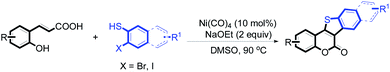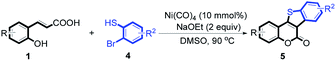Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A nickel-catalyzed tandem reaction involving cyclic esterification/C–S bond formation for synthesizing 5-oxa-11-thia-benzofluoren-6-ones†
Rongrong
Cai
,
Qicai
Wei
and
Runsheng
Xu
 *
*
Department of Biology and Environment, Jiyang College of Zhejiang A&F University, Shaoxing 311800, Zhejiang, China. E-mail: 20140041@zafu.edu.cn
First published on 14th July 2020
Abstract
A nickel-catalyzed tandem reaction involving cyclic esterification/C–S bond formation has been developed. Starting from samples containing 3-(2-hydroxy-phenyl)-acrylic acids with 2-halide-benzenethiols, versatile biologically active 5-oxa-11-thia-benzofluoren-6-one compounds were efficiently synthesized in good to high yields. This new methodology provides an economical approach toward C–S bond formation.
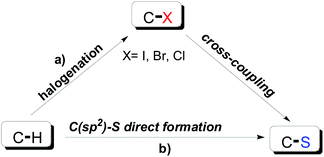
Sulfur-containing organic compounds have been widely applied in syntheses of pharmaceutical and functional materials.1 Due to its relatively large atomic radius and high electron density, sulfur displays relatively high reactivity and is easy to modify, at least in theory.2 In recent decades, the activation of the C–H bond is considered as one of the most useful C–S bond formation strategies (Scheme 1a). However, compared to C–X (X = I, Br, Cl) cross-coupling, direct C–S bond cross-coupling reactions require harsher conditions and more activated reaction systems (Scheme 1b).3 Given the present challenges, the development of more efficient and environmentally friendly chemical processes for drug discovery is required.4
5-Oxa-11-thia-benzofluoren-6-one constitutes the central core unit of a variety of natural polycyclic lactones with important biological activities, including anticancer, antibacterial, antimyotoxic, and phytoalexin effects.5 A wide range of biological properties make 5-oxa-11-thia-benzofluoren-6-ones interesting synthetic targets for chemists. Several synthetic methods have been developed for the construction of this privileged structural unit.6 Most of the reported procedures involve multiple steps with moderate overall yields. The starting materials are often not very readily available. And harsh reaction conditions are usually required. In view of these limitations, the development of an efficient strategy for synthesizing 5-oxa-11-thia-benzofluoren-6-ones is highly desirable. Herein, we report a novel nickel-catalyzed tandem reaction involving cyclic esterification/C–S bond formation (Scheme 2). Versatile biologically active 5-oxa-11-thia-benzofluoren-6-one compounds were efficiently synthesized in good to high yields under mild conditions. This new methodology was concluded to provide an economical approach toward C–S bond formation.
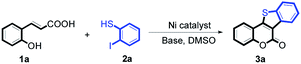
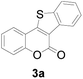
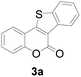
At first, the reaction conditions were screened based on the model reaction of 3-(2-hydroxy-phenyl)-acrylic acid 1a with 2-iodo-benzenethiol 2a (Table 1). The structure of 3a was confirmed from 1H NMR, 13C NMR, and HRMS analyses. Various nickel-containing catalysts were tested, and displayed good catalytic activities in the presence of Na2CO3 (entries 1–7), with the Ni(CO)4 catalyst exhibiting the best catalytic efficiency (entry 7). Various bases were also tested, and NaOEt was found to be the optimal base (entry 12), having produced the product 3a with an 83% yield. Better results were also obtained when using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 ratio of 1a to 2a than when using a 1
1.2 ratio of 1a to 2a than when using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (entries 10 and 12). Also, under these optimized conditions, the product yield was better when the reaction temperature was 90 °C than when it was 80 °C or 100 °C (entries 12, 13 and 14). Furthermore, the results also showed that the reaction yield was higher when using DMSO as the solvent than when using CHCl3 or DMF as the solvent (entries 12, 15 and 16). Thus, the optimum reaction condition was determined to be that involving reacting 1a and 2a in a 1
1 ratio (entries 10 and 12). Also, under these optimized conditions, the product yield was better when the reaction temperature was 90 °C than when it was 80 °C or 100 °C (entries 12, 13 and 14). Furthermore, the results also showed that the reaction yield was higher when using DMSO as the solvent than when using CHCl3 or DMF as the solvent (entries 12, 15 and 16). Thus, the optimum reaction condition was determined to be that involving reacting 1a and 2a in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 ratio in the presence of Ni(CO)4 (10 mol%) and NaOEt (2 equiv.) in DMSO (5 mL) at 90 °C for 10 hours (Table 1, entry 12).
1.2 ratio in the presence of Ni(CO)4 (10 mol%) and NaOEt (2 equiv.) in DMSO (5 mL) at 90 °C for 10 hours (Table 1, entry 12).
| Entry | Ni catalyst | Base |
1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a |
3a b (%) |
|---|---|---|---|---|
| a Unless otherwise noted, reaction conditions were 1a (0.5 mmol), 2a (0.5 mmol), nickel catalyst (10 mol%), base (2 equiv.), DMSO (5 mL), 90 °C, and a reaction time of 10 h. b Isolated yield. c At 80 °C. d At 100 °C. e In CHCl3. f In DMF. | ||||
| 1 | NiCl2 | Na2CO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
nr |
| 2 | NiBr2 | Na2CO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
36 |
| 3 | NiSO4 | Na2CO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
44 |
| 4 | (PCy3)2NiCl2 | Na2CO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
39 |
| 5 | (DPPE)NiCl2 | Na2CO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
55 |
| 6 | (PPh3)2NiCl2 | Na2CO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30 |
| 7 | Ni(CO)4 | Cs2CO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
77 |
| 8 | Ni(CO)4 | NaOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
65 |
| 9 | Ni(CO)4 | Na2SO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 |
| 10 | Ni(CO)4 | NaOEt | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
54 |
| 11 | Ni(CO)4 | NEt3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
46 |
| 12 | Ni(CO)4 | NaOEt | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
83 |
| 13 | Ni(CO)4 | NaOEt | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
68c |
| 14 | Ni(CO)4 | NaOEt | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
75d |
| 15 | Ni(CO)4 | NaOEt | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
73e |
| 16 | Ni(CO)4 | NaOEt | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
54f |
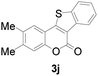
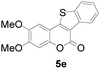
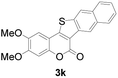
Next, a wide array of 3-(2-hydroxy-phenyl)-acrylic acids 1 and 2-iodo-benzenethiols 2 were subjected to this reaction, and provided the products 3 with good to excellent yields (69–89%, Table 2). 3-(2-Hydroxy-phenyl)-acrylic acids 1 bearing each an electron-donating group (Me and MeO) demonstrated better activity levels than did those bearing each an electron-withdrawing group (F, Cl, and Br). 2-Iodo-benzenethiols 2 bearing each an electron-withdrawing group also demonstrated better activity than did those bearing each an electron-donating group. Notably, use of very strong electron-withdrawing groups, such as trifluoromethyl and nitro groups, failed to lead to the corresponding products.
| Entry | R | R1 | 3 | Yieldb |
|---|---|---|---|---|
| a Unless otherwise noted, reaction conditions were 1 (0.5 mmol), 2 (0.6 mmol), Ni(CO)4 (10 mol%), NaOEt (2 equiv.), DMSO (5 mL), 90 °C and a reaction time of 10 h. b Isolated yield. | ||||
| 1 | H | H |
|
83 |
| 2 | 5-CH3 | H |
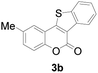
|
84 |
| 3 | 5-CH3 | Naphthyl |
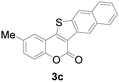
|
86 |
| 4 | 4-CH3O | H |
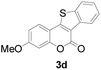
|
89 |
| 5 | 4-CH3O | 4,5-diCH3O |
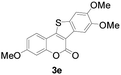
|
76 |
| 6 | 4-CH3O | Naphthyl |
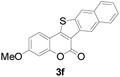
|
81 |
| 7 | 5-F | H |
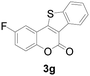
|
71 |
| 8 | 5-Cl | H |
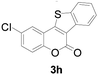
|
75 |
| 9 | 5-Br | H |
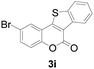
|
69 |
| 10 | 4,5-diCH3 | H |
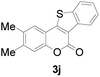
|
74 |
| 11 | 4,5-diCH3O | Naphthyl |
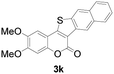
|
72 |
Furthermore, other 3-(2-hydroxy-phenyl)-acrylic acids 1 with 2-bromo-benzenethiols 4 also successfully provided the corresponding products (Table 3). 3-(2-Hydroxy-4,5-dimethoxy-phenyl)-acrylic acid displayed a moderate reactivity with chlorobenzene, and the corresponding yield was 64% (entry 4). Furthermore, to our delight, reactants with more substituents also proceeded smoothly (entry 6).
Conclusions
In summary, we have reported a nickel–catalyzed tandem reaction involving cyclic esterification/C–S bond formation. Starting from samples of 3-(2-hydroxy-phenyl)-acrylic acids with 2-halide-benzenethiols, versatile biologically active 5-oxa-11-thia-benzofluoren-6-one compounds were efficiently synthesized in good to high yields. This new methodology provides an economical approach toward C–S bond formation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support was provided by the Natural Science Foundation of China (No. 21702186).Notes and references
- (a) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400–5449 CrossRef CAS; (b) T. Itoh and T. Mase, Org. Lett., 2014, 6, 4587–4590 CrossRef; (c) M. A. Fernández-Rodríguez, Q. Shen and J. F. Hartwig, J. Am. Chem. Soc., 2006, 128, 2180–2181 CrossRef PubMed; (d) M. N. Birkholz, Z. Freixa and P. W. van Leeuwen, Chem. Soc. Rev., 2009, 38, 1099–1118 RSC; (e) I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2011, 111, 1596–1636 CrossRef CAS.
- (a) K. Pericherla, A. Jha and B. Khungar, Org. Lett., 2013, 15, 4304–4307 CrossRef CAS PubMed; (b) Z. H. Yang, Y. An, L. Y. Chen, Z. Y. Shao and S. Y. Zhao, Adv. Synth. Catal., 2016, 358, 3869–3875 CrossRef CAS; (c) K. Liao, F. Zhou, J. S. Yu, W. M. Gao and J. Zhou, Chem. Commun., 2015, 51, 16255–16258 RSC; (d) J. S. H. Yu, M. Huang, P. G. Ding, X. S. Hu, F. Zhou and J. Zhou, ACS Catal., 2016, 6, 5139–5144 Search PubMed.
- (a) R. S. Xu, J. P. Wan, H. Mao and Y. J. Pan, J. Am. Chem. Soc., 2010, 132, 15531–15533 CrossRef CAS; (b) R. S. Xu, L. Yue and Y. J. Pan, Tetrahedron, 2012, 68, 5046–5052 CrossRef CAS; (c) F. F. Duan, S. Q. Song and R. S. Xu, Chem. Commun., 2017, 53, 2737–2739 RSC; (d) R. R. Cai, Z. D. Zhou, Q. Q. Chai, Y. E. Zhu and R. S. Xu, RSC Adv., 2018, 8, 26828–26836 RSC.
- (a) M. C. Carreño, Chem. Rev., 1995, 95, 1717–1760 CrossRef; (b) N. S. Simpkins, Sulphones in Organic Synthesis, Pergamon Press, Oxford, 1993 Search PubMed; (c) M. N. Noshi, A. El-Awa, E. Torres and P. L. Fuchs, J. Am. Chem. Soc., 2007, 129, 11242–11247 CrossRef CAS PubMed; (d) A. López-Pérez, R. Robles-Machín, J. Adrio and J. C. Carretero, Angew. Chem., Int. Ed., 2007, 46, 9261–9264 CrossRef PubMed.
- (a) A. M. Myers, P. S. Charifson and C. E. Owens, et al. , J. Med. Chem., 1994, 37, 4109–4117 CrossRef CAS PubMed; (b) M. D. Collini and C. P. Miller, Tetrahedron Lett., 2001, 42, 8429–8843 CrossRef CAS; (c) H. J. Song, M. Kai, X. D. Liu, W. Yin, Q. Zeng, X. J. Yao and R. Wang, Anti-Cancer Drugs, 2012, 1 CrossRef PubMed; (d) L. L. Liu, Q. Q. Deng, S. J. Weng, X. L. Yang and Y. M. Zhong, Neuroscience, 2016, 332, 53–60 CrossRef CAS PubMed; (e) P. Zanos, R. Moaddel, P. J. Morris, L. M. Riggs, J. N. Highland, P. Georgiou, E. F. R. Pereira, E. X. Albuquerque, C. J. Thomas and C. A. Zarate, Pharmacol. Rev., 2018, 70, 621–660 CrossRef CAS PubMed.
- (a) C. P. Miller, M. D. Collini, H. A. Harris, and K. C. James, 5,11-dioxa-benzo[b]fluoren-10-one and 5-oxa-11-thia-benzo[b]fluoren-10-ones as estrogenic agents, US Pat., 20020183310, 2002 Search PubMed; (b) M. D. Collini and C. P. Miller, Tetrahedron Lett., 2001, 42, 8429–8431 CrossRef CAS; (c) J. D. Hepworth and B. M. Heron, Prog. Heterocycl. Chem., 1997, 9, 289–317 CAS; (d) M. A. Brimble, J. S. Gibson and J. Sperry, Comprehensive Heterocyclic Chemistry III, 2008, vol. 7, pp. 419–699 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra04367b |
| This journal is © The Royal Society of Chemistry 2020 |