Open Access Article
Open Access ArticleUltrafast broadband nonlinear optical properties and excited-state dynamics of two bis-chalcone derivatives
Lei Shen a,
Zhongguo Li*b,
Xingzhi Wuc,
Wenfa Zhou
a,
Zhongguo Li*b,
Xingzhi Wuc,
Wenfa Zhou d,
Junyi Yang*a and
Yinglin Song
d,
Junyi Yang*a and
Yinglin Song *ae
*ae
aSchool of Physical Science and Technology, Soochow University, Suzhou 215006, China. E-mail: 15962196650@163.com
bSchool of Electronic and Information Engineering, Changshu Institute of Technology, Changshu 215500, China. E-mail: claes.lee@gmail.com
cJiangsu Key Laboratory of Micro and Nano Heat Fluid Flow Technology and Energy Application, School of Mathematics and Physics, Suzhou University of Science and Technology, Suzhou 215009, China
dSchool of Optoelectronic Science and Engineering, Soochow University, Suzhou 215006, China
eDepartment of Physics, Harbin Institute of Technology, Harbin 150001, China. E-mail: ylsong@hit.edu.cn
First published on 17th April 2020
Abstract
The development of organic nonlinear optical (NLO) chromophores is vital for various fields such as two-photon biomedical imaging, optical limiting, etc. In this work, two bis-chalcone molecules 1,4-bis[3-(2,4-dimethoxyphenyl)-2-acryloyl]benzene (C1) and 4,4′-bis[3-(2,4-bimethoxy phenyl)-2-acryloyl]biphenyl (C2) were synthesized and characterized. The excited-state dynamics of these two chromophores were studied using femtosecond transient absorption (TA) measurements. And their broadband nonlinear absorption properties and optical limiting (OL) response were investigated by femtosecond open-aperture Z-scan and intensity-dependent transmittance measurements in the wavelength range from 515 nm to 800 nm, respectively. The TA results demonstrate that C2 has strong excited-state absorption behavior and longer lifetime. In addition, the nonlinear absorption response of C2 was found to be superior to that of C1 in the visible range after 500 nm, which is attributed to a two-photon-absorption induced excited-state absorption mechanism. These results indicate that the nonlinear optical response and excited-state dynamics in bis-chalcone compounds could be enhanced via intramolecular charge-transfer.
1. Introduction
Organic nonlinear optical (NLO) materials have attracted considerable attention in recent years due to their intriguing properties, such as structural flexibility, large π-conjugated systems, and biocompatibility, and are highly promising for applications in all-optical switching, optical limiting, bio-imaging, and so on.1–5 It is well known that the NLO response of conjugated organic materials is closely related to the structure of the molecule, such as the size of the π-conjugated system and the introduction of electron donor and electron acceptor.6–10 Hence, designing novel organic chromophores exhibiting superior NLO response is of great scientific and technological interest not only for shedding light on the nonlinear light–matter interaction but also for the development of novel photonic devices. Chalcone derivatives are a kind of π-conjugated molecules and have been widely investigated in biology and medical science.11–14 In recent years, they have received much interest due to the high tendency to crystallize and notable second and third-order NLO responses.15–17 Dharmaprakash et al. have synthesized numerous chalcone derivatives and mainly investigated the crystalline perfection, third-order NLO and OL properties.18–22 Despite these effects, however, the NLO mechanism and excited-state dynamics of these chalcone chromophores remains elusive.23 Gu et al. studied the NLO properties of chalcone derivatives and proposed that their NLO response could be attributed to the multi-photon absorption induced excited-state absorption mechanism (MPA induced ESA).24–26 Bis-chalcone is a derivative of chalcone, which has a larger π-conjugated electron system in its molecule skeleton. Although advances have been made towards investigating the NLO properties of bis-chalcones derivatives, however, the novel bis-chalcones compound with broadband ultrafast NLO response remains elusive.27–30Albota et al. reported that symmetric charge transfer from the end of a conjugated system to the middle or vice versa can lead to large two-photon cross-section.31 Following this idea, two bis-chalcone derivatives C1 (ref. 32) and C2 (Scheme 1) were synthesized and characterized. Their ultrafast excited-state dynamics were investigated using femtosecond TA measurements. And their broadband NLO responses and mechanism were also studied via femtosecond Z-scan technique in the visible regime. Our results show that the introduction of benzene ring to the bis-chalcone compound has a substantial impact on the excited-state absorption spectra and dynamics of these chromophores. And the nonlinear absorption properties of C2 is greatly enhanced compared to that of C1, indicating C2 compound is a promising OL material.
2. Experimental section and theoretical calculation
2.1 Synthesis and characterization of C1 and C2
2.2 Quantum chemical calculations
In order to investigate the influence of molecular structure on the NLO properties of the two compounds, quantum chemical calculation of density functional theory (DFT) was performed using the Gaussian 09 program package to obtain frontier molecular orbital distributions. The structures of the compounds was optimized by the B3LYP/6-31G (D, P) model. The energy and electron cloud distribution of frontier molecular orbitals were calculated. To further analyse the electron transition, the time-dependent density functional theory (TD-DFT)33 was performed using the CAM-B3LYP functional34 and the b3lyp/6-311g(d) basis set. The type of electron transition can be characterized through hole–electron analysis which was performed using Multiwfn 3.7 program.35 The data of the density isosurface were extracted from Multiwfn and visualized by the VMD program.362.3 Transient optical nonlinearity
Ultrafast TA measurements were performed in ambient atmosphere at room temperature. A detailed description of the experimental system is reported elsewhere.27 In brief, the 400 nm pump pulse was generated by an optical parametric amplifier (OPA, ORPHEUS, light conversion) pumped by a mode-locked Yb: KGW based fiber laser (PHAROS, light conversion). The laser pulse duration and repetition rate were 190 fs and 6 kHz, respectively. The probe light was the white-light supercontinuum generated using sapphire crystal. The instrument response function (IRF) was estimated to be around 250 fs. In order to minimize the high-order dynamics, the pump energy was kept below 12 mW.2.4 Nonlinear absorption experiments
The nonlinear absorption and optical limiting properties of C1 and C2 were measured by open-aperture Z-scan37 and intensity-dependent transmittance measurement with the same femtosecond laser source in fs TA experiments. The pulse duration and laser repetition rate were 190 fs and 20 Hz, respectively. The sample compounds were diluted in DMSO solvent with a concentration of 4 × 10−3 M and contained in 2 mm quartz cells. The neat DMSO solvent was also measured under the same experimental condition and the solvent's NLO response was removed in the data analysis.3. Results and discussions
3.1 UV-vis absorption and florescence spectra
The UV-vis absorption spectra and fluorescence spectra of bis-chalcone derivatives C1 and C2 are shown in Fig. 1. The measurements were carried out in dilute DMSO (2 × 10−5 M) at room temperature. The results demonstrate that the change in absorption peak of C1 and C2 is rather small (387 nm vs. 378 nm), but their emission maximum have dramatic changes (536 nm and 490 nm). The fluorescence spectrum band of C2 showed blue-shifted compared to that of C1, which indicate the strong intramolecular charge-transfer character in C2.38 This charge-transfer character can also be supported by the DFT calculation results in Fig. 2. | ||
| Fig. 1 UV-vis absorption (solid lines) and emission spectrum (dashed lines) of C1 (black) and C2 (blue). | ||
As can be seen in Fig. 2, the electron moiety transition is corresponded with the maximum absorption peak on the absorption spectra from HOMO to LUMO ability. The HOMO–LUMO energy gap of C1 and C2 are 3.40 eV and 3.46 eV, respectively. And these results corresponded to the UV-vis absorption.
Fig. 3 shows the hole and electron distributions of the two compounds. Red and grey isosurfaces denote where electrons increase (electron) and where electrons decrease (hole), respectively. The transition energies (between S0 and S1) of the two compounds were calculated to be 2.956 and 3.034 eV, respectively. The results of Fig. 3 clearly show that C2 has strong intramolecular charge-transfer. And compared with C1, C2 has more π-electrons.
3.2 Transient absorption spectrum
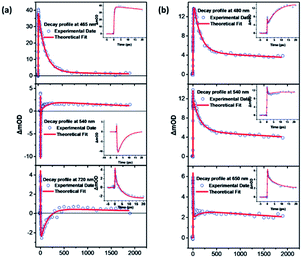
Fig. 4(a) and (b) show the TA spectra of C1 and C2 in DMSO solution, respectively. The evolution associated difference spectra (EADS) for C1 and C2 are plotted in Fig. 4(c) and (d), respectively. In Fig. 4(c), the TA spectrum at a delay of 0.55 picosecond (ps) after 400 nm laser excitation is characterized by an excited state absorption (ESA) band with peak before 460 nm and a stimulated emission (SE) peak at about 540 nm. The transient spectrum evolves in 20 ps, exhibiting a dynamic red shift of the SE band maximum to 610 nm which may attribute to the vibrational relaxation of S1. With further time delay, the ESA band and SE band decays up to about 400 ps. The TA spectral evolution of C1 suggests three distinct relaxation processes which occur in 0–0.25 ps, 0.25–20 ps, 20–350 ps, 350 ps–ns time scales. Through global analysis39 (see Fig. 5(a)), we obtain four dynamic processes and the lifetimes are about 0.25 ps, 4.9 ps, 142.8 ps and 2.9 ns, respectively. The first content is unable to be distinguished as the time resolution of our experiment is 0.25 ps. So we assign the ultrafast process of 0.25 ps to the internal conversion in the first singlet state (S1). The next component with a 4.9 ps lifetime corresponds to the vibrational relaxation of S1. The last two times are then assigned to the inter-system crossing (ISC) process between singlet and triplet state (142.8 ps) and lifetime of the triplet state (2.9 ns).40,41 | ||
| Fig. 4 TA spectra of C1 (a) and C2 (b) diluted in DMSO at excitation wavelength of 400 nm. (c and d) Plots of the TA spectra at various time delays of C1 and C2, respectively. | ||
As shown in Fig. 4(d), the TA spectra of C2 underwent dramatic changes compared to the TA spectra of C1. Following 400 nm laser excitation, the TA spectrum at 0.2 ps is characterized by an excited state absorption (ESA) band with peak at around 540 nm. The valley at about 485 nm attributes to the competition between ESA and the effect of fluorescence. The ESA peak at 540 nm exhibits a red shift with a decrease in intensity within 1.4 ps. With further time delay, a new ESA band grows in the 450–550 nm region. After 12 ps, on further delay, ESA decay in the 1100 ps time scale. And then follows a much longer ESA decay in the time scale of ns. Through global analysis (see Fig. 5(b)), we obtain four similar dynamic processes with C1 and the lifetimes are about 0.25 ps, 7.5 ps, 144.8 ps and 7 ns, respectively. It can be easily seen in Fig. 5, unlike C1, with the improvement of the molecule, the relaxation process of excited state has been changed and the lifetime becomes longer (from 2.9 ns to 7 ns). Furthermore, there's no SE in the TA spectrum of C2. And the intensity of ESA of C2 is stronger than that of C1 in the visible range after 500 nm which means the nonlinear optical properties of C2 can be improved because of more π-electrons in C2.42–44 The TA spectra indicate that the excited-state relaxation processes change dramatically with the molecule structure change from C1 to C2, which could be related to the stronger intramolecular charge-transfer character in C2.
3.3 Femtoseconds Z-scan experiment
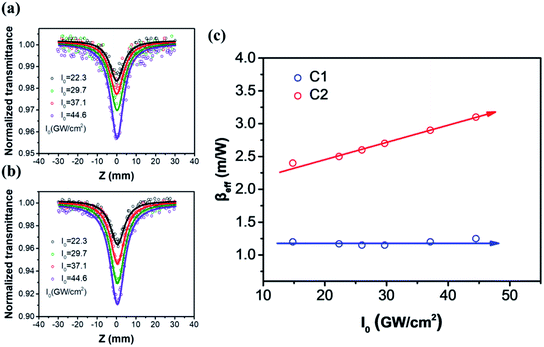
To investigate the nonlinear optical response of these two compounds, open-aperture Z-scan measurements were carried out at different laser intensity in wavelength range of 515–800 nm. The concentration of C1 and C2 were about 5.2 × 10−3 mol L−1. The contribution of solvent's NLO response was subtracted in the data fitting. The experimental results show that C2 has larger reverse saturation absorption (RSA) than C1 in the whole wavelength range. Due to the fs laser pulses used in the Z-scan measurements, the main RSA mechanism should be intrinsic multi-photon absorption. To further analyze the mechanism of the observed optical nonlinearities, all Z-scan measurements were carried out under different laser intensity. And the variation of nonlinear absorption coefficient with different laser irradiance at 600 nm was plotted in Fig. 6(c).By fitting to the Z-scan theory,37 the effective nonlinear absorption βeff at different intensities were obtained. From Fig. 6(c), the extracted βeff of C1 is observed to remain substantially unchanged, indicating that 2 PA may be the only mechanism under this wavelength. In contrast, the βeff of C2 is nearly a proportionally increasing function of the intensity I0, which means there was higher-order nonlinear absorption. Due to the laser wavelength is far away from the potential 3PA window (>1100 nm), the higher-order nonlinear absorption can be attributed to the 2PA-induced ESA.45–47 Therefore, the Z-scan results in Fig. 6 agree well with the fs TA measurements of the two compounds. According to the 2PA-induced ESA theory, the nonlinear absorption coefficient can be expressed as α = α0 + βI + γI2, where α0, β and γ represent the linear absorption, TPA, and the effective fifth-order nonlinear absorption coefficients, respectively. And the TPA cross section σTPA can be achieved by the equation σTPA = hωβ/N. Both β and γ can be determined by theoretical fitting the Z-scan curves in different laser intensity. All NLO parameters of the two compounds are summarized in Table 1. The experimental error was less than 10%.
| Wavelength (nm) | C1 | C2 | ||||
|---|---|---|---|---|---|---|
| β (10−2 cm GW−1) | σTPA (GM) | γ (10−4 cm3 GW−2) | β (10−2 cm GW−1) | σTPA (GM) | γ (10−4 cm3 GW−2) | |
| 515 | 0.50 | 80.2 | 2.0 | 0.63 | 101.0 | 2.9 |
| 532 | 0.95 | 139.7 | 2.5 | 1.1 | 170.7 | 2.7 |
| 580 | 0.9 | 128.2 | 0.75 | 1.99 | 283.4 | 2 |
| 600 | 1.19 | 163.8 | 0 | 1.99 | 274.0 | 2.4 |
| 650 | 0.4 | 50.8 | 0.2 | 0.7 | 88.9 | 0.25 |
| 700 | 0.66 | 77.9 | 0.15 | 0.95 | 112.1 | 0.2 |
| 800 | 0.6 | 67.1 | 0.17 | 0.9 | 92.9 | 0.25 |
From Table 1, it is shown that both compounds exhibit TPA induced ESA in the whole wavelength window from 515 nm to 800 nm, except for C1 compound in around 600 nm. The results also demonstrate that the two-photon absorption coefficients β and TPA cross section σTPA of C2 are substantially larger than C1, which indicate that C2 has promising nonlinear absorption response in the visible regime.
3.4 Optical limiting behaviour
From previous analysis, C2 compound has strong ICT and exhibits favorable TPA and ESA,45,46 which means it may have strong optical limiting response in the visible wavelength regime. Fig. 7 shows the variations of the transmittance of C2 diluted in DMSO as a function of the input laser fluence at 480, 532, 600, 700 and 800 nm. The sample solution was contained in a 10 mm thick cuvette. Fig. 7 shows that the C2 shows high linear transmittance at all wavelengths (0.85–0.98). And the transmittance of C2 solution decreases with increasing input irradiance. The properties of OL material is shown as the OL threshold, which is the laser fluence when the transmittance of the sample decreases to 50% of the original value. The OL threshold of C2 and other organic materials reported in recent literatures are summarized in Table 2.38,45,46,48–52 The results in Table 2 show that the C2 compound has strong OL response compared to other organic compounds and inorganic nanomaterials, which means it's a promising ultrafast broadband optical limiter. Based on the fs TA and Z-scan measurements above, the OL response of C2 can be attributed to the TPA induced ESA mechanism.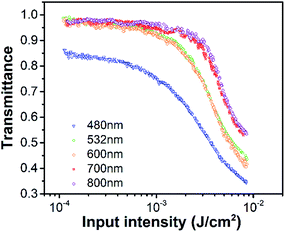 | ||
| Fig. 7 Ultrafast OL of C2 as a function of the input fluence at 480 nm, 532 nm, 600 nm, 700 nm and 800 nm with 190 fs excitation. | ||
| Sample | λ/pulse | Wavelength (nm) | T (%) | Threshold (10−3 J cm−2) | Ref. |
|---|---|---|---|---|---|
| C2/DMSO | 190 fs | 480 | 85 | 4.9 | This work |
| 532 | 98 | 6.5 | |||
| 600 | 98 | 5.9 | |||
| 700 | 98 | 10.3 | |||
| 800 | 98 | 12.9 | |||
| Pyrene-based molecules/DMSO | 190 fs | 515–900 | >91 | ≈19 | 45 |
| Hydrazone derivatives/DMF | 190 fs | 515–800 | 72–98 | 2.75–10.6 | 46 |
| Fluorescent stilbene dye/CHCl3 | 100 fs | 800 | — | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
38 |
| Silver nanoparticle-embedded polymer film | 110 fs | 800 | 94 | 154 | 48 |
| a-Se doped silica | 100 fs | 800 | 85 | 450 | 49 |
| QD | 8 ns | 532, 1064 | — | >720 | 50 |
| Grapheme/water | 8 ns | 532, 1064 | 70 | >500 | 51 |
| CNT/water | 3–80 ns | 430–1064 | — | 70–947 | 52 |
| CNT/chloroform | 3–80 ns | 430–1064 | — | 13–740 | 52 |
4. Conclusions
In summary, two bis-chalcone derivatives C1 and C2 were successfully synthesized and characterized by UV-vis, fluorescent spectra and DFT and TD-DFT calculation. And their nonlinear optical response and excited-state dynamics were characterized using broadband femtosecond Z-scan measurement and transient absorption measurements, respectively. The fs TA spectra demonstrate that C2 has strong excited-state absorption behaviour and longer excited-state lifetime compared to C1. Remarkably, the Z-scan measurements show that the nonlinear absorption response of C2 is stronger than C1 in range of 515 nm to 800 nm under the excitation of fs laser pulse, which is attributed to the 2PA-induced ESA mechanism. These results indicate that the nonlinear optical response and excited-state dynamics in bis-chalcone chromophore can be fine-tuned by intramolecular charge-transfer process. Our results clearly demonstrate the bis-chalcone derivatives C2 with excellent nonlinear absorption and longer lifetime can be promising candidate for future applications in optical limiting, bio-imaging, etc.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the support of the NSAF (Grant No. U1630103), Special Fund from State Key Laboratory of Intense Pulsed Radiation Simulation and Effect (Grant No. SKLIPR1715) and National Natural Science Foundation of China (Grant No. 11704048, 11804244).References
- Y. Song, Y. Chen, X. Jiang, W. Liang, K. Wang, Z. Liang, Y. Ge, F. Zhang, L. Wu and J. Zheng, Adv. Opt. Mater., 2018, 6, 1701287 CrossRef.
- X. Li, R. Liu, H. Xie, Y. Zhang, B. Lyu, P. Wang, J. Wang, Q. Fan, Y. Ma and S. Tao, Opt. Express, 2017, 25, 18346–18354 CrossRef CAS PubMed.
- I. I. Smolyaninov, J. Opt. Soc. Am. B, 2019, 36, 1629–1636 CrossRef CAS.
- S. J. Varma, J. Kumar, Y. Liu, K. Layne, J. Wu, C. Liang, Y. Nakanishi, A. Aliyan, W. Yang and P. M. Ajayan, Adv. Opt. Mater., 2017, 5, 1700713 CrossRef.
- Y. Tamgadge, A. Sunatkari, S. Talwatkar, V. Pahurkar and G. Muley, Opt. Mater., 2016, 51, 175–184 CrossRef CAS.
- B. Jędrzejewska, P. Krawczyk, M. Pietrzak, M. Gordel, K. Matczyszyn, M. Samoć and P. Cysewski, Dyes Pigm., 2013, 99, 673–685 CrossRef.
- M. Tasior, K. Hassanein, L. M. Mazur, I. Sakellari, D. Gray, M. Farsari, M. Samoć, F. Santoro, B. Ventura and D. T. Gryko, Phys. Chem. Chem. Phys., 2018, 20, 22260–22271 RSC.
- L. M. Mazur, T. Roland, S. Leroy-Lhez, V. Sol, M. Samoc, I. D. Samuel and K. Matczyszyn, J. Phys. Chem. B, 2019, 123, 4271–4277 CrossRef CAS PubMed.
- J. Jia, J. Zhang, C. Zhou, M. Zheng, D. Feng, G. Liang and Y. She, Dyes Pigm., 2019, 166, 314–322 CrossRef CAS.
- M. de Torres, S. Semin, I. Razdolski, J. Xu, J. A. Elemans, T. Rasing, A. E. Rowan and R. J. Nolte, Chem. Commun., 2015, 51, 2855–2858 RSC.
- Z. Rozmer and P. Perjési, Phytochem. Rev., 2016, 15, 87–120 CrossRef CAS.
- P. M. Sivakumar, S. Cometa, M. Alderighi, V. Prabhawathi, M. Doble and F. Chiellini, Carbohydr. Polym., 2012, 87, 353–360 CrossRef CAS.
- A. Foroumadi, S. Emami, M. Sorkhi, M. Nakhjiri, Z. Nazarian, S. Heydari, S. K. Ardestani, F. Poorrajab and A. Shafiee, Chem. Biol. Drug Des., 2010, 75, 590–596 CrossRef CAS PubMed.
- S. Chowdhary, P. Bhoj, S. Bahekar, N. Togre, K. Goswami and H. Chandak, J. Mahatma Gandhi Inst. Med. Sci., 2017, 22, 128 Search PubMed.
- S. R. Maidur, P. S. Patil, S. V. Rao, M. Shkir and S. Dharmaprakash, Opt. Laser Technol., 2017, 97, 219–228 CrossRef CAS.
- P. Patil, S. R. Maidur, S. V. Rao and S. Dharmaprakash, Opt. Laser Technol., 2016, 81, 70–76 CrossRef CAS.
- P. S. Patil, S. R. Maidur, M. Shkir, S. AlFaify, V. Ganesh, K. N. Krishnakanth and S. V. Rao, J. Appl. Crystallogr., 2018, 51, 1035–1042 CrossRef CAS.
- P. Patil, M. Bannur, D. Badigannavar and S. Dharmaprakash, Opt. Laser Technol., 2014, 55, 37–41 CrossRef CAS.
- T. C. S. Shetty, S. Raghavendra, C. C. Kumar, S. Naveen, S. R. Maidur, P. S. Patil, S. Chandraju, G. Ananthnag and S. Dharmaprakash, Opt. Mater., 2018, 86, 138–147 CrossRef.
- T. C. S. Shetty, S. Raghavendra, C. C. Kumar and S. Dharmaprakash, Appl. Phys. B: Lasers Opt., 2016, 122, 205 CrossRef.
- P. Patil, S. Dharmaprakash, H.-K. Fun and M. Karthikeyan, J. Cryst. Growth, 2006, 297, 111–116 CrossRef CAS.
- P. Patil, S. Dharmaprakash, K. Ramakrishna, H.-K. Fun, R. S. S. Kumar and D. N. Rao, J. Cryst. Growth, 2007, 303, 520–524 CrossRef CAS.
- H. Song, Z. Kuang, X. Wang, Y. Guo, Q. Guo, H. Zhang and A. Xia, J. Phys. Chem. C, 2018, 122, 15108–15117 CrossRef CAS.
- B. Gu, W. Ji, X.-Q. Huang, P. Patil and S. Dharmaprakash, Opt. Express, 2009, 17, 1126–1135 CrossRef CAS PubMed.
- B. Gu, W. Ji, P. Patil, S. Dharmaprakash and H.-T. Wang, Appl. Phys. Lett., 2008, 92, 091118 CrossRef.
- B. Gu, W. Ji, H.-Z. Yang and H.-T. Wang, Appl. Phys. Lett., 2010, 96, 081104 CrossRef.
- Y. Yang, X. Wu, J. Jia, L. Shen, W. Zhou, J. Yang and Y. Song, Opt. Laser Technol., 2020, 123, 105903 CrossRef CAS.
- P. Poornesh, K. Ravi, G. Umesh, P. K. Hegde, M. Manjunatha, K. Manjunatha and A. Adhikari, Opt. Commun., 2010, 283, 1519–1527 CrossRef CAS.
- A. M. Asiri and S. A. Khan, Mater. Lett., 2011, 65, 1749–1752 CrossRef CAS.
- M. S. Kiran, B. Anand, S. S. S. Sai and G. N. Rao, J. Photochem. Photobiol., A, 2014, 290, 38–42 CrossRef.
- M. Albota, D. Beljonne, J.-L. Brédas, J. E. Ehrlich, J.-Y. Fu, A. A. Heikal, S. E. Hess, T. Kogej, M. D. Levin and S. R. Marder, Science, 1998, 281, 1653–1656 CrossRef CAS PubMed.
- S. Tsukerman, V. Maslennikova, V. Nikitchenko and V. Lavrushin, J. Appl. Spectrosc., 1970, 12, 76–80 CrossRef.
- F. Furche and R. Ahlrichs, J. Chem. Phys., 2002, 117, 7433–7447 CrossRef CAS.
- T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS.
- M. Sheik-Bahae, A. A. Said, T.-H. Wei, D. J. Hagan and E. W. Van Stryland, IEEE J. Quantum Electron., 1990, 26, 760–769 CrossRef CAS.
- T. He, S. Sreejith, Y. Gao, A. C. Grimsdale, Y. Zhao, X. Lin and H. Sun, Appl. Phys. Lett., 2015, 106, 111904 CrossRef.
- I. H. Van Stokkum, D. S. Larsen and R. Van Grondelle, Biochim. Biophys. Acta, Bioenerg., 2004, 1657, 82–104 CrossRef CAS PubMed.
- R. M. van der Veen, A. Cannizzo, F. Van Mourik, A. Vlcek Jr and M. Chergui, J. Am. Chem. Soc., 2011, 133, 305–315 CrossRef CAS PubMed.
- Y. p. Wang, S. Zhang, S. m. Sun, K. Liu and B. Zhang, Chin. J. Chem. Phys., 2013, 26, 651–655 CrossRef CAS.
- F. Guo, W. Sun, D. Wang, L. Zhao, Z. Lu and Y. Nie, Appl. Opt., 2001, 40, 1386–1388 CrossRef CAS PubMed.
- J. Jia, X. Wu, Y. Fang, J. Yang, Y. Han, J. Xiao, X. Zhang, Y. Wang and Y. Song, J. Phys. Chem. C, 2020, 124, 4701–4708 CrossRef CAS.
- T. C. Lin, Y. H. Lee, C. Y. Liu, B. R. Huang, M. Y. Tsai, Y. J. Huang, J. H. Lin, Y. K. Shen and C. Y. Wu, Chem. - Eur. J., 2013, 19, 749–760 CrossRef CAS PubMed.
- Z. Xiao, Y. Shi, R. Sun, J. Ge, Z. Li, Y. Fang, X. Wu, J. Yang, M. Zhao and Y. Song, J. Mater. Chem. C, 2016, 4, 4647–4653 RSC.
- J. Jia, X. Wu, Y. Fang, J. Yang, X. Guo, Q. Xu, Y. Han and Y. Song, J. Phys. Chem. C, 2018, 122, 16234–16241 CrossRef CAS.
- R. Niu, Q. Chang, X. Wu, Y. Han, J. Jia, S. Chen and Y. Song, Opt. Mater., 2018, 85, 319–328 CrossRef CAS.
- S. Porel, N. Venkatram, D. N. Rao and T. Radhakrishnan, J. Appl. Phys., 2007, 102, 033107 CrossRef.
- K. A. Mary, N. Unnikrishnan and R. Philip, RSC Adv., 2015, 5, 14034–14041 RSC.
- Z. Xie, F. Wang and C. y. Liu, Adv. Mater., 2012, 24, 1716–1721 CrossRef CAS.
- M. Feng, H. Zhan and Y. Chen, Appl. Phys. Lett., 2010, 96, 033107 CrossRef.
- L. Vivien, D. Riehl, P. Lancon, F. Hache and E. Anglaret, Opt. Lett., 2001, 26, 223–225 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |