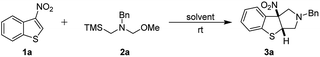
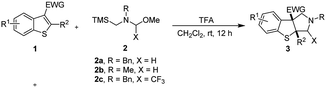
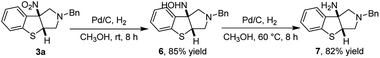
Open Access Article
Open Access ArticleDearomative [3 + 2] cycloaddition reaction of nitrobenzothiophenes with nonstabilized azomethine ylides†
Kai-Kai Wang a,
Yan-Xin Xieb,
Yan-Li Lic,
Rongxiang Chena and
Zhan-Yong Wang
a,
Yan-Xin Xieb,
Yan-Li Lic,
Rongxiang Chena and
Zhan-Yong Wang *a
*a
aSchool of Pharmacy, Xinxiang University, Xinxiang 453000, P. R. China. E-mail: zhanyongw@126.com
bSchool of Chemistry and Materials Engineering, Xinxiang University, Xinxiang 453000, P. R. China
cMedical College, Xinxiang University, Xinxiang 453000, P. R. China
First published on 4th August 2020
Abstract
A highly diastereoselective dearomative [3 + 2] 1,3-dipolar cycloaddition reaction of nitrobenzothiophenes with an in situ-generated nonstabilized azomethine ylides has been developed. The transformation provides a series of functionalized fused tricyclic benzo[4,5]thieno[2,3-c]pyrroles in good yields (up to 92%) under mild reaction conditions. In addition, a gram-scale experiment and the synthetic transformation of the cycloadduct further highlighted the synthetic utility. The relative configurations of the typical products were clearly confirmed by X-ray crystallography.
The dearomative cycloaddition reactions are a powerful synthetic strategy to obtain valuable structural motifs which exist in numerous biologically active natural products, pharmaceutical agents, and also in synthetic and materials building blocks.1 Among them, indole substrates gained more and more research interest to develop effective methods for the construction of indole-based skeletons and their functionalization via dearomative transformation.2 Because the unique properties of indole ring systems are ubiquitous in biologically active alkaloids,3 the range of methodologies that have been explored to access dearomatized indole heterocycles is extremely extensive. In contrast to the dearomative reactions of indole substrates, the analogous benzothiophenes derivatives have been less explored.4 In addition, the benzo[b]thiophene derivatives that have found widespread application are frequently found in many bioactive compounds, pharmaceuticals, and as synthetic building blocks.5 Therefore, the development of efficient synthetic method to realize the dearomatization of benzo[b]thiophenes for the construction of diverse functionalized heteroarenes molecules continues to be an important and highly desirable in the organic synthetic community.
On the other hand, 1,3-dipolar cycloaddition of azomethine ylides with electron deficient dipolarophiles that have a wide range of applications in organic synthesis, is a useful and facile synthetic process for five or six membered heterocyclic rings in one step.6 Especially, nonstabilized azomethine ylides generated in situ are highly active intermediates,7 with electron-deficient benzoheterocycles, including 2-nitroindoles or 3-nitroindoles (Scheme 1a)8 and benzo[b]thiophene 1,1-dioxides (Scheme 1b)9 as robust electrophiles to construct various polycyclic heterocyclic skeletons via the dearomative [3 + 2] 1,3-dipolar cycloaddition reaction in the simple way. However, 3- and 2-nitrobenzothiophenes have been uncovered as electrophiles for the dearomative 1,3-dipolar cycloaddition reactions with nonstabilized azomethine ylides to provide S-containing polyheterocyclic compounds. Enormous efforts have been devoted to the development of ever more efficient synthetic methods for the construction and direct functionalization of these heteroaromatic compounds. Herein, we describe a dearomative [3 + 2] cycloaddition reaction of 3-nitrobenzothiophenes with nonstabilized azomethine ylides without metal catalysts under mild reaction conditions, providing a convenient and efficient access to functionalized fused tricyclic benzo[4,5]thieno[2,3-c]pyrroles derivatives bearing two contiguous stereocenters. Additionally, we also successfully extended this new protocol to 2-nitrobenzothiophene and 2-nitrobenzofuran for the corresponding dearomatization products.
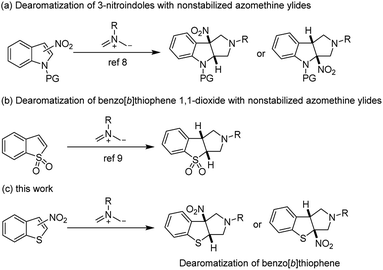 | ||
| Scheme 1 Dearomative cycloaddition reaction of electron-deficient heteroarenes with nonstabilized azomethine ylides. | ||
Initially, we chosed 3-nitrobenzothiophene 1a and N-(methoxymethyl)-N-(trimethylsilyl-methyl)-benzyl-amine 2a which generated in situ nonstabilized azomethine ylide in the presence of trifluoroacetic acid (TFA) as model substrates to optimize the reaction conditions. As the results are shown in Table 1. To our delight, the cycloaddition reaction proceeded smoothly and the desired [3 + 2] cycloadduct 3a was obtained in 90% yield with in high diastereoselective CH2Cl2 (entry 1). Moreover, the yield could not be further improved when the reaction time was prolonged (entry 2). The yields were decreased (5–81%) when we employed other organic solvents (entries 3–10). In order to further improve the yield, the reaction was performed at 40 °C in CH2Cl2. However, the yield of the product decreased to 72% (entry 11), which the possible reason may be that the nonstabilized azomethine ylide was unstable at high temperature that resulted in unexpected side reactions. When the amount of trifluoroacetic acid was loaded to 1.2 equiv., the reaction does not improve the yield of product 3a (entry 12). Lowering the amount of trifluoroacetic acid led to a decreased yield despite with a prolonged reaction time (entry 13). In addition, the yield of the product 3a was 64% when the reaction was carried out for 6 h (entry 14) (Table 1).
| Entry | Solvent | Time | Yield of 3ab (%) |
|---|---|---|---|
a Unless noted otherwise, reactions were performed with 3-nitrobenzothiophene 1a (0.1 mmol) and 2a (0.12 mmol), TFA (0.1 mmol, 1 equiv.) in solvent (1.0 mL) at rt.b Yield of the isolated product and dr >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by 1H NMR analysis.c The reaction was performed at 40 °C.d 1.2 equiv. TFA were used.e 0.5 equiv. TFA were used. 1 by 1H NMR analysis.c The reaction was performed at 40 °C.d 1.2 equiv. TFA were used.e 0.5 equiv. TFA were used. |
|||
| 1 | CH2Cl2 | 12 | 90 |
| 2 | CH2Cl2 | 24 | 90 |
| 3 | CHCl3 | 24 | 81 |
| 4 | DCE | 24 | 80 |
| 5 | EtOAc | 24 | <5 |
| 6 | CH3CN | 24 | <5 |
| 7 | Toluene | 24 | <5 |
| 8 | THF | 24 | 22 |
| 9 | Et2O | 24 | 20 |
| 10 | Dioxane | 24 | 73 |
| 11c | CH2Cl2 | 12 | 72 |
| 12d | CH2Cl2 | 12 | 90 |
| 13e | CH2Cl2 | 24 | 62 |
| 14 | CH2Cl2 | 6 | 64 |
| Entry | R1 | R2 | Substrate | Yieldb (%) |
|---|---|---|---|---|
a Unless noted otherwise, reactions were performed with 3-nitrobenzothiophene 1 (0.1 mmol), 2 (0.12 mmol), TFA (0.1 mmol, 1 equiv.) in CH2Cl2 (1.0 mL) at rt for 12 h, EWG = NO2.b Yield of the isolated product and dr >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by 1H NMR analysis.c The relative configuration of 3d was determined by X-ray analysis. The other products were assigned by analogy.d EWG = CN. 1 by 1H NMR analysis.c The relative configuration of 3d was determined by X-ray analysis. The other products were assigned by analogy.d EWG = CN. |
||||
| 1 | H | H | 2a | 3a, 90 |
| 2 | 5-Me | H | 2a | 3b, 92 |
| 3 | 4-Cl | H | 2a | 3c, 90 |
| 4c | 5-Cl | H | 2a | 3d, 91 |
| 5 | 4-Br | H | 2a | 3e, 92 |
| 6 | 5-Br | H | 2a | 3f, 88 |
| 7 | 6-Br | H | 2a | 3g, 87 |
| 8 | 7-Br | H | 2a | 3h, 89 |
| 9 | H | H | 2b | 3i, 84 |
| 10 | 4-Cl | H | 2b | 3j, 86 |
| 11 | 5-Cl | H | 2b | 3k, 85 |
| 12 | 4-Br | H | 2b | 3l, 82 |
| 13 | 5-Br | H | 2b | 3m, 83 |
| 14 | 6-Br | H | 2b | 3n, 81 |
| 15 | 7-Br | H | 2b | 3o, 84 |
| 16 | H | H | 2c | 3p, 0 |
| 17d | H | H | 2a | 3q, 0 |
| 18 | H | Me | 2a | 3r, 0 |
With the optimized conditions in hand, we set out to investigate the scope and limitation of 3-nitrobenzothiophenes 1 with nonstabilized azomethine ylides via dearomative [3 + 2] cycloaddition reaction to provide fused tricyclic benzo[4,5]thieno[2,3-c]pyrroles. The representative results are summarized in Table 2. Under the optimized conditions, the dearomative [3 + 2] cycloaddition reactions were tolerated all the screening various 3-nitrobenzothiophenes 1, regardless of the different positions and electronic properties of substituents into the aryl ring of 3-nitrobenzothiophenes when the 3-nitrobenzothiophenes 1 reacted smoothly with the precursor of nonstabilized azomethine ylides 2a or 2b. The reaction afforded the corresponding products 3 (3a–o) in high isolated yields with excellent diastereoselectivities (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). In addition, the relative configuration of product 3d was determined unambiguously as (3S,8S) or (3R,8R) via single-crystal X-ray diffraction analysis (CCDC 2006826†).12 For its structural details, see the ESI.†10 The other products were assigned by analogy. However, when the 2c substrate reacts with 3-nitrobenzothiophene 1a and 3-cyanobenzothiophene 1q reacts with N-(methoxymethyl)-N-(trimethylsilyl-methyl)-benzyl-amine 2a under the standard conditions (entries 16 and 17). These reactions didn't work. These reactions revealed that the compounds 2c and 1q had significantly lower reactivity. Subsequently, when we tried the reaction of 3-2-methyl-3-nitrobenzothiophene 1r with N-(methoxymethyl)-N-(trimethylsilyl-methyl)-benzyl-amine 2a under the standard conditions (entry 18). Unfortunately, it was observed that the reaction did not take place. The possible reason may be due to the increased steric hindrance at the C2-position of the 2-methyl-3-nitrobenzothiophene, inhibiting the cycloaddition reaction.
1 dr). In addition, the relative configuration of product 3d was determined unambiguously as (3S,8S) or (3R,8R) via single-crystal X-ray diffraction analysis (CCDC 2006826†).12 For its structural details, see the ESI.†10 The other products were assigned by analogy. However, when the 2c substrate reacts with 3-nitrobenzothiophene 1a and 3-cyanobenzothiophene 1q reacts with N-(methoxymethyl)-N-(trimethylsilyl-methyl)-benzyl-amine 2a under the standard conditions (entries 16 and 17). These reactions didn't work. These reactions revealed that the compounds 2c and 1q had significantly lower reactivity. Subsequently, when we tried the reaction of 3-2-methyl-3-nitrobenzothiophene 1r with N-(methoxymethyl)-N-(trimethylsilyl-methyl)-benzyl-amine 2a under the standard conditions (entry 18). Unfortunately, it was observed that the reaction did not take place. The possible reason may be due to the increased steric hindrance at the C2-position of the 2-methyl-3-nitrobenzothiophene, inhibiting the cycloaddition reaction.
 | ||
| Scheme 2 Dearomative cycloaddition reaction of 2-nitrobenzothiophene and 2-nitrobenzofuran with nonstabilized azomethine ylide. | ||
Having proven the effectiveness of our protocol for dearomative [3 + 2] cycloaddition of 3-nitrobenzothiophenes with nonstabilized azomethine ylides. Then, we next turned our attention to dearomative annulation of other heteroaromatic ring bearing nitro group to confirm the practicability of the methodology (Scheme 2). The results show that the 2-nitrobenzothiophene and 2-nitrobenzofuran proved to be well compatible with the dearomative [3 + 2] cycloaddition reaction and underwent the transformation successfully to provide the expected products in the 91% and 90% yield, respectively.
Moreover, in order to highlight the synthetic utility of our methodology, a gram scale experiment between 5 mmol of 3-nitrobenzothiophene 1a and 6 mmol of N-(methoxymethyl)-N-(trimethylsilyl-methyl)-benzyl-amine 2a proceeded smoothly under the standard conditions and offered compound 3a (1.373 g) in 88% yield with dr >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Scheme 3). Subsequently, the attempt to reduce the nitro group and remove the benzyl group of 3a through Pd/C-catalyzed hydrogenation. However, the benzyl group was not removed,11a while the nitro group on the quaternary carbon center in 3a was reduced to give an NHOH intermediate 6 in 85% yield at room temperature.11b Next, Pd/C-catalyzed hydrogenation of the NHOH intermediate 6 was successfully conducted at 60 °C to give a free amine 7 in 82% yield (Scheme 4).11
1 (Scheme 3). Subsequently, the attempt to reduce the nitro group and remove the benzyl group of 3a through Pd/C-catalyzed hydrogenation. However, the benzyl group was not removed,11a while the nitro group on the quaternary carbon center in 3a was reduced to give an NHOH intermediate 6 in 85% yield at room temperature.11b Next, Pd/C-catalyzed hydrogenation of the NHOH intermediate 6 was successfully conducted at 60 °C to give a free amine 7 in 82% yield (Scheme 4).11
In conclusion, we have successfully developed an efficient dearomative [3 + 2] cycloaddition reaction of nitrobenzothiophenes with nonstabilized azomethine ylides generated in situ. The functionalized fused tricyclic benzo[4,5]thieno[2,3-c]pyrroles frameworks were efficiently constructed in high yields (up to 92%) with excellent diastereoselectivities (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) under mild reaction condition without metal catalyst. The potential synthetic utility and practicality of the approach were also highlighted by the gram-scale experiment and the synthetic transformation of the product into other polycyclic heterocyclic compounds. The further application of this strategy is presently under bioactive investigation in our laboratory.
1 dr) under mild reaction condition without metal catalyst. The potential synthetic utility and practicality of the approach were also highlighted by the gram-scale experiment and the synthetic transformation of the product into other polycyclic heterocyclic compounds. The further application of this strategy is presently under bioactive investigation in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation (21801214) and the Higher Education Institution Key Research Project Plan of Henan Province of China (18A150014).Notes and references
- For reviews, see: (a) C.-X. Zhuo, W. Zhang and S.-L. You, Catalytic Asymmetric Dearomatization Reactions, Angew. Chem., Int. Ed., 2012, 51, 12662 CrossRef PubMed; (b) W. C. Wertjes, E. H. Southgate and D. Sarlah, Recent advances in chemical dearomatization of nonactivated arenes, Chem. Soc. Rev., 2018, 47, 7996 RSC; (c) C. Li, S. S. Ragab, G. Liu and W. Tang, Enantioselective formation of quaternary carbon stereocenters in natural product synthesis: a recent update, Nat. Prod. Rep., 2020, 37, 276 RSC; (d) C. Zheng and S.-L. You, Catalytic asymmetric dearomatization (CADA) reaction-enabled total synthesis of indole-based natural products, Nat. Prod. Rep., 2019, 36, 1589 RSC.
- For selected examples, see: (a) K. Liu, W. Song, Y. Deng, H. Yang, C. Song, T. Abdelilah, S. Wang, H. Cong, S. Tang and A. Lei, Electrooxidation enables highly regioselective dearomative annulation of indole and benzofuran derivatives, Nat. Commun., 2020, 11, 3 CrossRef PubMed; (b) J.-Q. Zhang, F. Tong, B.-B. Sun, W.-T. Fan, J.-B. Chen, D. Hu and X.-W. Wang, Pd-Catalyzed Asymmetric Dearomative Cycloaddition for Construction of Optically Active Pyrroloindoline and Cyclopentaindoline Derivatives: Access to 3a-Aminopyrroloindolines, J. Org. Chem., 2018, 83, 2882 CrossRef CAS PubMed; (c) L. Birbaum, L. Gillard, H. Gerard, H. Oulyadi, G. Vincent, X. Moreau, M. De Paolis and I. Chataigner, Dearomatization of 3-Nitroindoles with Highly gamma-Functionalized Allenoates in Formal (3 + 2) Cycloadditions, Chem.–Eur. J., 2019, 25, 13688 CrossRef CAS PubMed; (d) J.-J. Suo, J. Du, Y.-J. Jiang, D. Chen, C.-H. Ding and X.-L. Hou, Diastereo- and enantioselective palladium-catalyzed dearomative 4 + 2 cycloaddition of 3-nitroindoles, Chin. Chem. Lett., 2019, 30, 1512 CrossRef CAS; (e) P. V. Santhini, S. A. Babu, A. R. Krishnan, E. Suresh and J. John, Heteroannulation of 3-Nitroindoles and 3-Nitrobenzo[b]thiophenes: A Multicomponent Approach toward Pyrrole-Fused Heterocycles, Org. Lett., 2017, 19, 2458 CrossRef CAS PubMed; (f) Y. S. Gee, D. J. Rivinoja, S. M. Wales, M. G. Gardiner, J. H. Ryan and C. J. T. Hyland, Pd-Catalyzed Dearomative 3 + 2 Cycloaddition of 3-Nitroindoles with 2-Vinylcyclopropane-1,1-dicarboxylates, J. Org. Chem., 2017, 82, 13517 CrossRef CAS PubMed; (g) D.-F. Yue, J.-R. Zhao, X.-Z. Chen, Y. Zhou, X.-M. Zhang, X.-Y. Xu and W.-C. Yuan, Multiple Hydrogen-Bonding Bifunctional Thiourea-Catalyzed Asymmetric Dearomative 4 + 2 Annulation of 3-Nitroindoles: Highly Enantioselective Access to Hydrocarbazole Skeletons, Org. Lett., 2017, 19, 4508 CrossRef CAS PubMed; (h) X. Zhao, X. Liu, H. Mei, J. Guo, L. Lin and X. Feng, Asymmetric Dearomatization of Indoles through a Michael/Friedel-Crafts-Type Cascade To Construct Polycyclic Spiroindolines, Angew. Chem., Int. Ed., 2015, 54, 4032 CrossRef CAS PubMed; (i) Y.-C. Zhang, J.-J. Zhao, F. Jiang, S.-B. Sun and F. Shi, Organocatalytic Asymmetric Arylative Dearomatization of 2,3-Disubstituted Indoles Enabled by Tandem Reactions, Angew. Chem., Int. Ed., 2014, 53, 13912 CrossRef CAS PubMed; (j) W. Shao, H. Li, C. Liu, C.-J. Liu and S.-L. You, Copper-Catalyzed Intermolecular Asymmetric Propargylic Dearomatization of Indoles, Angew. Chem., Int. Ed., 2015, 54, 7684 CrossRef CAS PubMed; (k) C. Liu, Q. Yin, L.-X. Dai and S.-L. You, Synthesis of pyrroloindolines and furoindolines via cascade dearomatization of indole derivatives with carbenium ion, Chem. Commun., 2015, 51, 5971 RSC.
- (a) M. Bandini and A. Eichholzer, Catalytic Functionalization of Indoles in a New Dimension, Angew. Chem., Int. Ed., 2009, 48, 9608 CrossRef CAS PubMed; (b) N. R. Ball-Jones, J. J. Badillo and A. K. Franz, Strategies for the enantioselective synthesis of spirooxindoles, Org. Biomol. Chem., 2012, 10, 5165 RSC; (c) J. Ling, D. Mara, B. Roure, M. Laugeois and M. R. Vitale, Copper(I)-Catalyzed Dearomative (3 + 2) Cycloaddition of 3-Nitroindoles with Propargylic Nucleophiles: An Access to Cyclopenta[b]indolines, J. Org. Chem., 2020, 85, 3838 CrossRef CAS PubMed.
- For selected examples, see: (a) J.-R. Zhuo, B.-X. Quan, J.-Q. Zhao, M.-L. Zhang, Y.-Z. Chen, X.-M. Zhang and W.-C. Yuan, Base-mediated 4 + 2 annulation of electron-deficient nitrobenzoheterocycles and alpha,alpha-dicyanoalkenes in water: Facile access to structurally diverse functionalized dibenzoheterocyclic compounds, Tetrahedron, 2020, 76, 131115 CrossRef CAS; (b) X.-J. Zhou, J.-Q. Zhao, X.-M. Chen, J.-R. Zhuo, Y.-P. Zhang, Y.-Z. Chen, X.-M. Zhang, X.-Y. Xu and W.-C. Yuan, Organocatalyzed Asymmetric Dearomative Aza-Michael/Michael Addition Cascade of 2-Nitrobenzofurans and 2-Nitrobenzothiophenes with 2-Aminochalcones, J. Org. Chem., 2019, 84, 4381 CrossRef CAS PubMed; (c) J.-Q. Zhao, L. Yang, Y. You, Z.-H. Wang, K.-X. Xie, X.-M. Zhang, X.-Y. Xu and W.-C. Yuan, Phosphine-catalyzed dearomative (3 + 2) annulation of 2-nitrobenzofurans and nitrobenzothiophenes with allenoates, Org. Biomol. Chem., 2019, 17, 5294 RSC; (d) D.-F. Yue, J.-Q. Zhao, Y.-Z. Chen, X.-M. Zhang, X.-Y. Xu and W.-C. Yuan, Zinc-Catalyzed Enantioselective Dearomative 3 + 2 Cycloaddition Reaction of 3-Nitrobenzothiophenes and 3-Nitrothieno-2,3-b-yridine with 3-Isothiocyanato Oxindoles, Adv. Synth. Catal., 2018, 360, 1420 CrossRef CAS; (e) H. Wang, Q. Hu, M. Wang and C. Guo, Enantioselective 4 + 2 Annulation to the Concise Synthesis of Chiral Dihydrocarbazoles, Iscience, 2020, 23, 100840 CrossRef CAS PubMed; (f) P. V. Santhini, A. R. Krishnan, S. A. Babu, B. S. Simethy, G. Das, V. K. Praveen, S. Varughese and J. John, One-Pot MCR-Oxidation Approach toward Indole-Fused Heteroacenes, J. Org. Chem., 2017, 82, 10537 CrossRef CAS PubMed; (g) Y. Li, F. Tur, R. P. Nielsen, H. Jiang, F. Jensen and K. A. Jorgensen, Enantioselective Formal 4 + 2 Cycloadditions to 3-Nitroindoles by Trienamine Catalysis: Synthesis of Chiral Dihydrocarbazoles, Angew. Chem., Int. Ed., 2016, 55, 1020 CrossRef CAS PubMed; (h) X.-M. Chen, C.-W. Lei, D.-F. Yue, J.-Q. Zhao, Z.-H. Wang, X.-M. Zhang, X.-Y. Xu and W.-C. Yuan, Organocatalytic Asymmetric Dearomatization of 3-Nitroindoles and 3-Nitrobenzothiophenes via Thiol-Triggered Diastereo- and Enantioselective Double Michael Addition Reaction, Org. Lett., 2019, 21, 5452 CrossRef CAS PubMed; (i) D. Cao, A. Ying, H. Mo, D. Chen, G. Chen, Z. Wang and J. Yang, 4 + 2 Annulation of 3-Nitroindoles with Alkylidene Malononitriles: Entry to Substituted Carbazol-4-amine Derivatives, J. Org. Chem., 2018, 83, 12568 CrossRef CAS PubMed.
- For selected examples, see: (a) S.-M. Wen, C.-H. Lin, C.-C. Chen and M.-J. Wu, Efficient synthesis of 3-benzoyl Benzo[b]thiophenes and raloxifene via Mercury(II)-Catalyzed cyclization of 2-alkynylphenyl alkyl sulfoxides, Tetrahedron, 2018, 74, 2493 CrossRef CAS; (b) W. E. Noland, H. V. Kumar, Y. Reddi, C. J. Cramer, A. V. Novikov, H. Kim, Y. Zhu, Y. C. Chin, Y. Zhou, P. Radakovic, A. Uprety, J. Xie and G. C. Flick, Diels-Alder/Ene Reactivities of 2-(1'-Cycloalkenyl)thiophenes and 2-(1'-Cycloalkenyl)benzo[b]thiophenes with N-Phenylmaleimides: Role of Cycloalkene Ring Size on Benzothiophene and Dibenzothiophene Product Distributions, J. Org. Chem., 2020, 85, 5265 CrossRef CAS PubMed; (c) M. Zhang, H. Xu, C. Peng, H. Huang, S. Bo, J. Liu, X. Liu, Z. Zhen and L. Qiu, Novel NLO-phores containing dihexyl amino benzo[b]thiophene exhibiting good transparency and enhanced electro-optical activity dagger, RSC Adv., 2014, 4, 15870 RSC; (d) C. Viglianisi, L. Di Pietro, V. Meoni, R. Amorati and S. Menichetti, From simple phenols to potent chain-breaking antioxidants by transposition of benzo-1,4-oxathiines to benzo[b]thiophenes, Arkivoc, 2019, 65 Search PubMed.
- For reviews, see: (a) R. Narayan, M. Potowski, Z.-J. Jia, A. P. Antonchick and H. Wadmann, Catalytic Enantioselective 1,3-Dipolar Cycloadditions of Azomethine Ylides for Biology-Oriented Synthesis, Acc. Chem. Res., 2014, 47, 1296 CrossRef CAS PubMed; (b) C. Najera, J. M. Sansano and M. Yus, 1,3-Dipolar cycloadditions of azomethine imines, Org. Biomol. Chem., 2015, 13, 8596 RSC; (c) K. Martina, S. Tagliapietra, V. V. Veselov and G. Cravotto, Green Protocols in Heterocycle Syntheses via 1,3-Dipolar Cycloadditions, Front. Chem., 2019, 7, 1 CrossRef PubMed; (d) T. Hashimoto and K. Maruoka, Recent Advances of Catalytic Asymmetric 1,3-Dipolar Cycloadditions, Chem. Rev., 2015, 115, 5366 CrossRef CAS PubMed; (e) M. S. Singh, S. Chowdhury and S. Koley, Progress in 1,3-dipolar cycloadditions in the recent decade: an update to strategic development towards the arsenal of organic synthesis, Tetrahedron, 2016, 72, 1603 CrossRef CAS.
- For selected examples, see: (a) K.-K. Wang, Y.-L. Li, Z.-Y. Wang, M.-W. Hu, T.-T. Qiu and B.-K. Zhu, Cross 1,3-dipolar cycloadditions of C,N-cyclic azomethine imines with an N-benzyl azomethine ylide: facile access to fused tricyclic 1,2,4-hexahydrotriazines, Org. Biomol. Chem., 2019, 17, 244 RSC; (b) S.-n. Li, B. Yu, J. Liu, H.-l. Li and R. Na, Bronsted Acid or Lewis Acid Catalyzed 3 + 3 Cycloaddition of Azomethine Imines with N-Benzyl Azomethine Ylide: A Facile Access to Bicyclic N-Heterocycles, Synlett, 2016, 27, 282 CrossRef CAS; (c) K.-K. Wang, Y.-L. Li, Z.-Y. Wang, X. Ma, Y.-L. Mei, S.-S. Zhang and R. Chen, Formal [3 + 2] cycloaddition of azomethine ylides generated in situ with unactivated cyclic imines: A facile approach to tricyclic imidazolines derivatives, J. Heterocycl. Chem., 2020, 57, 1456 CrossRef CAS; (d) E. M. Buev, V. S. Moshkin and V. Y. Sosnovskikh, Nonstabilized Azomethine Ylides in the Mannich Reaction: Synthesis of 3,3-Disubstituted Pyrrolidines, Including Oxindole Alkaloids, J. Org. Chem., 2017, 82, 12827 CrossRef CAS PubMed; (e) E. M. Buev, V. S. Moshkin and V. Y. Sosnovskikh, Reagents for Storage and Regeneration of Nonstabilized Azomethine Ylides: Spiroanthraceneoxazolidines, Org. Lett., 2016, 18, 1764 CrossRef CAS PubMed; (f) A. M. D'Souza, N. Spiccia, J. Basutto, P. Jokisz, L. S. M. Wong, A. G. Meyer, A. B. Holmes, J. M. White and J. H. Ryan, 1,3-Dipolar Cycloaddition-Decarboxylation Reactions of an Azomethine Ylide with Isatoic Anhydrides: Formation of Novel Benzodiazepinones, Org. Lett., 2011, 13, 486 CrossRef PubMed; (g) V. S. Moshkin, E. M. Buev and V. Y. Sosnovskikh, Nonstabilized azomethine ylides as reagents for alkylaminomethylation of aromatic ketones via 5-aryloxazolidines, Tetrahedron Lett., 2015, 56, 5278 CrossRef CAS.
- (a) S. Roy, T. L. S. Kishbaugh, J. P. Jasinski and G. W. Gribble, 1,3-Dipolar cycloaddition of 2- and 3-nitroindoles with azomethine ylides. A new approach to pyrrolo-3,4-b-indoles, Tetrahedron Lett., 2007, 48, 1313 CrossRef CAS; (b) S. Lee, S. Diab, P. Queval, M. Sebban, I. Chataigner and S. R. Piettre, Aromatic C=C Bonds as Dipolarophiles: Facile Reactions of Uncomplexed Electron-Deficient Benzene Derivatives and Other Aromatic Rings with a Non-Stabilized Azomethine Ylide, Chem.–Eur. J., 2013, 19, 7181 CrossRef CAS PubMed.
- K.-K. Wang, Y.-L. Li, G.-Y. Ma, M.-H. Yi and B.-K. Zhu, Highly Efficient and Diastereoselective Construction of Tricyclic Pyrrolidine-Fused Benzo[b]thiophene 1,1-dioxide Derivatives via 1,3-Dipolar [3 + 2] Cycloaddition, J. Heterocycl. Chem., 2019, 56, 2274 CrossRef CAS.
- For more details, see the ESI†.
- (a) J.-Y. Guo, Z.-Y. Zhang, T. Guan, L.-W. Mao, Q. Ban, K. Zhao and T.-P. Loh, Photoredox-catalyzed stereoselective alkylation of enamides with N-hydroxyphthalimide esters via decarboxylative cross-coupling reactions, Chem. Sci., 2019, 10, 8792 RSC; (b) G.-Y. Ran, P. Wang, W. Du and Y.-C. Chen, α-Regioselective [3 + 2] annulations with Morita–Baylis–Hillman carbonates of isatins and 2-nitro-1,3-enynes, Org. Chem. Front., 2016, 3, 861 RSC; (c) Z. Wang, D.-C. Wang, M.-S. Xie, G.-R. Qu and H.-M. Guo, Enantioselective Synthesis of Fused Polycyclic Tropanes via Dearomative 3 + 2 Cycloaddition Reactions of 2-Nitrobenzofurans, Org. Lett., 2020, 22, 164 CrossRef CAS PubMed; (d) H. Wang, J. Zhang, Y. Tu and J. Zhang, Phosphine-Catalyzed Enantioselective Dearomative 3 + 2 -Cycloaddition of 3-Nitroindoles and 2-Nitrobenzofurans, Angew. Chem., Int. Ed., 2019, 58, 5422 CrossRef CAS PubMed.
- CCDC 2006826† for 3d contains the supplementary crystallographic data for this paper.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data for new compounds, and crystallographic data. CCDC 2006826 for 3d. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra05687a |
| This journal is © The Royal Society of Chemistry 2020 |