covalent binding and catalytic applications.23 From the physicomechanical aspect, the suitable stability of acacia gum led us to apply it as an appropriate substrate for immobilization of magnetic nanoparticles. Previously, acacia gum was used as a matrix for catalytic systems. For instance, Banerjee and Chen used this polymer to design a nanoscale absorbent system for the removal of copper ions from water resources. They also magnetized acacia gum through the composition of Fe3O4 NPs for an easy separation from the mixture.24 In this work, we attempted to perform a chemical modification of acacia gum by sulfonation of the hydroxyl functional groups and use the product as an acidic catalytic system for the facilitated synthesis of HHA derivatives. The chemical structure of the acacia gum polymer is presented in Fig. 1(a).
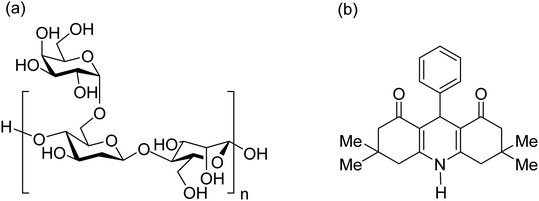 | ||
| Fig. 1 (a) Chemical structure of the acacia gum polymer and (b) general structure of tetramethyl-9-phenyl-hexahydroacridine-1,8(2H,5H)-dione. | ||
To date, various types of hydroacridine derivatives have been developed and investigated for their therapeutic properties. For example, it has been revealed that 5,6-dihydroacridine derivatives possess antidiabetic and antioxidant properties.25,26 Therefore, it is highly important to prepare appropriate conditions for fast and direct synthesis of hydroacridine derivatives. Generally, HHAs and a wide spectrum of active pharmaceutical ingredients (APIs) are synthesized via multicomponent coupling reactions. Today, to obtain purer products with high reaction yields and to shorten the reaction time, many strategies are being introduced and applied. One of the most effective strategies is to use heterogeneous metallic catalytic systems.27–29 Briefly, through the existence of heteroatoms in the structure of the preliminary reactants of multicomponent reactions, constructive electronic interactions provide suitable conditions for chemical bonding. In this regard, sulfonated polymeric networks appear to be efficient for catalysis of organic synthesis reactions. For instance, a polymer-impregnated sulfonated carbon composite was recently reported as an acidic catalytic system for assisting the alkylation of phenol.30 In this study, our aim was to sulfonate acacia gum and apply it to facilitate the multicomponent synthesis reactions of tetramethyl-9-phenyl-hexahydroacridine-1,8(2H,5H)-dione. The general structure of these pharmaceuticals is presented in Fig. 1(b).
Concisely, we introduce a convenient method to synthesize an Fe3O4@acacia–SO3H heterogeneous magnetic catalytic system. Then, it is clearly shown that the synthesis of HHA derivatives is highly facilitated through applying this efficient catalyst. 87–94% reaction yields were obtained for different derivatives of HHA in reaction times of less than two hours. Moreover, convenient separation and excellent reusability were observed for this system through its magnetic properties.
2. Results and discussion
2.1. Preparation method of the Fe3O4@acacia–SO3H nano-powder
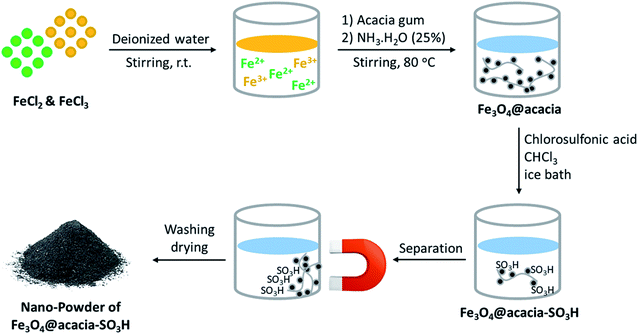
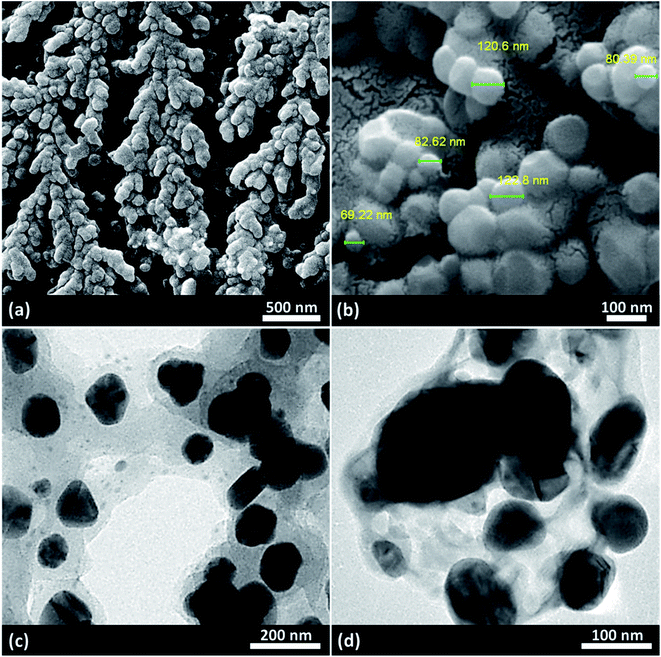
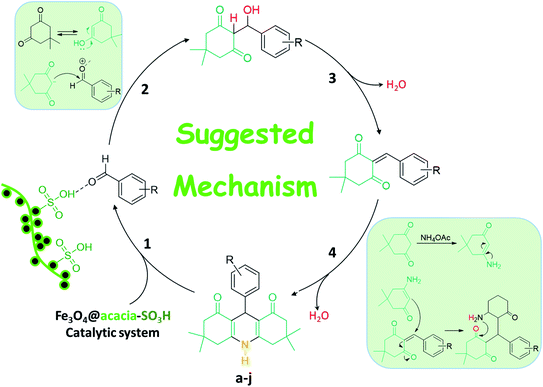
As presented in Fig. 2, iron(II) and iron(III) chloride salts were dissolved in deionized water at room temperature. Then, acacia gum powder was added and was also dissolved. In the next stage, iron ions were precipitated via co-deposition and produced Fe3O4 nanoparticles, which were well composed with the polymeric texture of acacia.31 Via this in situ method, a better composition was obtained, and the dark particles of Fe3O4 were well immobilized. For this purpose, ammonia solution was used to raise the pH value. Moreover, after separation and drying of the precipitate, the particles of Fe3O4@acacia were dispersed in chloroform and the temperature was reduced by an ice bath. Due to the exothermic reaction of sulfonic acid, gentle addition of this material at cool temperatures is required. During the preparation process, the Fe3O4 nanoparticles appear to electrostatically combine with the acacia textures because both species contain several hydroxyl groups in their chemical structures. In the case of sulfone groups, they are most likely covalently attached to the acacia and Fe3O4 nanoparticles.32 In the next stage, after completion of the addition of sulfonic acid and stirring for 120 min, the particles of Fe3O4@acacia–SO3H composite were magnetically separated, washed, and dried in an oven.2.2. Characterization of the Fe3O4@acacia–SO3H nano-powder
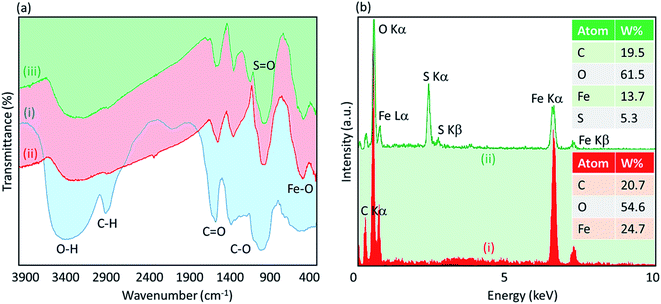
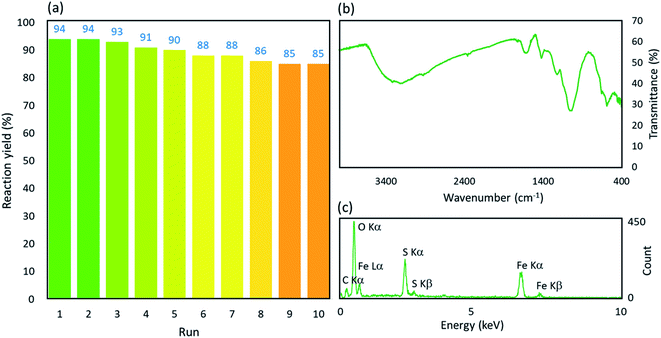
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. This claim is proven by the peak that appeared at ∼1660 cm−1 in the spectrum (i). The composition of the Fe3O4 NPs was confirmed by the peak appearing at ∼590 cm−1 in the spectrum (ii), which is related to the Fe–O bond. As observed in the spectrum (ii), the sharp broad peak of hydroxyl groups in the structure of acacia gum became deformed; this result may be due to the physicochemical composition of the Fe3O4 NPs. According to literature, the peak related to the S
O. This claim is proven by the peak that appeared at ∼1660 cm−1 in the spectrum (i). The composition of the Fe3O4 NPs was confirmed by the peak appearing at ∼590 cm−1 in the spectrum (ii), which is related to the Fe–O bond. As observed in the spectrum (ii), the sharp broad peak of hydroxyl groups in the structure of acacia gum became deformed; this result may be due to the physicochemical composition of the Fe3O4 NPs. According to literature, the peak related to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond is appeared in the range of 1000–1200 cm−1. Accordingly, as shown in the spectrum (iii), this peak appeared and confirmed the successful sulfonation of the Fe3O4@acacia binary composite. To obtain more confirmation of the successful execution of the sulfonation process, energy-dispersive X-ray (EDX) analysis was also performed. As Fig. 3(b) shows, 5.3% of the total weight of the Fe3O4@acacia–SO3H nanocomposite was formed of sulfur after carrying out the sulfonation process. The existence of the other essential elements, such as carbon, oxygen, and iron, related to the desired structures of the Fe3O4@acacia binary composite and Fe3O4@acacia–SO3H nano-powder are also proven by EDX analysis.
O bond is appeared in the range of 1000–1200 cm−1. Accordingly, as shown in the spectrum (iii), this peak appeared and confirmed the successful sulfonation of the Fe3O4@acacia binary composite. To obtain more confirmation of the successful execution of the sulfonation process, energy-dispersive X-ray (EDX) analysis was also performed. As Fig. 3(b) shows, 5.3% of the total weight of the Fe3O4@acacia–SO3H nanocomposite was formed of sulfur after carrying out the sulfonation process. The existence of the other essential elements, such as carbon, oxygen, and iron, related to the desired structures of the Fe3O4@acacia binary composite and Fe3O4@acacia–SO3H nano-powder are also proven by EDX analysis.
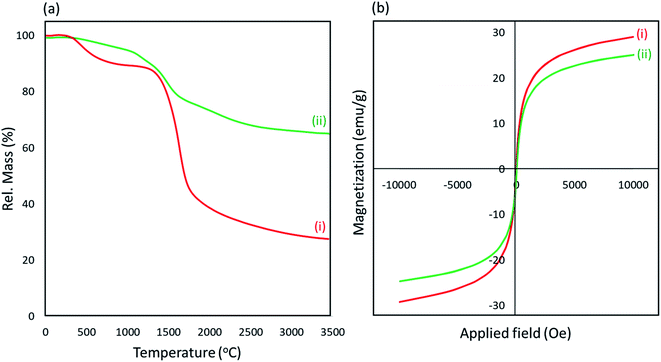
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (Oe) power, and this value is enough to perform a convenient magnetic separation process.
000 (Oe) power, and this value is enough to perform a convenient magnetic separation process.
2.3. Catalytic application of the Fe3O4@acacia–SO3H nano-powder in the organic synthesis of 9-phenyl hexahydroacridine pharmaceutical derivatives
As discussed in the Introduction section, the main goal of the design and fabrication of the Fe3O4@acacia–SO3H nano-powder was to provide a suitably active substrate with high heterogeneity to increase the convenience of the organic synthesis of 9-phenyl hexahydroacridine pharmaceutical derivatives. Here, it is clearly demonstrated that high reaction yields were obtained through applying the present catalytic system. Moreover, the reaction time significantly decreased in comparison with the catalyst-free conditions. A brief comparison was made between our novel designed catalytic system and other recently reported systems that highlights the high efficiency of the present nanocomposite in organic catalysis (Table 1). Scheme 1 presents a general view of the targeted organic reaction that was intended to be catalyzed by the Fe3O4@acacia–SO3H nano-powder.| Entry | Cat. system | Cat. weight (g) | Medium | Temp. (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Abbreviations: Cat.: catalyst; Temp.: temperature, DMF: dimethylformamide; DCM: dichloromethane; N.R.: no reaction. The reaction progress was controlled by thin-layer chromatography, and the desired hexahydroacridine product was purified via flash-column chromatography.b Isolated yield.c Optimum conditions. | ||||||
| 1 | — | — | EtOH | 25 | 110 | N.R. |
| 2 | — | — | EtOH | 75 | 110 | N.R. |
| 3 | Fe3O4 NPs | 0.02 | EtOH | 75 | 110 | Trace |
| 4 | Acacia gum | 0.02 | EtOH | 75 | 110 | Trace |
| 5 | Fe3O4@acacia–SO3H | 0.01 | EtOH | 75 | 110 | 88 |
| 6 | Fe3O4@acacia–SO3H | 0.02 | EtOH | 75 | 110 | 94c |
| 7 | Fe3O4@acacia–SO3H | 0.02 | EtOH | 75 | 300 | 94 |
| 8 | Fe3O4@acacia–SO3H | 0.03 | EtOH | 75 | 110 | 94 |
| 9 | Fe3O4@acacia–SO3H | 0.03 | EtOH | 50 | 110 | 91 |
| 10 | Fe3O4@acacia–SO3H | 0.02 | H2O | 80 | 110 | 62 |
| 11 | Fe3O4@acacia–SO3H | 0.02 | DMF | 130 | 110 | 75 |
| 12 | Fe3O4@acacia–SO3H | 0.02 | DCM | 35 | 110 | 79 |
| 13 | Fe3O4@acacia–SO3H | 0.02 | Toluene | 130 | 110 | 76 |
| 14 | Fe3O4@acacia–SO3H | 0.02 | CH3CN | 75 | 110 | 79 |
| 15 | Nano-Fe3O4–TiO2–SO3H | 0.01 | Solvent free | 110 | 55 | 86 (ref. 35) |
| 16 | Fe3O4@SiO2–MoO3H | 0.02 | Solvent free | 90 | 40 | 90 (ref. 36) |
| 17 | Cell-Pr-NHSO3H | 0.05 | Ethanol | Reflux | 48 | 88 (ref. 37) |
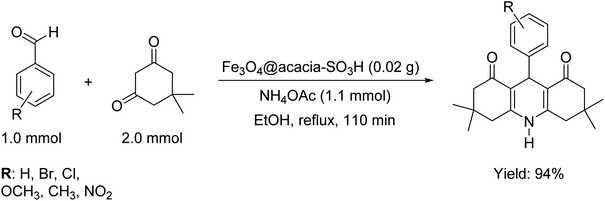 | ||
| Scheme 1 General schematic of the organic synthesis reaction of the 9-phenyl hexahydroacridine derivatives catalyzed by the Fe3O4@acacia–SO3H nanocatalyst. | ||
| Entry | Product structure | Product code | Time (min) | Yielda (%) | Melting point (°C) | Ref. | |
|---|---|---|---|---|---|---|---|
| Found | Reported | ||||||
| a Isolated yield. | |||||||
| 1 | 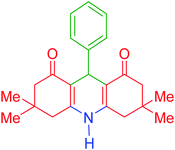 |
a | 110 | 93 | 279–281 | 277–279 | 38 |
| 2 | 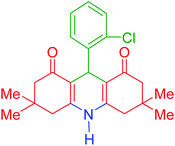 |
b | 145 | 87 | 264–266 | 263–264 | 39 |
| 3 | 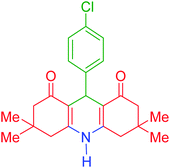 |
c | 135 | 91 | 290–292 | 295–297 | 40 |
| 4 | 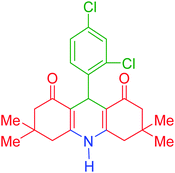 |
d | 150 | 87 | 318–320 | 319–321 | 41 |
| 5 | 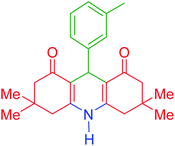 |
e | 125 | 91 | 211–213 | 210–213 | 41 |
| 6 | 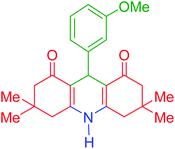 |
f | 120 | 92 | 301–303 | 300–302 | 42 |
| 7 | 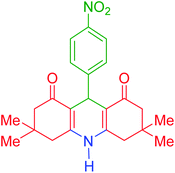 |
g | 150 | 86 | 286–288 | 287–289 | 36 |
| 8 | 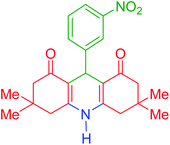 |
h | 150 | 86 | 281–283 | 282–284 | 43 |
| 9 | 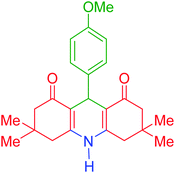 |
i | 110 | 94 | 288–290 | 287–290 | 44 |
| 10 | 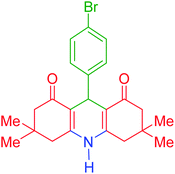 |
j | 140 | 90 | 321–324 | 322–324 | 45 |
3. Experimental
3.1. Materials and equipment
All commercially available chemicals, solvents, reagents and were purchased from Sigma-Aldrich and Merck Company. All the applied materials and equipment are summarized in Table S1.†3.2. Practical methods
3.3. Spectral data for selected products
3,3,6,6-Tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (product a): M. P (°C): 279–281. 1H NMR (300 MHz, DMSO), δ (ppm): 9.30 (s, 1H), 7.00–7.13 (m, 5H), 4.60 (s, 1H), 2.40–2.48 (m, 2H), 2.27 (d, 2H), 2.12 (d, 2H), 2.00 (d, 2H), 0.98 (s, 6H), 0.83 (s, 6H). 13C NMR (75 MHz, DMSO), δ (ppm): 194.3, 149.3, 147.1, 127.6, 127.5, 125.4, 111.4, 50.2, 32.9, 32.2, 29.1, 26.4.9-(2-Chlorophenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (product b): M. P (°C): 264–266. 1H NMR (300 MHz, DMSO), δ (ppm): 9.59 (s, 1H), 7.25–7.27 (d, 1H), 7.10–7.20 (m, 1H), 7.04–7.08 (m, 1H), 6.99–7.01 (m, 1H), 5.05 (s, 1H), 2.71–2.10 (m, 8H), 0.98 (s, 6H), 0.92 (s, 6H). 13C NMR (75 MHz, DMSO), δ (ppm): 196.4, 152.4, 142.6, 132.0, 129.7, 128.2, 115.2, 50.6, 40.8, 32.2, 31.4, 29.3, 27.2.
9-(4-Chlorophenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (product c): M. P (°C): 290–292. 1H NMR (300 MHz, DMSO), δ (ppm): 9.92 (s, 1H), 7.27–7.30 (d, 2H), 7.18–7.19 (d, 2H), 4.50 (s, 1H), 2.50–2.59 (dd, 4H), 2.25–2.28 (d, 2H), 2.07–2.10 (d, 2H), 1.04 (s, 6H), 0.90 (s, 6H). 13C NMR (75 MHz, DMSO), δ (ppm): 196.1, 149.3, 147.1, 131.7, 129.6, 129.3, 128.2, 113.1, 50.2, 33.6, 32.8, 29.1, 27.3.
9-(2,4-Dichlorophenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (product d): M. P (°C): 318–320. 1H NMR (300 MHz, DMSO), δ (ppm): 9.95 (s, 1H), 7.34 (s, 1H), 7.28–7.33 (d, 1H), 7.19–7.22 (d, 1H), 5.58 (s, 1H), 2.38–2.43 (m, 2H), 2.34 (d, 2H), 2.32 (d, 2H), 2.31 (d, 2H), 1.14 (s, 6H), 1.07 (s, 6H). 13C NMR (75 MHz, DMSO), δ (ppm): 189.8, 135.3, 134.1, 130.0, 129.5, 126.7, 115.4, 47.0, 46.9, 46.3, 31.7, 31.2, 28.9, 27.8.
9-(3-Methylphenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (product e): M. P (°C): 211–213. 1H NMR (300 MHz, DMSO), δ (ppm): 8.07 (s, 1H), 7.26 (s, 1H), 7.09–7.11 (d, 1H), 7.03–7.08 (m, 1H), 6.86–6.88 (d, 1H), 5.05 (s, 1H), 2.32–2.40 (m, 2H), 2.26 (d, 2H), 2.22 (d, 2H), 2.16 (d, 2H), 1.05 (s, 6H), 0.95 (s, 6H). 13C NMR (75 MHz, DMSO), δ (ppm): 196.1, 149.5, 146.7, 137.3, 129.1, 128.0, 126.9, 125.2, 113.3, 51.1, 40.8, 33.6, 32.7, 29.8, 27.2, 21.8.
9-(3-Methoxyphenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (product f): M. P (°C): 301–303. 1H NMR (300 MHz, DMSO), δ (ppm): 8.94 (s, 1H), 7.25 (s, 1H), 7.22 (d, 1H), 7.01 (m, 1H), 6.98 (d, 1H), 5.07 (s, 1H), 3.91 (s, 3H), 2.22–2.27 (m, 2H), 2.17 (d, 2H), 2.11 (d, 2H), 2.04 (d, 2H), 1.12 (s, 6H), 0.95 (s, 6H). 13C NMR (75 MHz, DMSO), δ (ppm): 196.1, 158.72, 149.9, 143.8, 134.9, 128.5, 127.7, 125.2, 112.8, 50.8, 40.2, 33.0, 32.3, 29.4, 27.9, 20.9.
9-(4-Nitrophenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (product g): M. P (°C): 286–288. 1H NMR (300 MHz, DMSO), δ (ppm): 9.90 (s, 1H), 7.19–7.21 (d, 2H), 7.12–7.14 (d, 2H), 4.76 (s, 1H), 2.42–2.49 (d, 4H), 2.14–2.32 (d, 2H), 1.95–1.98 (d, 2H), 0.99 (s, 6H), 0.84 (s, 6H). 13C NMR (75 MHz, DMSO), δ (ppm): 194.4, 149.5, 146.1, 129.9, 129.5, 127.5, 115.5, 111.1, 50.1, 40.0, 32.6, 32.2, 29.0, 26.4.
3,3,6,6-Tetramethyl-9-(3-nitrophenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (product h): M. P (°C): 281–283. 1H NMR (300 MHz, DMSO), δ (ppm): 9.31 (s, 1H), 8.00–8.01 (d, 2H), 7.65–7.66 (d, 1H), 7.54–7.57 (t, 1H), 4.65 (s, 1H), 2.50–2.59 (dd, 4H), 2.27–2.30 (d, 2H), 2.08–2.12 (d, 2H), 1.04 (s, 6H), 0.91 (s, 6H). 13C NMR (75 MHz, DMSO), δ (ppm): 196.0, 149.4, 149.0, 131.1, 129.7, 122.3, 112.9, 51.0, 40.9, 33.9, 32.8, 29.7, 27.3, 21.1.
9-(4-Methoxyphenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (product i): M. P (°C): 288–290. 1H NMR (300 MHz, DMSO), δ (ppm): 9.20 (s, 1H), 7.05–7.07 (t, 2H), 6.76–6.80 (t, 2H), 4.46 (s, 1H), 3.70 (s, 3H), 2.56 (d, 2H), 2.49–2.51 (m, 2H), 2.27 (d, 2H), 2.09 (d, 2H), 1.03 (s, 6H), 0.91 (s, 6H). 13C NMR (75 MHz, DMSO), δ (ppm): 196.2, 157.5, 149.6, 139.2, 128.8, 125.2, 113.0, 50.8, 40.36, 32.6, 32.4, 29.5, 26.9.
9-(4-Bromophenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (product j): M. P (°C): 321–324. 1H NMR (300 MHz, DMSO), δ (ppm): 9.33 (s, 1H), 7.23–7.26 (dd, 2H), 6.87–6.92 (m, 2H), 4.73 (s, 1H), 2.26–2.46 (m, 4H), 2.19–2.22 (m, 2H), 2.15 (m, 4H), 1.10 (s, 6H), 0.99 (s, 6H). 13C NMR (75 MHz, DMSO), δ (ppm): 194.4, 149.6, 149.3, 147.1, 127.9, 127.8, 126.0, 111.9, 50.7, 32.2, 31.2, 29.2, 27.3.
4. Conclusions
In this work, we designed and fabricated a novel catalytic system with high heterogeneity and magnetic features to facilitate the MCR synthetic reactions of 9-phenyl hexahydroacridine pharmaceutical derivatives. A combination of acacia gum (gum arabic) with iron oxide magnetic particles on the nanoscale was used as a magnetized natural matrix. From the physicochemical aspect, through effective H-binding interactions, the organic and inorganic ingredients were firmly fixed and combined well with each other. EM imaging approaches indeed disclosed the composition of the composite. Then, the prepared Fe3O4@acacia binary composite was equipped with sulfone groups, which are considered to be the main active catalytic sites. Afterward, the high catalytic performance of the formed cluster-shaped composite was investigated in the organic synthesis reactions of 9-phenyl hexahydroacridine derivatives. The mechanical and thermal stability of the fabricated Fe3O4@acacia–SO3H nano-powder was also studied, and this substantial stability was highlighted in the recycling process. Overall, herein, we have made an effort to comprehensively study the structural features of the Fe3O4@acacia–SO3H nano-powder and demonstrate the catalytic performance of this product. Due to the high convenience of the synthesis process and the low prices of the used raw materials, this product is recommended for industrial applications.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors gratefully acknowledge the partial support from the Research Council of the Iran University of Science and Technology (IUST). Furthermore, AES is grateful for the National Research grants from MINECO “Juan de la Cierva” [FJCI-2018-037717].References
- A. Gulati, J. Malik, Mandeep and R. Kakkar, Peanut shell biotemplate to fabricate porous magnetic Co3O4 coral reef and its catalytic properties for p-nitrophenol reduction and oxidative dye degradation, Colloids Surf., A, 2020, 604, 125328 CAS.
- R. Taheri-Ledari, J. Rahimi and M. Ali, Mater. Res. Express, 2020, 7, 015067 CrossRef CAS.
- W. Zhang, R. Taheri-Ledari, Z. Hajizadeh, E. Zolfaghari, M. R. Ahghari, A. Maleki, M. R. Hamblin and Y. Tian, Enhanced activity of vancomycin by encapsulation in hybrid magnetic nanoparticles conjugated to a cell-penetrating peptide, Nanoscale, 2020, 12, 3855–3870 RSC.
- N. Kang, D. Xu, Y. Han, X. Lv, Z. Chen, T. Zhou, L. Ren and X. Zhou, Magnetic targeting core/shell Fe3O4/Au nanoparticles for magnetic resonance/photoacoustic dual-modal imaging, Mater. Sci. Eng., C, 2019, 98, 545–549 CrossRef CAS.
- R. T. Ledari, W. Zhang, M. Radmanesh, S. S. Mirmohammadi, A. Maleki, N. Cathcart and V. Kitaev, Small, 2020, 2002733 CrossRef.
- Z. Hajizadeh, K. Valadi, R. Taheri-Ledari and A. Maleki, Convenient Cr(VI) Removal from Aqueous Samples: Executed by a Promising Clay-Based Catalytic System, Magnetized by Fe3O4 Nanoparticles and Functionalized with Humic Acid, ChemistrySelect, 2020, 5, 2441–2448 CrossRef CAS.
- A. Maleki, F. Hassanzadeh-Afruzi, Z. Varzi and M. S. Esmaeili, Magnetic dextrin nanobiomaterial: an organic–inorganic hybrid catalyst for the synthesis of biologically active polyhydroquinoline derivatives by asymmetric Hantzsch reaction, Mater. Sci. Eng., C, 2020, 109, 110502 CrossRef CAS.
- A. Maleki, R. Taheri-Ledari and M. Soroushnejad, Surface functionalization of magnetic nanoparticles via palladium-catalyzed Diels-Alder approach, ChemistrySelect, 2018, 3, 13057–13062 CrossRef CAS.
- A. E. Shalan, M. Rasly and M. M. Rashad, Organic acid precursor synthesis and environmental photocatalysis applications of mesoporous anatase TiO2 doped with different transition metal ions, J. Mater. Sci.: Mater. Electron., 2014, 25(7), 3141–3146 CrossRef CAS.
- S. Parvaz, R. Taheri-Ledari, M. S. Esmaeili, M. Rabbani and A. Maleki, A brief survey on the advanced brain drug administration by nanoscale carriers: with a particular focus on AChE reactivators, Life Sci., 2020, 240, 117099 CrossRef CAS.
- A. Maleki, R. Taheri-Ledari, J. Rahimi, M. Soroushnejad and Z. Hajizadeh, Facile peptide bond formation: effective interplay between isothiazolone rings and silanol groups at silver/iron oxide nanocomposite surfaces, ACS Omega, 2019, 4, 10629–10639 CrossRef CAS.
- A. Maleki, R. Taheri-Ledari, R. Ghalavand and R. Firouzi-Haji, Palladium-decorated o-phenylenediamine-functionalized Fe3O4/SiO2 magnetic nanoparticles: a promising solid-state catalytic system used for Suzuki–Miyaura coupling reactions, J. Phys. Chem. Solids, 2020, 136, 109200 CrossRef CAS.
- R. Taheri-Ledari, A. Maleki, E. Zolfaghari, M. Radmanesh, H. Rabbani, A. Salimi and R. Fazel, High-performance sono/nano-catalytic system: Fe3O4@Pd/CaCO3-DTT core/shell nanostructures, a suitable alternative for traditional reducing agents for antibodies, Ultrason. Sonochem., 2020, 61, 104824 CrossRef CAS.
- R. Taheri-Ledari, J. Rahimi and A. Maleki, Synergistic catalytic effect between ultrasound waves and pyrimidine-2,4-diamine-functionalized magnetic nanoparticles: applied for synthesis of 1,4-dihydropyridine pharmaceutical derivatives, Ultrason. Sonochem., 2019, 59, 104737 CrossRef.
- K. Valadi, S. Gharibi, R. Taheri-Ledari and A. Maleki, Ultrasound-assisted synthesis of 1,4-dihydropyridine derivatives by an efficient volcanic-based hybrid nanocomposite, Solid State Sci., 2020, 101, 106141 CrossRef CAS.
- J. Rahimi, R. Taheri-Ledari, M. Niksefat and A. Maleki, Enhanced reduction of nitrobenzene derivatives: effective strategy executed by Fe3O4/PVA-10% Ag as a versatile hybrid nanocatalyst, Catal. Commun., 2020, 134, 105850 CrossRef CAS.
- A. Maleki, M. Niksefat, J. Rahimi and R. Taheri-Ledari, Multicomponent synthesis of pyrano[2,3-d]pyrimidine derivatives via a direct one-pot strategy executed by novel designed cooperated Fe3O4@polyvinyl alcohol magnetic nanoparticles, Mater. Today Chem., 2019, 13, 110–120 CrossRef CAS.
- R. Taheri-Ledari, S. M. Hashemi and A. Maleki, High-performance sono/nano-catalytic system: CTSN/Fe3O4–Cu nanocomposite, a promising heterogeneous catalyst for the synthesis of N-arylimidazoles, RSC Adv., 2019, 9, 40348–40356 RSC.
- S. S. Soltani, R. Taheri-Ledari, S. M. F. Farnia, A. Maleki and A. Foroumadi, Synthesis and characterization of a supported Pd complex on volcanic pumice laminates textured by cellulose for facilitating Suzuki–Miyaura cross-coupling reactions, RSC Adv., 2020, 10, 23359–23371 RSC.
- R. Taheri-Ledari and A. Maleki, Antimicrobial therapeutic enhancement of levofloxacin via conjugation to a cell-penetrating peptide: an efficient sonochemical catalytic process, J. Pept. Sci., 2020, 26, e3277 CrossRef CAS.
- G. U. Rehman, M. Tahir, P. S. Goh, A. F. Ismail and I. U. Khan, Controlled synthesis of reduced graphene oxide supported magnetically separable Fe3O4@rGO@AgI ternary nanocomposite for enhanced photocatalytic degradation of phenol, Powder Technol., 2019, 356, 547–558 CrossRef CAS.
- V. Javanbakht, S. M. Ghoreishi, N. Habibi and M. Javanbakht, A novel magnetic chitosan/clinoptilolite/magnetite nanocomposite for highly efficient removal of Pb(II) ions from aqueous solution, Powder Technol., 2016, 302, 372–383 CrossRef CAS.
- A. M. lslam, G. O. Phillipsl, A. Sljivo, M. J. Snowden and P. A. Williams, A review of recent developments on the regulatory, structural and functional aspects of gum Arabic, Food Hydrocolloids, 1997, 11, 493–505 CrossRef.
- S. S. Banerjee and D. H. Chen, Fast removal of copper ions by gum Arabic modified magnetic nano-adsorbent, J. Hazard. Mater., 2007, 147, 792–799 CrossRef CAS.
- Á. Magyar and Z. Hell, Molecular Sieve Supported Lanthanum Catalyst for the Efficient Synthesis of Polyhydroquinolines via Hantzsch Synthesis, Period. Polytech., Chem. Eng., 2017, 61, 278–282 CrossRef.
- K. T. Naveen, K. B. Sai and K. Chandana, Synthesis, Characterization and screening of novel 5,6-dihydroacridine derivatives as potent antidiabetic and antioxidant agent, J. Basic Appl. Res. Int., 2016, 2, 176–184 Search PubMed.
- A. Maleki, Z. Varzi and F. Hassanzadeh-Afruzi, Preparation and characterization of an eco-friendly ZnFe2O4@alginic acid nanocomposite catalyst and its application in the synthesis of 2-amino-3-cyano-4H-pyran derivatives, Polyhedron, 2019, 171, 193–202 CrossRef CAS.
- Z. Varzi and A. Maleki, Design and preparation of ZnS-ZnFe2O4: a green and efficient hybrid nanocatalyst for the multicomponent synthesis of 2,4,5-triaryl-1H-imidazoles, Appl. Organomet. Chem., 2019, 33, e5008 CrossRef.
- H. Veisi, N. Dadres, P. Mohammadi and S. Hemmati, Green synthesis of silver nanoparticles based on oil-water interface method with essential oil of orange peel and its application as nanocatalyst for A3 coupling, Mater. Sci. Eng., C, 2019, 105, 110031 CrossRef CAS.
- P. K. Khatri, M. Manchanda, I. K. Ghosh and S. L. Jain, Polymer impregnated sulfonated carboncomposite solid acid catalyst for alkylation of phenol with methyl-tert-butyl ether, RSC Adv., 2015, 5, 3286–3290 RSC.
- M. S. Esmaeili, Z. Varzi, R. Eivazzadeh-Keihan, A. Maleki and H. Ghafuri, Design and development of natural and biocompatible raffinose-Cu2O magnetic nanoparticles as a heterogeneous nanocatalyst for the selective oxidation of alcohols, Mol. Catal., 2020, 492, 111037 CrossRef CAS.
- A. Bagheri, P. Salarizadeh, M. S. Asre Hazer, P. Hosseinabadi, S. Kashefi and H. Beydaghi, The effect of adding sulfonated SiO2 nanoparticles and polymer blending on properties and performance of sulfonated poly ether sulfone membrane: fabrication and optimization, Electrochim. Acta, 2019, 295, 875–890 CrossRef CAS.
- C. Işik, G. Arabaci, Y. I. Doğaç, İ. Deveci and M. Teke, Synthesis and characterization of electrospun PVA/Zn2+ metal composite nanofibers for lipase immobilization with effective thermal, pH stabilities and reusability, Mater. Sci. Eng., C, 2019, 99, 1226–1235 CrossRef.
- T. Şişmanoğlu, S. Karakuş, Ö. Birer, G. S. P. Soylu, A. Kolan, E. Tan, Ö. Ürk, G. Akdut and A. Kilislioglu, Preparation and characterization of antibacterial Senegalia (Acacia) senegal/iron–silica bio-nanocomposites, Appl. Surf. Sci., 2015, 354, 250–255 CrossRef.
- A. Amoozadeh, S. Golian and S. Rahmani, TiO2-coated magnetite nanoparticle-supported sulfonic acid as a new, efficient, magnetically separable and reusable heterogeneous solid acid catalyst for multicomponent reactions, RSC Adv., 2015, 5, 45974–45982 RSC.
- M. Kiani and M. Mohammadipour, Fe3O4@SiO2–MoO3H nanoparticles: a magnetically recyclable nanocatalyst system for the synthesis of 1,8-dioxo-decahydroacridine derivatives, RSC Adv., 2017, 7, 997–1007 RSC.
- S. Karhale, C. Bhenki, G. Rashinkar and V. Helavi, Covalently anchored sulfamic acid on cellulose as heterogeneous solid acid catalyst for the synthesis of structurally symmetrical and unsymmetrical 1,4-dihydropyridine derivatives, New J. Chem., 2017, 41, 5133–5141 RSC.
- G. M. Ziarani, A. Badiei, M. Hassanzadeh and S. Mousavi, Synthesis of 1,8-dioxo-decahydroacridine derivatives using sulfonic acid functionalized silica (SiO2–Pr–SO3H) under solvent free conditions, Arabian J. Chem., 2014, 7, 335–339 CrossRef CAS.
- D. Patil, D. Chandam, A. Mulik, P. Patil, S. Jagadale, R. Kant, V. Gupta and M. Deshmukh, Novel Brønsted Acidic Ionic Liquid ([CMIM][CF3COO]) Prompted Multicomponent Hantzsch Reaction for the Eco-Friendly Synthesis of Acridinediones: An Efficient and Recyclable Catalyst, Catal. Lett., 2014, 144, 949–958 CrossRef CAS.
- A. Magyar and Z. Hell, An Efficient One-Pot Four-Component Synthesis of 9-Aryl-Hexahydroacridine-1,8-Dione Derivatives in the Presence of a Molecular Sieves Supported Iron Catalyst, Catal. Lett., 2019, 149, 2528–2534 CrossRef CAS.
- S. Asgharnasl, R. Eivazzadeh-Keihan, F. Radinekiyan and A. Maleki, Preparation of a novel magnetic bionanocomposite based on factionalized chitosan by creatine and its application in the synthesis of polyhydroquinoline, 1,4-dyhdropyridine and 1,8-dioxo-decahydroacridine derivatives, Int. J. Biol. Macromol., 2020, 144, 29–46 CrossRef CAS.
- E. Eidi, M. Z. Kassaee and Z. Nasresfahani, Nanocrystalline TiO2, via green combustion synthesis, as an efficient and reusable catalyst for the preparation of 1,8-dioxooctahydroxanthenes and 1,8-dioxodecahydroacridines, Appl. Organomet. Chem., 2015, 29, 793–797 CrossRef CAS.
- M. S. Mirhosseyni, F. Nemati and A. Elhampour, Hollow Fe3O4@DA–SO3H: an efficient and reusable heterogeneous nano-magnetic acid catalyst for synthesis of dihydropyridine and dioxodecahydroacridine derivatives, J. Iran. Chem. Soc., 2017, 14, 791–801 CrossRef CAS.
- A. Amoozadeh, S. Rahmani, M. Bitaraf, F. B. Abadi and E. Tabrizian, Nano-zirconia as an excellent nano support for immobilization of sulfonic acid: a new, efficient and highly recyclable heterogeneous solid acid nanocatalyst for multicomponent reactions, New J. Chem., 2016, 40, 770–780 RSC.
- M. A. Zolfigol, F. Karimi, M. Yarie and M. Torabi, Catalytic application of sulfonic acid-functionalized titana-coated magnetic nanoparticles for the preparation of 1,8-dioxodecahydroacridines and 2,4,6-triarylpyridines via anomeric-based oxidation, Appl. Organomet. Chem., 2018, 32, e4063 CrossRef.
- S. Karhale, C. Bhenki, G. Rashinkar and V. Helavi, Covalently anchored sulfamic acid on cellulose as heterogeneous solid acid catalyst for the synthesis of structurally symmetrical and unsymmetrical 1,4-dihydropyridine derivatives, New J. Chem., 2017, 41, 5133–5141 RSC.
- A. Bamoniri and S. Fouladgar, SnCl4-functionalized nano-Fe3O4 encapsulated-silica particles as a novel heterogeneous solid acid for the synthesis of 1,4-dihydropyridine derivatives, RSC Adv., 2015, 5, 78483–78490 RSC.
- A. Bamoniri, B. B. F. Mirjalili and S. Fouladgar, Sonochemically synthesis of 1,4-dihydropyridine derivatives using nano-silica supported tin tetrachloride as a reusable solid acid catalyst, J. Taiwan Inst. Chem. Eng., 2016, 63, 396–403 CrossRef CAS.
- Z. Zarei and B. Akhlaghinia, ZnII doped and immobilized on functionalized magnetic hydrotalcite (Fe3O4/HT-SMTU-ZnII): a novel, green and magnetically recyclable bifunctional nanocatalyst for the one-pot multi-component synthesis of acridinediones under solvent-free conditions, New J. Chem., 2017, 41, 15485–15500 RSC.
- A. Zhu, R. Liu, C. Du and L. Li, Betainium-based ionic liquids catalysed multicomponent Hantzsch reactions for the efficient synthesis of acridinediones, RSC Adv., 2017, 7, 6679–6684 RSC.
- L. M. Ramos, M. O. Rodrigues and B. A. D. Neto, Org. Biomol. Chem., 2019, 17, 7260–7269 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The table of the materials and equipment applied in this project and the original spectra of the selected synthesized 9-phenyl hexahydroacridine compounds. This section can be found in the online version. See DOI: 10.1039/d0ra07986c |
| This journal is © The Royal Society of Chemistry 2020 |