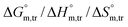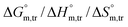Open Access Article
Open Access ArticleEffects of temperature and polyols on the ciprofloxacin hydrochloride-mediated micellization of sodium dodecyl sulfate†
Shamim Mahbubab,
Sayma Aktera,
Luthfunnessaa,
Parul Aktera,
Md. Anamul Hoque b,
Malik Abdul Rub
b,
Malik Abdul Rub cd,
Dileep Kumar
cd,
Dileep Kumar *ef,
Yousef G. Alghamdic,
Abdullah M. Asiri
*ef,
Yousef G. Alghamdic,
Abdullah M. Asiri cd and
Hurija Džudžević-Čančarg
cd and
Hurija Džudžević-Čančarg
aDepartment of Chemistry & Physics, Gono Bishwabidyalay, Savar, Dhaka-1344, Bangladesh
bDepartment of Chemistry, Jahangirnagar University, Savar, Dhaka-1342, Bangladesh
cChemistry Department, Faculty of Science, King Abdulaziz University, Jeddah-21589, Saudi Arabia
dCenter of Excellence for Advanced Materials Research, King Abdulaziz University, Jeddah-21589, Saudi Arabia
eDivision of Computational Physics, Institute for Computational Science, Ton Duc Thang University, Ho Chi Minh City, Vietnam. E-mail: dileepkumar@tdtu.edu.vn; Tel: +84943720085
fFaculty of Applied Sciences, Ton Duc Thang University, Ho Chi Minh City, Vietnam
gDepartment of Natural Science in Pharmacy, Faculty of Pharmacy, University of Sarajevo, Zmaja od Bosne 8, 71 000 Sarajevo, Bosnia and Herzegovina
First published on 9th April 2020
Abstract
Herein, a conductivity method was engaged to explore the effects of a fluoroquinolone drug, namely ciprofloxacin hydrochloride (CFH)/CFH + polyols (organic compounds with multiple hydroxyl groups (glucose and fructose)), on the aggregation phenomenon of sodium dodecyl sulfate (SDS) at different temperatures (298.15–318.15 K) while maintaining a gap of 5 K. In this study, the critical micelle concentration (cmc) of the SDS/SDS + CFH mixture in water and polyols media was determined from plots of the specific conductivity versus the concentration of SDS to gain knowledge of the effects of CFH/CFH + polyols on the micelle formation behavior of SDS. The cmc value of the surfactant decreases in the presence of CFH in an aqueous medium; thus, CFH favors the micellization of SDS. The cmc values of SDS and the SDS + CFH mixture were enhanced in polyols media. The cmc values of SDS/SDS + CFH show a U-shaped behavior with temperature. The counterion dissociation (α) of the pure surfactant is higher in the presence of the drug and is further enhanced through an increase in the CFH concentration in water/polyols media. Different thermodynamic parameters, such as the Gibbs free energy of micellization 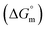 , standard enthalpy
, standard enthalpy 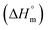 , entropy
, entropy 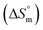 , different transfer energies and enthalpy–entropy compensation parameters of micellization were determined and illustrated in detail to compare these parameters between the pure SDS and SDS + CFH mixture in polyols media. The negative values of
, different transfer energies and enthalpy–entropy compensation parameters of micellization were determined and illustrated in detail to compare these parameters between the pure SDS and SDS + CFH mixture in polyols media. The negative values of 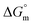 for the SDS/SDS + CFH mixture in all cases indicate spontaneous micelle formation. The
for the SDS/SDS + CFH mixture in all cases indicate spontaneous micelle formation. The  and
and 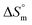 values indicate the presence of both hydrophobic and electrostatic interactions amongst the studied components.
values indicate the presence of both hydrophobic and electrostatic interactions amongst the studied components.
1. Introduction
A surfactant with a structure that contains both hydrophilic and hydrophobic moieties in the same molecule possesses unique solubility properties, i.e., solubility in both polar and nonpolar media. In an aqueous medium, a surfactant can form aggregates at a certain concentration due to a delicate balance of hydrophilic interactions and hydrophobic interactions.1 This surfactant aggregate is known as a micelle, and the concentration at which a surfactant micelle formed is known as cmc.2–4 This micellar aggregation of an amphiphilic substance can augment the solubility of weakly soluble organic compounds by altering their microenvironment properties e.g. polarity, surface tension, viscosity etc.5 The solubility enhancement property of surfactant micelles is the key factor in their wide use in industrial, commercial and technological applications.6–11 Surfactants are extensively found in products (medicines, food, cosmetics, paints etc.) are used in daily life. Surfactants are widely used in the textile and pharmaceutical industries as solubility enhancers, diluents, emulsifying agents, and stabilizing agents.12–16 In drug formulation and development as well as drug delivery, the study of the molecular interactions between the surfactant and desired drug is interesting and important because the efficiency of drug release and drug delivery depend on the molecular interactions of the surfactants and drug. The release of sparingly water-soluble drugs is enhanced in the presence of surfactant. In the presence of a lower concentration of surfactant, penetration of water molecules into the drug mass is facilitated due to the lower surface tension and thus wettability of drug increases. At higher concentrations (above cmc), the dissolution of the drug is higher due to the solubilization phenomenon of the surfactant micelles. This solubilization phenomenon enhances the rate of entry of drug molecules into the bloodstream; however, excess use of surfactant lowers the drug absorption level by lowering the chemical potential of the drug. This occurs when the amount of surfactant exceeds the required amount to solubilize the drug,15 i.e., it exceeds the cmc under the corresponding conditions. Thus, it is important to accurately determine the cmc of a surfactant in the presence of a drug in different media for drug formulation. Again, the structures of surfactants are comparable with the biological membrane; therefore, they can be considered as model biomembranes, and the interactions of drugs with surfactants in the presence of different additives provide important information about the behavior of drugs with biological membranes.17,18 In different biomedical applications, fluorescent organic nanoparticles (FON) are significantly employed and have advantages over conventional fluorescent inorganic nanoparticles.19–23 However, a drawback of FON is their hydrophobic properties and insolubility in water.19,20 Thus, the synthesis of FON with amphiphilic properties as well as increased hydrophilicity is desirable in the field of bioengineering. In the synthesis of amphiphilic FON, surfactants with a combination of aggregation-induced emission components play a significant role.19,20SDS is an anionic surfactant; it is the most common surfactant used in detergents and is highly effective to remove oily stains. It is used as a food additive and is generally recognized as a safe ingredient. SDS is potentially effective to hinder and avert infections caused by numerous viruses, such as HIV, herpes simplex and the Semliki Forest virus.24,25 It is also utilized as a cell lysis agent to extract DNA/RNA and to denature proteins. Aqueous solutions of SDS with Triton X-100 and sodium dodecylbenzene sulfonate are popular for suspending nanotubes, such as carbon nanotubes.26 The currently employed drug CFH is a fluoroquinolone antibiotic drug that is utilized to treat different bacterial diseases, such as skin, respiratory/sinus, bone and joint and urinary tract infections. It can also be exploited for the treatment of gonorrhea and to treat persons affected by anthrax or plague. However, CFH tends to reduce blood sugar levels. Diabetic patients are at very high risk for numerous bacterial infections, e.g. skin infections, respiratory infections, and urinary tract infections.27,28 Skin infection, as well as delay of wound healing, are very common in diabetic patients;29,30 interestingly, CFH is used for the treatment of these infections.31 Thus, the interaction of CFH with a model surfactant such as SDS (or other pharmaceutical ingredients) in the presence of different sugars such as glucose and fructose, which are also known as polyols, is important. The transportation of hydrophobic drugs in the human body is accomplished by the incorporation of surfactant micelles. Again, the micelle formation of a surfactant is a function of the additive concentration. The uptake of CFH by a patient can alter the cmc of the used surfactant; thus, the drug transportation properties can be altered. Thus, the amount of surfactant that needs to be used in a drug formulation can be understood from the cmc values obtained at varying conditions.
Rub et al.32 investigated the behavior of SDS with the amphiphilic drug promazine hydrochloride in the presence of electrolyte and urea; they reported that the cmc was reduced in electrolyte medium and enhanced in urea medium. Our group also investigated the interactions between SDS and CFH in H2O/electrolyte solutions at different temperatures and observed favorable micellization in the electrolyte medium.33 The behavior of tetradecyltrimethylammonium bromide (TTAB) and an antibiotic drug, levofloxacin hemihydrate (LFH), in the presence of monohydroxy/polyhydroxy organic compounds was also investigated by our group.34 Although a large number of investigations about the interactions of different ionic surfactants with various drugs have been reported,32–35 studies of the interactions of SDS with CFH in the presence of polyols are rare. Accordingly, we planned here to investigate the association behavior of SDS in media containing CFH/CFH + polyols. In the current study, different physico-chemical parameters, such as cmc, α, standard free energy change 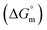 , enthalpy change
, enthalpy change 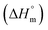 , entropy change
, entropy change 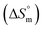 , and intrinsic enthalpy gain
, and intrinsic enthalpy gain 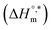 , have been assessed for SDS aggregation in the presence of CFH/CFH + polyols mixtures to understand the effects of CFH and polyols as well as to elucidate the modes of interaction between the employed components at different temperatures. Here, we apply the surfactant as a drug carrier in glucose or fructose medium as a model drug delivery system. Glucose and fructose are also found in the human body; therefore, their presence may affect the micellization tendencies of surfactant and surfactant–drug mixtures because surfactants are usually used as drug carriers.
, have been assessed for SDS aggregation in the presence of CFH/CFH + polyols mixtures to understand the effects of CFH and polyols as well as to elucidate the modes of interaction between the employed components at different temperatures. Here, we apply the surfactant as a drug carrier in glucose or fructose medium as a model drug delivery system. Glucose and fructose are also found in the human body; therefore, their presence may affect the micellization tendencies of surfactant and surfactant–drug mixtures because surfactants are usually used as drug carriers.
2. Experimental
2.1 Materials
In this study, we utilized analytical grade chemicals without any purification. SDS (CAS number: 151-21-3) with a mass fraction purity of 0.98 was collected from Scharlau Chemie S. A. (E.U.). CFH (CAS number: 86483-48-9) was collected from Gonoshasthaya Pharmaceuticals Ltd., Bangladesh. Glucose (CAS number: 50-99-7) and fructose (CAS number: 57-48-7) were collected from Merck, Germany. The mass fraction purities of CFH, glucose, and fructose were 0.98, 0.99 and 0.98, respectively. All employed system solutions were prepared using double-distilled deionized water with a specific conductivity of less than 2 μS cm−1 over the temperature range from 298.15 to 318.15 K.2.2 Conductivity method
Conductivity measurements were performed to elucidate the interactions between SDS (shown in Scheme 1) and CFH (shown in Scheme 2) in the presence/absence of polyols according to a process mentioned in the literature.36–40 The specific conductivities of the SDS and SDS + CFH mixed systems with different concentrations of CFH in H2O and polyols (glucose/fructose) media were recorded with a conductivity meter (digital HI 2315, Hanna, Germany) with a dip cell; the cell constant provided by the company was 1.0 cm−1. The precision of the employed conductivity meter was about ±0.5%. The temperature of the system was sustained at the desired value with an RM6 Lauda H2O thermostatted bath with a precision of ± 0.2 K. For calibration of the conductivity meter, 0.1 N KCl solution was utilized prior to each experiment. 50 mL of solvent (H2O/10 mmol kg−1 glucose/10 mmol kg−1 fructose) in the absence/presence of CFH was placed in a large test tube, and the specific conductivity of the corresponding system was measured. Later, a fixed concentration of surfactant solution prepared in the corresponding solvent was progressively added to the solvent. After appropriate mixing and sufficient time to attain temperature equilibration, the conductivity of the ensuing solution was noted. After that, the observed specific conductivity (κ) reading versus [SDS] was plotted by Origin 17 software, and the cmc of that system was estimated from the sharp contravention point.3. Results and discussion
3.1 Critical micelle concentration (cmc) and extent of counterion dissociation (α)
Self-assembly is a general route for the formation of nanoparticles and surfactants that are used in smart materials. Thus, the effects of the concentration of the surfactant, such as sodium dodecyl sulfate (SDS), on the formation of self-assemblies should be evaluated, i.e. it is necessary to evaluate the critical concentration of self-assembly (cmc) of SDS in different conditions. For this purpose, in the current study, the cmc values of pure surfactant/surfactant + CFH mixed systems in the absence/presence of polyols (glucose or fructose) at different temperatures were evaluated. A simple and reliable conductometric technique was employed to determine the cmc of SDS in different additive media. The specific conductivity of the solution of the ionic surfactant shows a linear relationship with the concentration of the surfactant. The conductivity of SDS solution increases with increasing concentration of SDS because the concentrations of Na+ and DS− (dodecyl sulfate ion) originate from the increasing disintegration of SDS. However, disruption of the linear relationship between the conductivity and the concentration of SDS was observed at a certain concentration due to micelle formation of SDS because SDS micelles has lower mobility than monomeric SDS molecules. Thus, the contravention point in the specific conductivity (κ) vs. [SDS] is considered to be the cmc of the corresponding surfactant system.34,41,42 Fig. 1 shows the plots of κ vs. surfactant concentration.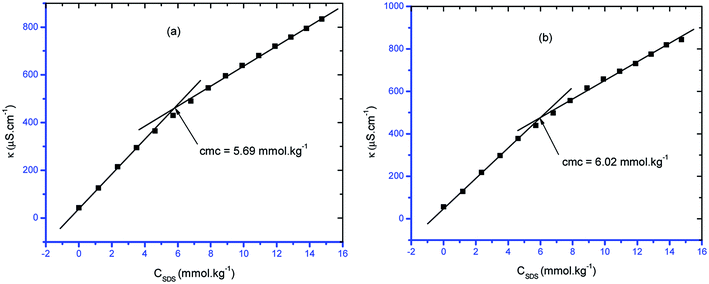 | ||
| Fig. 1 Representative graphs of κ vs. [SDS] containing 0.5 mmol kg−1 CFH in (a) glucose (10 mmol kg−1) and (b) fructose (10 mmol kg−1) at temperature = 303.15 K. | ||
The observed cmc of pure SDS at 298.15 K by conductivity measurements was 8.44 mmol kg−1, which is comparable with the value estimated by NMR spectroscopy (8.22 mmol kg−1).43 The observed cmc of pure SDS at 313.15 K by conductivity measurements was 7.08 mmol kg−1, whereas in the literature, the cmc was observed to be 8.0 mmol kg−1 by dynamic light scattering.44 Obtained cmc values of pure SDS in the range of 7.75 to 8.25 mM were reported using different techniques by Baloch et al.45 The cmc of pure SDS reported by Kumar et al. was 8.1 mmol kg−1.46 All the literature values support the experimental values obtained herein.
From Fig. 1, it can be observed that the slope in the post-micellar region is lower than that in the pre-micellar region; this occurs due to reduced counterion binding in the stern layer after micellization. Thus, these two slopes can be exploited for the calculation of α by the equation α = S2/S1, where S1 and S2 are the slopes in the pre- and post-micellar regions, correspondingly.47,48 Buckingham et al.49 established the α value valuation via the conductivity method, which was confirmed by another research group (Kale et al.)50 as well as by Bandhopadhyay and Moulik51 through an ion-selective electrode method. The measurement of the value of α is significant to distinguish the micellar behavior of surfactants. The stability along with the shape changeover of micelles from spherical to rod-like structures is responsible for the viscoelastic behavior of the surfactant and depends on the α value.52–54 Again, in real applications, where the charge of the micelle surface plays a vital role, e.g. DNA transportation, estimation of the α value is important.55 The rate of reaction of an organic molecule with a hydrophilic ion while maintaining the binding capability to the micelle is critically dependent on the α value.52,53 Again, the thermodynamic parameters of the micellization phenomenon are critically dependent on the α value.56 The fraction of counterion binding (β) is estimated from the relation β = (1 − α).57,58
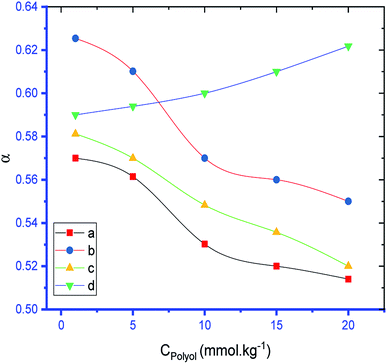
The cmc and α values observed in our experiments are outlined in Tables 1 and 2. The observed cmc values of SDS were found to dwindle in the presence of CFH; this reduction proceeds via enhancement of the CFH concentration in the water system in the entire studied temperature range. However, the cmc values were found to be enhanced in the presence of CFH in the cases of polyols (glucose or fructose) medium; this continued with further increase of [CFH]. There is an opportunity to obtain a positively charged N atom (N+ ion) in the structure of CFH by rearrangement, which neutralizes the micellar surface charge and favors micellization. On the other hand, the oxygen of the quinolone group of CFH repels the SO42− group of the surfactant; thus, the presence of CFH disfavors micellization. In water, the first factor predominates over the second; thus, the cmc decreases in the presence of CFH and increases in the presence of polyols. For ionic surfactants, the cmc decreases as the temperature augments until it reaches a minimum; subsequently, it increases with further increment of the temperature.59
| CCFH (mmol kg−1) | Water | 10 mmol kg−1 glucose | 10 mmol kg−1 fructose | ||||||
|---|---|---|---|---|---|---|---|---|---|
| cmc, mmol kg−1 | α | β | cmc, mmol kg−1 | α | β | cmc, mmol kg−1 | α | β | |
| a Relative standard uncertainty (ur) limits are ur(cmc) = ±3%, ur(α) = ±4% and ur(β) = ±4%. | |||||||||
| T = 298.15 K | |||||||||
| 0.0 | 8.44 | 0.59 | 0.41 | 4.99 | 0.54 | 0.46 | 5.67 | 0.57 | 0.43 |
| 0.5 | 8.02 | 0.60 | 0.40 | 5.92 | 0.58 | 0.42 | 6.34 | 0.61 | 0.39 |
| 1.0 | 7.67 | 0.66 | 0.34 | 6.33 | 0.59 | 0.41 | 6.89 | 0.63 | 0.37 |
| 2.0 | 6.99 | 0.75 | 0.25 | 6.95 | 0.61 | 0.39 | 7.41 | 0.64 | 0.36 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| T = 303.15 K | |||||||||
| 0.0 | 7.97 | 0.58 | 0.42 | 4.48 | 0.53 | 0.47 | 4.98 | 0.55 | 0.45 |
| 0.5 | 7.62 | 0.61 | 0.39 | 5.69 | 0.57 | 0.43 | 6.02 | 0.60 | 0.40 |
| 1.0 | 7.40 | 0.65 | 0.35 | 6.01 | 0.58 | 0.42 | 6.43 | 0.62 | 0.38 |
| 2.0 | 5.92 | 0.74 | 0.26 | 6.72 | 0.60 | 0.40 | 6.98 | 0.63 | 0.37 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| T = 308.15 K | |||||||||
| 0.0 | 7.33 | 0.57 | 0.43 | 4.04 | 0.52 | 0.48 | 4.62 | 0.54 | 0.46 |
| 0.5 | 7.02 | 0.60 | 0.40 | 5.13 | 0.56 | 0.44 | 5.26 | 0.59 | 0.41 |
| 1.0 | 6.81 | 0.62 | 0.38 | 5.66 | 0.57 | 0.43 | 5.92 | 0.60 | 0.40 |
| 2.0 | 6.21 | 0.71 | 0.29 | 6.15 | 0.58 | 0.42 | 6.47 | 0.61 | 0.39 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| T = 313.15 K | |||||||||
| 0.0 | 7.08 | 0.57 | 0.43 | 4.55 | 0.54 | 0.46 | 4.99 | 0.55 | 0.45 |
| 0.5 | 6.63 | 0.59 | 0.41 | 5.49 | 0.57 | 0.43 | 5.97 | 0.60 | 0.40 |
| 1.0 | 6.42 | 0.64 | 0.36 | 6.04 | 0.59 | 0.41 | 6.51 | 0.61 | 0.39 |
| 2.0 | 5.94 | 0.69 | 0.31 | 6.61 | 0.60 | 0.40 | 6.98 | 0.63 | 0.37 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| T = 318.15 K | |||||||||
| 0.0 | 7.47 | 0.56 | 0.44 | 4.97 | 0.55 | 0.45 | 5.51 | 0.56 | 0.44 |
| 0.5 | 6.91 | 0.62 | 0.38 | 5.89 | 0.59 | 0.41 | 6.39 | 0.61 | 0.39 |
| 1.0 | 6.56 | 0.66 | 0.34 | 6.46 | 0.60 | 0.40 | 6.82 | 0.62 | 0.38 |
| 2.0 | 6.32 | 0.75 | 0.25 | 6.93 | 0.62 | 0.38 | 7.41 | 0.64 | 0.36 |
| CPolyols, mmol kg−1 | CCFH, mmol kg−1 | Glucose | Fructose | ||||
|---|---|---|---|---|---|---|---|
| cmc/mmol kg−1 | α | β | cmc/mmol kg−1 | α | β | ||
| a Relative standard uncertainty (ur) limits are ur(cmc) = ±3%, ur(α) = ±4% and ur(β) = ±4%. | |||||||
| 1.00 | 0.00 | 5.16 | 0.57 | 0.43 | 5.57 | 0.58 | 0.42 |
| 5.00 | 0.00 | 4.79 | 0.56 | 0.44 | 5.31 | 0.57 | 0.43 |
| 10.00 | 0.00 | 4.48 | 0.53 | 0.47 | 4.98 | 0.55 | 0.45 |
| 15.00 | 0.00 | 4.23 | 0.52 | 0.48 | 4.87 | 0.54 | 0.46 |
| 20.00 | 0.00 | 4.06 | 0.51 | 0.49 | 4.75 | 0.52 | 0.48 |
| 1.00 | 0.50 | 6.83 | 0.63 | 0.37 | 6.97 | 0.59 | 0.41 |
| 5.00 | 0.50 | 6.33 | 0.61 | 0.39 | 6.48 | 0.59 | 0.41 |
| 10.00 | 0.50 | 5.69 | 0.57 | 0.43 | 6.02 | 0.60 | 0.40 |
| 15.00 | 0.50 | 5.35 | 0.56 | 0.44 | 6.91 | 0.61 | 0.39 |
| 20.00 | 0.50 | 5.07 | 0.55 | 0.45 | 7.49 | 0.62 | 0.38 |
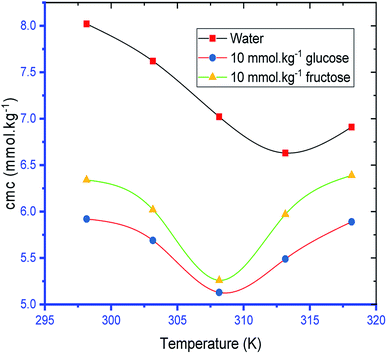
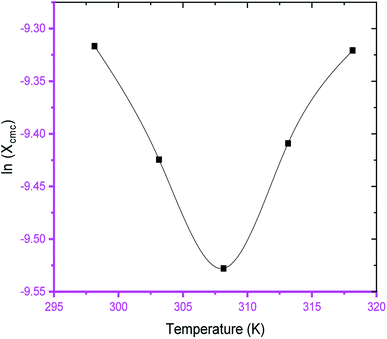
The variation of cmc of SDS/SDS + CFH as a function of temperature was also observed to be U-shaped, e.g. cmc decreases as the temperature increases, reaches a minimum, and then increases with the successive upsurge of temperature (Fig. 2). In aqueous medium, the minimum was observed at 313.15 K, whereas in polyols medium, it was observed at 308.15 K (Table 1). The obtained alteration of cmc of amphiphiles via temperature can be explained in the following two ways: (a) the enhanced dehydration of the hydrophilic heads at elevated temperature favors micellization; (b) the enhanced solubility of the surfactant at elevated temperature opposes micellization. These two opposite effects determine whether the cmc of the ionic surfactant will increase or decline at a certain temperature. The obtained U-shaped change of cmc with changing temperature reveals that the first factor is predominant at lower temperature, and reduction of cmc is observed. At elevated temperature, the second factor is predominant over the first one; thus, the cmc is enhanced.
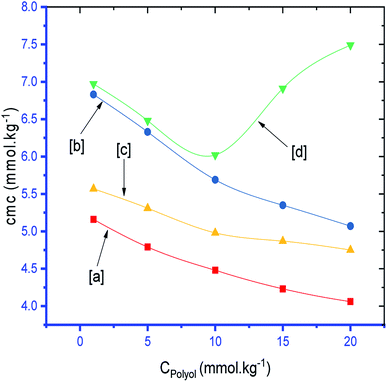
The observed cmc values of SDS and SDS + CFH dwindled in the presence of polyols (glucose/fructose). The hydroxyl groups of the polyols strongly attract water molecules; thus, the solubility of the surfactants is effectively reduced, which increases the hydrophobic interaction amongst the surfactant monomers. Thus, micellization starts to occur at lower concentration, i.e. cmc is lower in polyols solution. The attained cmc values follow the order cmcwater > cmcfructose > cmcglucose (Table 1 and Fig. 2). The decrease of cmc of the amphiphile in the presence of polyols was also reported by other researchers.34 Again, the observed cmc values of SDS/SDS + CFH decrease as the glucose concentration increases (Fig. 3), whereas the cmc of pure SDS decreases with the enhancement of fructose concentration; however, for SDS + CFH, the cmc value shows U-shaped behavior with changing fructose concentration (Fig. 3). Because the cmc of the SDS/SDS + CFH systems decreases with increasing glucose concentration and dissolution of the drug decreases above cmc, a smaller amount of SDS should be used to formulate the drug for diabetic patients to obtain better drug activity. The attained outcomes indicate that micellization is favored in the presence of CFH in water but disfavored in polyols media. Polyols that are water-soluble can be employed as cosolvents, and these polyols are not incorporated in the micelles. Thus, the addition of polyols to water is the cause of modification of the aqueous phase, which alters the micellar properties of the surfactants. The higher density of polyols compared to that of water is the cause of the increment of the volume fraction of surfactant in polyols solvent, which rises with increasing concentration of polyols. The interlayer spacing of the surfactant is reduced with augmentation of the volume fraction of the surfactant, which reduces the value of cmc in the presence of polyols (Table 1).
Table 1 shows that the values of α of the SDS and SDS + CFH mixed systems were reduced in the presence of polyols in almost all cases. The value of α of the surfactant was perceived to be greater in CFH solution, and the value increased further with increasing [CFH] in water/polyols solvents (Table 1). For pure SDS, the values of α were found to decrease monotonically in aqueous medium, whereas a U-shaped trend was observed in polyols medium with temperature variation. For the SDS + CFH mixture in the aqueous system, the values of α did not show any trend with temperature; however, their values in the presence of polyols decreased initially with temperature, after which the values increased as the temperature increased further (Table 1). The α value decreased with increasing glucose content for both SDS and the SDS + CFH mixture. However, in the presence of fructose, the α value decreased with increasing fructose content in pure SDS solution but was enhanced for the SDS + CFH mixture (Table 2 and Fig. 4).
Because there are no reported values about the behavior of CFH with surfactant in the presence of polyols, especially glucose, the formulation of drugs for diabetic patients is difficult. Again, excess use of surfactants in drug formulations can cause problems; thus, more study is required to obtain equal activity using less surfactant. The cmc values of the currently employed surfactant along with its mixture with CFH were reduced in polyols solutions; this helps to achieve better drug delivery of a hydrophobic drug by incorporating it in micelles at a lower surfactant concentration and minimizing the use of surfactants. Thus, the findings of our current study provide ideas for drug formulation, especially for diabetic patients, which is very rare in the existing literature in this field.
3.2 Thermodynamic properties of SDS and the SDS + CFH mixture
Thermodynamic parameters are important tools to understand the micellization phenomenon, the interactions between the drug and surfactant and the influence of different additives. Also, the drug delivery and release rate are functions of the molecular interaction of a drug with surfactants; thus, these molecular interactions can be explained in terms of thermodynamic parameters, and the values of different thermodynamic parameters can be utilized in drug formulation to achieve better drug delivery and drug release rates. The spontaneity or non-spontaneity of micellization can be measured from the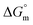 values of micellization, which can be executed based on the pseudo-phase partition model60–63 through the following relation:
values of micellization, which can be executed based on the pseudo-phase partition model60–63 through the following relation:
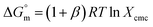 | (1) |
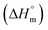 for pure SDS along with CFH-mediated micellization of SDS were estimated utilizing the subsequent equation:
for pure SDS along with CFH-mediated micellization of SDS were estimated utilizing the subsequent equation:
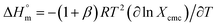 | (2) |
The alteration of Xcmc, which is dependent on temperature, is demonstrated to be a parabolic arc through relation (3):
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xcmc = A + BT + CT2 Xcmc = A + BT + CT2
| (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xcmc vs. T, which was subsequently exploited to measure
Xcmc vs. T, which was subsequently exploited to measure  of the currently studied system. The estimated constant values attained from eqn (3) are summarized in Table S1 (ESI)† and were exploited accordingly to measure the values of
of the currently studied system. The estimated constant values attained from eqn (3) are summarized in Table S1 (ESI)† and were exploited accordingly to measure the values of  through the following relation:64–66
through the following relation:64–66
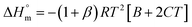 | (4) |
The estimated 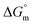 and
and  values were subsequently used for the measurement of the entropy
values were subsequently used for the measurement of the entropy 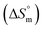 under analogous conditions utilizing the following equation:
under analogous conditions utilizing the following equation:
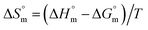 | (5) |
All the thermodynamic parameters evaluated in the current study are summarized in Table 3. The 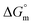 values for all systems (SDS/SDS + CFH in aqueous as well as in polyols (glucose or fructose) media) were negative, which shows that the micellization phenomena are thermodynamically spontaneous.2,11,67 The observed
values for all systems (SDS/SDS + CFH in aqueous as well as in polyols (glucose or fructose) media) were negative, which shows that the micellization phenomena are thermodynamically spontaneous.2,11,67 The observed 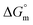 value of SDS alone was found to be higher than those of the SDS + CFH mixed system both in aqueous and polyols (glucose or fructose) media, which signifies that pure SDS undergoes micellization more spontaneously than the SDS + CFH mixture. The negative
value of SDS alone was found to be higher than those of the SDS + CFH mixed system both in aqueous and polyols (glucose or fructose) media, which signifies that pure SDS undergoes micellization more spontaneously than the SDS + CFH mixture. The negative 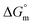 for the micellization of individual SDS in aqueous medium is enhanced as the temperature elevates, indicating that the association phenomena are additionally spontaneous at the higher studied temperatures; therefore, cmc is lower at higher temperature (Table 1). However, for the SDS + CFH mixture in H2O, the negative values of
for the micellization of individual SDS in aqueous medium is enhanced as the temperature elevates, indicating that the association phenomena are additionally spontaneous at the higher studied temperatures; therefore, cmc is lower at higher temperature (Table 1). However, for the SDS + CFH mixture in H2O, the negative values of 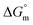 increase initially with temperature, reach a maximum, and then dwindle with the successive upsurge of the temperature. In polyols media, the negativity of
increase initially with temperature, reach a maximum, and then dwindle with the successive upsurge of the temperature. In polyols media, the negativity of 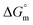 in the cases of the surfactant and the surfactant and CFH mixture increase initially with increasing temperature; after a certain temperature, their values start to decrease with the subsequent increase in temperature, with few exceptions (Table 3). In the aqueous system, the estimated value of
in the cases of the surfactant and the surfactant and CFH mixture increase initially with increasing temperature; after a certain temperature, their values start to decrease with the subsequent increase in temperature, with few exceptions (Table 3). In the aqueous system, the estimated value of  of SDS alone was found to be positive at subordinate temperature; however, on elevating the temperature, the value became negative, which signifies that micellization of SDS in aqueous medium is endothermic and exothermic at lower and higher temperature, respectively. In the case of SDS alone, this type of variation of
of SDS alone was found to be positive at subordinate temperature; however, on elevating the temperature, the value became negative, which signifies that micellization of SDS in aqueous medium is endothermic and exothermic at lower and higher temperature, respectively. In the case of SDS alone, this type of variation of  can also be found in the literature.39 The
can also be found in the literature.39 The  value in the case of CFH-mediated micellization of SDS in H2O was positive at both lower and higher CFH concentrations, whereas at the intermediate employed CFH concentration, a negative value was obtained; this signifies that the micellization phenomenon is endothermic at lesser and greater concentrations and exothermic at the intermediate concentration (Table 3). The
value in the case of CFH-mediated micellization of SDS in H2O was positive at both lower and higher CFH concentrations, whereas at the intermediate employed CFH concentration, a negative value was obtained; this signifies that the micellization phenomenon is endothermic at lesser and greater concentrations and exothermic at the intermediate concentration (Table 3). The  values for the SDS and SDS + CFH mixed systems in the presence of polyols were found to be positive and negative at lower and elevated temperature, respectively, in almost all cases (Table 3); this implies that micellization of SDS/SDS + CFH is endothermic and exothermic at lower and elevated temperature, respectively. The
values for the SDS and SDS + CFH mixed systems in the presence of polyols were found to be positive and negative at lower and elevated temperature, respectively, in almost all cases (Table 3); this implies that micellization of SDS/SDS + CFH is endothermic and exothermic at lower and elevated temperature, respectively. The  value is the outcome of different types of interactions, e.g. hydrophobic as well as hydrophilic interactions, counterion binding and hydration of the polar head groups of the surfactants. Negative values of
value is the outcome of different types of interactions, e.g. hydrophobic as well as hydrophilic interactions, counterion binding and hydration of the polar head groups of the surfactants. Negative values of  arise when hydration of the hydrophilic portion (head groups) of the surfactant dominates the disruption of the H2O structure around the hydrophobic chains of the monomeric surfactant and vice versa. The attained
arise when hydration of the hydrophilic portion (head groups) of the surfactant dominates the disruption of the H2O structure around the hydrophobic chains of the monomeric surfactant and vice versa. The attained 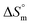 value for SDS alone was positive in H2O and declined as the temperature increased; this implies that SDS molecules are arranged in a more orderly fashion at higher temperatures, and therefore micellization is favored and cmc is lowered (Table 1). The
value for SDS alone was positive in H2O and declined as the temperature increased; this implies that SDS molecules are arranged in a more orderly fashion at higher temperatures, and therefore micellization is favored and cmc is lowered (Table 1). The 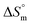 value for SDS + CFH was positive at the lower and higher employed CFH concentrations and negative at the intermediate concentration. Again, in glucose solution, at a lower selected temperature, the
value for SDS + CFH was positive at the lower and higher employed CFH concentrations and negative at the intermediate concentration. Again, in glucose solution, at a lower selected temperature, the 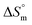 value for the surfactant alone was found to be positive; meanwhile, the value was negative at a higher temperature and positive in all cases for SDS in fructose solution. The positive
value for the surfactant alone was found to be positive; meanwhile, the value was negative at a higher temperature and positive in all cases for SDS in fructose solution. The positive 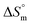 values in the presence of polyols decreased with elevation of the temperature. The
values in the presence of polyols decreased with elevation of the temperature. The 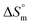 values for the SDS + CFH mixed system in the presence of polyols (glucose or fructose) were found to be positive in almost all cases. The attained
values for the SDS + CFH mixed system in the presence of polyols (glucose or fructose) were found to be positive in almost all cases. The attained 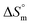 values for the SDS + CFH mixture decreased as the temperature was elevated, signifying more ordered SDS + CFH systems at elevated temperature. Positive values of
values for the SDS + CFH mixture decreased as the temperature was elevated, signifying more ordered SDS + CFH systems at elevated temperature. Positive values of 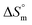 arise when the hydrophobic portion of the drug transfers from the aqueous vicinity to the micelle interior.68 It is well known that the H-bonding of water molecules in the immediate vicinity of a hydrophobic portion is stronger than that of normal water, i.e. the H2O molecules in the immediate vicinity of a hydrophobic moiety attract each other more strongly than normal H2O molecules; due to tightening of the H2O structure,69 the internal torsional vibration of the hydrophobic chain is reduced. This highly ordered H2O structure along with the reduced internal torsional vibration leads to the reduction of entropy. The removal of a non-polar moiety (hydrophobic chain) from the aqueous vicinity is entropically favorable, which disrupts the highly ordered H2O structure.68
arise when the hydrophobic portion of the drug transfers from the aqueous vicinity to the micelle interior.68 It is well known that the H-bonding of water molecules in the immediate vicinity of a hydrophobic portion is stronger than that of normal water, i.e. the H2O molecules in the immediate vicinity of a hydrophobic moiety attract each other more strongly than normal H2O molecules; due to tightening of the H2O structure,69 the internal torsional vibration of the hydrophobic chain is reduced. This highly ordered H2O structure along with the reduced internal torsional vibration leads to the reduction of entropy. The removal of a non-polar moiety (hydrophobic chain) from the aqueous vicinity is entropically favorable, which disrupts the highly ordered H2O structure.68
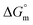 (kJ mol−1),
(kJ mol−1),  (kJ mol−1) and
(kJ mol−1) and 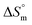 (J K−1 mol−1)) of all studied systemsa
(J K−1 mol−1)) of all studied systemsa
| CCFH mmol kg−1 | |||||
|---|---|---|---|---|---|
| 298.15 K | 303.15 K | 308.15 K | 313.15 K | 318.15 K | |
a Relative standard uncertainty (ur) limits are 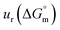 = ±3%, = ±3%, 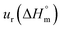 = ±4% and = ±4% and 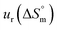 = ±5%. = ±5%. |
|||||
| Water | |||||
| 0.0 | −30.73/7.49/128.19 | −31.67/0.21/105.15 | −32.72/−7.69/81.25 | −33.38/−16.10/55.19 | −33.95/−25.22/27.45 |
| 0.5 | −7.18/36.58/146.70 | −7.41/32.23/130.77 | −7.85/28.02/116.39 | −8.22/23.39/100.95 | −8.04/17.8/81.29 |
| 1.0 | −7.01/−14.18/−24.06 | −7.30/−18.93/−38.37 | −7.80/−24.24/−53.32 | −8.05/−29.27/−67.76 | −7.99/−34.27/−82.62 |
| 2.0 | −6.79/38.27/151.15 | −7.41/31.12/127.09 | −7.58/23.84/101.96 | −7.95/15.38/74.50 | −7.54/5.71/41.65 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| 10 mmol kg−1 glucose | |||||
| 0.0 | −33.72/9.13/143.71 | −34.92/−10.71/79.84 | −36.13/−32.18/12.82 | −35.77/−54.21/−58.89 | −35.75/−77.53/−131.34 |
| 0.5 | −32.19/35.65/227.55 | −33.11/25.10/191.99 | −34.27/13.61/155.37 | −34.33/1.13/113.24 | −34.13/−11.90/69.87 |
| 1.0 | −31.52/−7.97/77.69 | −32.68/34.94/223.04 | −33.67/25.06/190.60 | −33.50/14.02/151.77 | −33.55/2.59/113.59 |
| 2.0 | −30.96/32.70/213.51 | −31.82/24.42/185.54 | −33.13/15.50/157.84 | −32.93/5.51/122.78 | −32.81/−4.84/87.91 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| 10 mmol kg−1 fructose | |||||
| 0.0 | −32.57/64.67/326.15 | −34.06/48.96/273.84 | −35.14/31.34/215.73 | −35.17/−12.05/150.79 | −35.11/−8.25/84.42 |
| 0.5 | −31.28/48.67/268.15 | −32.21/35.71/224.04 | −33.47/21.57/178.61 | −33.31/6.14/125.97 | −33.35/−10.08/73.12 |
| 1.0 | −31.73/43.98/253.93 | −32.68/34.94/223.04 | −32.80/−21.62/36.30 | −32.75/−35.77/−9.62 | −32.87/−50.59/55.69 |
| 2.0 | −30.08/32.44/209.66 | −31.01/22.26/175.74 | −32.25/11.27/141.24 | −32.03/−0.82/99.69 | −32.09/−13.42/58.68 |
Taken together, the magnitudes of  and
and 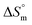 indicate that micellization of pure SDS is entropically controlled at lower temperature and both entropically and enthalpically controlled at greater temperature in H2O/fructose solution. In glucose medium, the micellization is governed by both entropy and enthalpy at lower temperature, whereas it is entirely enthalpically controlled at elevated temperature. In the aqueous system, the magnitudes of
indicate that micellization of pure SDS is entropically controlled at lower temperature and both entropically and enthalpically controlled at greater temperature in H2O/fructose solution. In glucose medium, the micellization is governed by both entropy and enthalpy at lower temperature, whereas it is entirely enthalpically controlled at elevated temperature. In the aqueous system, the magnitudes of  and
and 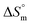 for the SDS + CFH mixed system indicate that micellization is entropically governed at both lower and higher drug concentrations, but enthalpically governed at the intermediate concentration of the drug. The
for the SDS + CFH mixed system indicate that micellization is entropically governed at both lower and higher drug concentrations, but enthalpically governed at the intermediate concentration of the drug. The  and
and 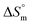 values for the SDS + CFH mixed system in the presence of glucose/fructose elicits that micellization is governed by entropy at lower temperature but becomes governed by both enthalpy and entropy at elevated temperature. Negative
values for the SDS + CFH mixed system in the presence of glucose/fructose elicits that micellization is governed by entropy at lower temperature but becomes governed by both enthalpy and entropy at elevated temperature. Negative  and positive
and positive 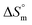 values were also observed for SDS in hexanediol + water medium in a microcalorimetric study.70 It is reported that positive enthalpy and entropy values of a system reveal the presence of hydrophobic bonding, while negative enthalpy and entropy values are indicative of both hydrogen bonding and electrostatic interactions.71,72 Other researchers have reported the presence of hydrophobic interactions between the surfactant and solutes based on negative enthalpies and positive entropies.73 Thus, the binding forces between SDS and CFH involve hydrophobic interactions as well as electrostatic interactions such as hydrogen bonding and ion-dipole interactions.
values were also observed for SDS in hexanediol + water medium in a microcalorimetric study.70 It is reported that positive enthalpy and entropy values of a system reveal the presence of hydrophobic bonding, while negative enthalpy and entropy values are indicative of both hydrogen bonding and electrostatic interactions.71,72 Other researchers have reported the presence of hydrophobic interactions between the surfactant and solutes based on negative enthalpies and positive entropies.73 Thus, the binding forces between SDS and CFH involve hydrophobic interactions as well as electrostatic interactions such as hydrogen bonding and ion-dipole interactions.
3.3 Thermodynamic transfer properties of the SDS/SDS + CFH mixed systems
Diverse thermodynamic transfer properties, e.g. free energy of transfer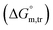 , enthalpy of transfer
, enthalpy of transfer 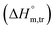 and entropy of transfer
and entropy of transfer 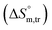 , during the micellization of SDS/SDS + CFH mixtures in the different employed solvents can be measured from the following equations:74,75
, during the micellization of SDS/SDS + CFH mixtures in the different employed solvents can be measured from the following equations:74,75
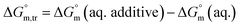 | (6) |
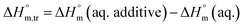 | (7) |
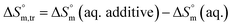 | (8) |
All measured 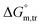 ,
, 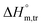 and
and 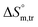 values in all exploited solvents are provided in Table 4. In the aqueous medium, in the presence of CFH, the magnitude of
values in all exploited solvents are provided in Table 4. In the aqueous medium, in the presence of CFH, the magnitude of 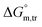 was found to be positive; this illustrates the lower spontaneity of micelle formation in the presence of CFH. The
was found to be positive; this illustrates the lower spontaneity of micelle formation in the presence of CFH. The 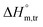 and
and 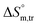 values in H2O were found to be positive at lower and greater concentrations of the drug and negative at an intermediate concentration of the drug. In the presence of polyols (glucose or fructose), the
values in H2O were found to be positive at lower and greater concentrations of the drug and negative at an intermediate concentration of the drug. In the presence of polyols (glucose or fructose), the 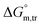 values obtained were negative for SDS alone at all investigated temperatures, implying the greater spontaneity of micelle formation in the presence of polyols (glucose or fructose). The
values obtained were negative for SDS alone at all investigated temperatures, implying the greater spontaneity of micelle formation in the presence of polyols (glucose or fructose). The 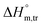 and
and 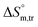 values for SDS alone in glucose medium were negative at almost all temperatures; however, these values were positive in fructose solution. The
values for SDS alone in glucose medium were negative at almost all temperatures; however, these values were positive in fructose solution. The 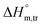 and
and 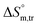 values for SDS + CFH were positive in glucose medium at all temperatures and CFH concentrations employed. Again, the
values for SDS + CFH were positive in glucose medium at all temperatures and CFH concentrations employed. Again, the 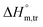 and
and 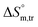 values for the SDS + CFH mixtures were positive at lower and greater concentrations of CFH and negative at intermediate concentration (Table 4).
values for the SDS + CFH mixtures were positive at lower and greater concentrations of CFH and negative at intermediate concentration (Table 4).
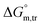 (kJ mol−1),
(kJ mol−1), 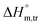 (kJ mol−1) and
(kJ mol−1) and 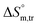 (J K−1 mol−1)] of the currently studied systemsa
(J K−1 mol−1)] of the currently studied systemsa
| CCFH (mmol kg−1) | T (K) | |||
|---|---|---|---|---|
| Water | 10 mmol kg−1 glucose | 10 mmol kg−1 fructose | ||
a Relative standard uncertainty (ur) limits are 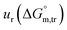 = ±3%, = ±3%, 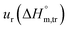 = ±4% and = ±4% and 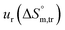 = ±5%. = ±5%. |
||||
| 0.0 | 298.15 | −2.99/1.64/15.52 | −1.85/57.18/197.96 | |
| 0.0 | 303.15 | −3.25/−10.92/−25.31 | −2.39/48.75/168.69 | |
| 0.0 | 308.15 | −3.40/−24.49/−68.43 | −2.41/39.03/134.48 | |
| 0.0 | 313.15 | −2.38/−38.11/−114.09 | −1.79/28.15/95.59 | |
| 0.0 | 318.15 | −1.80/−52.32/−158.78 | −1.16/16.97/56.97 | |
| 0.5 | 298.15 | 23.54/29.08/18.59 | −1.47/28.16/99.36 | −0.55/41.18/139.96 |
| 0.5 | 303.15 | 24.26/32.03/25.63 | −1.44/24.89/86.85 | −0.54/35.50/118.90 |
| 0.5 | 308.15 | 24.88/35.71/35.14 | −1.55/21.30/74.12 | −0.74/29.26/97.36 |
| 0.5 | 313.15 | 25.17/39.49/45.75 | −0.95/17.23/58.05 | 0.08/22.24/70.77 |
| 0.5 | 318.15 | 25.91/43.04/53.84 | −0.18/13.32/42.42 | 0.60/15.13/45.67 |
| 1.0 | 298.15 | 23.72/−21.67/−152.25 | −1.01/36.4/125.74 | 0.18/−3.00/−10.66 |
| 1.0 | 303.15 | 24.37/−19.14/−143.52 | −1.01/34.73/117.89 | 0.15/−8.18/−27.45 |
| 1.0 | 308.15 | 24.92/−16.55/−134.57 | −0.95/32.75/109.35 | −0.08/−13.93/−44.95 |
| 1.0 | 313.15 | 25.33/−13.17/−122.96 | −0.12/30.13/96.57 | 0.63/−19.67/−64.81 |
| 1.0 | 318.15 | 25.96/−9.06/−110.07 | 0.41/27.81/86.14 | 1.08/−25.37/−83.14 |
| 2.0 | 298.15 | 23.94/30.78/22.96 | −0.23/25.21/85.33 | 0.65/24.94/81.48 |
| 2.0 | 303.15 | 24.26/30.91/21.94 | −0.16/24.21/80.39 | 0.66/22.06/70.60 |
| 2.0 | 308.15 | 25.14/31.52/20.71 | −0.41/23.19/76.59 | 0.47/18.96/59.99 |
| 2.0 | 313.15 | 25.43/31.48/19.31 | 0.45/21.61/67.58 | 1.35/15.29/44.50 |
| 2.0 | 318.15 | 26.41/30.93/14.21 | 1.14/20.38/60.46 | 1.86/11.79/31.23 |
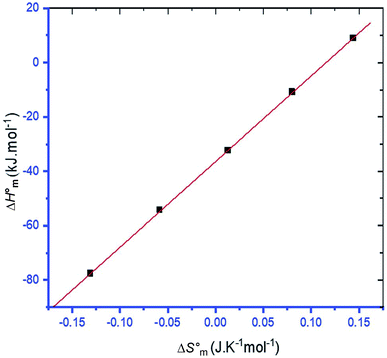
3.4 Enthalpy–entropy compensation
The linear relationship between and
and 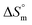 is called enthalpy–entropy compensation and can be assessed by exploiting the following relation:76,77
is called enthalpy–entropy compensation and can be assessed by exploiting the following relation:76,77
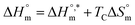 | (9) |
In eqn (9), the intercept 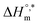 and the slope TC indicate the intrinsic enthalpy and compensation temperature, respectively. A representative graph of
and the slope TC indicate the intrinsic enthalpy and compensation temperature, respectively. A representative graph of  vs.
vs. 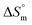 is presented in Fig. 6. The solute–solute and solute–solvent interactions can be explained by the assessed magnitudes of
is presented in Fig. 6. The solute–solute and solute–solvent interactions can be explained by the assessed magnitudes of 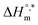 and TC, respectively. Enthalpy–entropy compensation was observed both for the surfactant alone and for its mixture with CFH in water/polyols media, and the attained values of the compensation parameters are summarized in Table S2 (ESI).† The estimated
and TC, respectively. Enthalpy–entropy compensation was observed both for the surfactant alone and for its mixture with CFH in water/polyols media, and the attained values of the compensation parameters are summarized in Table S2 (ESI).† The estimated 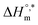 value was found to be negative in all systems (SDS/SDS + CFH) in the absence/presence of polyols (Table S2 (ESI)†). The obtained negative
value was found to be negative in all systems (SDS/SDS + CFH) in the absence/presence of polyols (Table S2 (ESI)†). The obtained negative 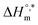 value indicates that micellization is privileged even at
value indicates that micellization is privileged even at 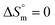 . The augmentation of the negative
. The augmentation of the negative 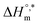 value signifies enhancement of the micelle stability.78,79 The estimated values of TC were in the range of 288 K to 345 K. The obtained values of TC in the range of 270 K to 330 K can be exploited to study the effects of H2O in the protein solution.80 Thus, the estimated values are comparable with biological fluids in almost all cases.
value signifies enhancement of the micelle stability.78,79 The estimated values of TC were in the range of 288 K to 345 K. The obtained values of TC in the range of 270 K to 330 K can be exploited to study the effects of H2O in the protein solution.80 Thus, the estimated values are comparable with biological fluids in almost all cases.
4. Conclusions
A conductometric study has been performed herein to study the self-aggregation properties of SDS and a mixture of SDS + CFH in the absence/presence of polyols (glucose or fructose) at different temperatures. The decrease in cmc in the presence of CFH is due to the establishment of additional hydrophobic interactions between the hydrophobic moieties of SDS and CFH. The addition of glucose/fructose enhances the self-aggregation of SDS + CFH. Micellar parameters such as cmc and α of the SDS + CFH mixture were observed to be dependent on the additive (polyols) and temperature variation. The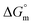 ,
,  and
and 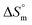 values for SDS and the SDS + CFH mixture reveal that the micellization process is thermodynamically spontaneous and that the interaction forces between SDS and CFH are hydrophobic, hydrogen bonding and ion-dipole type. The greater negative values of
values for SDS and the SDS + CFH mixture reveal that the micellization process is thermodynamically spontaneous and that the interaction forces between SDS and CFH are hydrophobic, hydrogen bonding and ion-dipole type. The greater negative values of 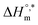 disclose the formation of more stable micelles in glucose/fructose compared to in aqueous medium. This study will aid the formulation of the drug CFH using the most commonly utilized surfactant, SDS, while considering different target patients, including diabetic patients, to achieve maximum activity of the drug; however, more study is still required to consider all the ingredients in body fluid during drug formulation. Because glucose reduces the cmc of SDS and the SDS + CFH mixture, the use of SDS can be reduced for a particular set of formulations. Also, the SDS + CFH mixture can be further studied in additives media which are present in body fluid using SEM/TEM for morphological studies and MD simulations to observe the interaction sites.
disclose the formation of more stable micelles in glucose/fructose compared to in aqueous medium. This study will aid the formulation of the drug CFH using the most commonly utilized surfactant, SDS, while considering different target patients, including diabetic patients, to achieve maximum activity of the drug; however, more study is still required to consider all the ingredients in body fluid during drug formulation. Because glucose reduces the cmc of SDS and the SDS + CFH mixture, the use of SDS can be reduced for a particular set of formulations. Also, the SDS + CFH mixture can be further studied in additives media which are present in body fluid using SEM/TEM for morphological studies and MD simulations to observe the interaction sites.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia under grant no. (KEP-51-130-38). The authors, therefore, acknowledge with thanks DSR technical and financial support.References
- D. Kumar and M. A. Rub, RSC Adv., 2019, 9, 22129 RSC.
- D. Kumar and M. A. Rub, J. Phys. Org. Chem., 2016, 29, 394 CrossRef CAS.
- D. Kumar and M. A. Rub, J. Surfactants Deterg., 2019, 22, 1299 CrossRef CAS.
- U. Ashraf, O. A. Chat, M. Maswal, S. Jabeen and A. A. Dar, RSC Adv., 2015, 5, 83608 RSC.
- M. Akram, S. Anwar, F. Ansari, I. A. Bhat and Kabir-ud-Din, RSC Adv., 2016, 6, 21697 RSC.
- M. Akram, I. A. Bhat and Kabir-ud-Din, RSC Adv., 2015, 5, 102780 RSC.
- D. Kumar, S. Hidayathulla and M. A. Rub, J. Mol. Liq., 2018, 271, 254 CrossRef CAS.
- S. Das, S. Mondal and S. Ghosh, RSC Adv., 2016, 6, 30795 RSC.
- P. Shi, H. Zhang, L. Lin, C. Song, Q. Chen and Z. Li, RSC Adv., 2019, 9, 3224 RSC.
- D. Kumar and M. A. Rub, J. Mol. Liq., 2019, 274, 639 CrossRef CAS.
- M. A. Rub, N. Azum, F. Khan and A. M. Asiri, J. Chem. Thermodyn., 2018, 121, 199 CrossRef.
- S. He, X. Liu, P. Yan, A. Wang, J. Su and X. Su, RSC Adv., 2019, 9, 4908 RSC.
- D. Kumar and M. A. Rub, J. Mol. Liq., 2018, 250, 329 CrossRef CAS.
- D. Kumar and M. A. Rub, J. Phys. Org. Chem., 2019, 32, e3918 CrossRef.
- V. Bhardwaj, T. Bhardwaj, K. Sharma, A. Gupta, S. Chauhan, S. S. Cameotra, S. Sharma, R. Guptad and P. Sharma, RSC Adv., 2014, 4, 24935 RSC.
- M. F. Ahmed, M. R. Molla, M. Saha, I. Shahriar, M. S. Rahman, M. A. Halim, M. A. Rub, M. A. Hoque and A. M. Asiri, RSC Adv., 2019, 9, 6556 RSC.
- M. Fresta, S. Guccione, A. R. Beccari, P. M. Furneri and G. Puglisi, Bioorg. Med. Chem., 2002, 10, 3871 CrossRef CAS.
- J. N. Israelachvili, Intermolecular and Surface Forces, Academic Press, New York, 1995 Search PubMed.
- Z. Huang, X. Zhang, X. Zhang, C. Fu, K. Wang, J. Yuan, L. Tao and Y. Wei, Polym. Chem., 2015, 5, 607 RSC.
- H. Huang, M. Liu, R. Jiang, J. Chen, L. Mao, Y. Wen, J. Tian, N. Zhou, X. Zhang and Y. Wei, J. Colloid Interface Sci., 2018, 513, 198 CrossRef CAS PubMed.
- J. Chen, M. Liu, Q. Huang, L. Huang, H. Huang, F. Deng, Y. Wen, J. Tian, X. Zhang and Y. Wei, Chem. Eng. J., 2018, 337, 82 CrossRef CAS.
- R. Jiang, M. Liu, T. Chen, H. Huang, Q. Huang, J. Tian, Y. Wen, Q. Cao, X. Zhang and Y. Wei, Dyes Pigm., 2017, 148, 52 CrossRef.
- X. Zhang, X. Zhang, B. Yang, M. Liu, W. Liu, Y. Chen and Y. Wei, Polym. Chem., 2014, 5, 356 RSC.
- J. Piret, A. Désormeaux and M. G. Bergeron, Curr. Drug Targets, 2002, 3, 17 CAS.
- J. Piret, J. Lamontagne, J. Bestman-Smith, S. Roy, P. Gourde, A. Désormeaux, R. F. Omar, J. Juhász and M. G. Bergeron, J. Clin. Microbiol., 2000, 38, 110 CAS.
- M. F. Islam, E. Rojas, D. M. Bergey, A. T. Johnson and A. G. Yodh, Nano Lett., 2003, 3, 269 CrossRef CAS.
- S. Knapp, Gerontology, 2013, 59, 99 CrossRef CAS PubMed.
- G. C. Koh, S. J. Peacock, T. van der Poll and W. J. Wiersinga, Eur. J. Clin. Microbiol. Infect. Dis., 2012, 31, 379 CrossRef CAS PubMed.
- H. Liao, J. Zakhaleva and W. Chen, Biomaterials, 2009, 30, 1689 CrossRef CAS PubMed.
- S. Mori, H. K. Takahashi, K. Liu, H. Wake, J. Zhang, R. Liu, T. Yoshino and M. Nishibori, Br. J. Pharmacol., 2010, 161, 229 CrossRef CAS PubMed.
- L. Boyanova and I. Mitov, Expert Rev. Anti-Infect. Ther., 2013, 11, 411 CrossRef CAS PubMed.
- M. A. Rub, N. Azum, S. B. Khan, H. M. Marwani and A. M. Asiri, J. Mol. Liq., 2015, 212, 532 CrossRef.
- M. A. Ahsan, M. R. Amin, S. Mahbub, M. R. Molla, S. Aktar, M. N. Arshad, M. A. Hoque, M. A. Rub and M. A. Khan, Chin. J. Chem. Eng., 2020, 28, 216 CrossRef.
- M. R. Amin, S. Mahbub, S. Hidayathulla, M. M. Alam, M. A. Hoque and M. A. Rub, J. Mol. Liq., 2018, 269, 417 CrossRef CAS.
- M. A. Hoque, M. M. Alam, M. R. Molla, S. Rana, M. A. Rub, M. A. Halim, M. A. Khanand and A. Ahmed, J. Mol. Liq., 2017, 244, 512 CrossRef CAS.
- S. Mahbub, M. A. Rub, M. A. Hoque and M. A. Khan, J. Phys. Org. Chem., 2018, 31, e3872 CrossRef.
- S. Mahbub, M. Rahman, S. Rana, M. A. Rub, M. A. Hoque, M. A. Khan and A. M. Asiri, J. Surfactants Deterg., 2018, 22, 137 CrossRef.
- S. Mahbub, M. A. Rub, M. A. Hoque and M. A. Khan, J. Phys. Org. Chem., 2019, 32, e3917 CrossRef.
- S. Mahbub, M. R. Molla, M. Saha, I. Shahriar, M. A. Hoque, M. A. Halim, M. A. Rub and M. A. Khan, J. Mol. Liq., 2019, 283, 263 CrossRef CAS.
- S. Mahbub, M. A. Rub, M. A. Hoque, M. A. Khan and D. Kumar, J. Phys. Org. Chem., 2019, 32, e3967 CrossRef.
- M. A. Hoque, M.-O.-F. Patoary, M. M. Rashid, M. R. Molla and M. A. Rub, J. Solution Chem., 2017, 46, 682 CrossRef CAS.
- F. Akhtar, M. A. Hoque and M. A. Khan, J. Chem. Thermodyn., 2008, 40, 1082 CrossRef CAS.
- G. Manzo, M. Carboni, A. C. Rinaldi, M. Casu and M. A. Scorciapino, Magn. Reson. Chem., 2013, 51, 176 CrossRef CAS PubMed.
- K. Ogino, T. Kubota, H. Uchiyama and M. Abe, J. Jpn. Oil Chem. Soc., 1988, 37, 588 CrossRef CAS.
- M. K. Baloch, G. Hameed and A. Bano, J. Chem. Soc. Pak., 2002, 24, 77 CAS.
- S. Kumar, Z. A. Khan, N. Parveen and Kabir-ud-Din, Colloids Surf., A, 2005, 268, 45 CrossRef CAS.
- D. Kumar and M. A. Rub, J. Mol. Liq., 2017, 238, 389 CrossRef CAS.
- S. M. I. H. Sristy, S. Mahbub, M. M. Alam, M. A. Rub and M. A. Hoque, J. Mol. Liq., 2019, 284, 12 CrossRef CAS.
- S. A. Buckingham, C. J. Garve and G. G. Warr, J. Phys. Chem., 1993, 97, 10236 CrossRef CAS.
- K. M. Kale, E. L. Cussler and D. F. Evans, J. Phys. Chem., 1980, 84, 593 CrossRef CAS.
- A. Bandhopadhyay and S. P. Moulik, Colloid Polym. Sci., 1988, 266, 455 CrossRef.
- L. S. Romsted, Rate Enhancements in Micellar Systems, PhD thesis, Indiana University, Bloomington, IN, 1975 Search PubMed.
- V. Soldi, J. Keiper, L. S. Romsted, I. M. Cuccovia and H. Chaimovich, Langmuir, 2000, 16, 59 CrossRef CAS.
- R. Oda, J. Narayanan, P. A. Hassan, C. Manohar, R. A. Salkar, F. Kern and S. J. Candau, Langmuir, 1998, 14, 4364 CrossRef CAS.
- Y. Wang, P. L. Dubin and H. Zhang, Langmuir, 2001, 17, 1670 CrossRef CAS.
- D. G. Hall, J. Chem. Soc., Faraday Trans. 1, 1981, 77, 1121 RSC.
- J. M. Kuiper, R. T. Buwalda, R. Hulstand and J. B. F. N. Engberts, Langmuir, 2001, 17, 5216 CrossRef CAS.
- L. Wang, Y. Zhang, L. Ding, J. Liu, B. Zhao, Q. Deng and T. Yan, RSC Adv., 2015, 5, 74764 RSC.
- G. C. Kresheck, Water: A Comprehensive Treatise, ed. F. Franks, Plenum, New York, vol. 4, 1975 Search PubMed.
- M. Rahman, M. A. Khan, M. A. Rub and M. A. Hoque, J. Mol. Liq., 2016, 223, 716 CrossRef CAS.
- Z. Medoš and M. B. Rogač, J. Chem. Thermodyn., 2015, 83, 117 CrossRef.
- F. Khan, M. A. Rub, N. Azum, D. Kumar and A. M. Asiri, J. Solution Chem., 2015, 44, 1937 CrossRef CAS.
- M. R. Molla, S. Rana, M. A. Rub, A. Ahmed and M. A. Hoque, J. Surfactants Deterg., 2018, 21, 231 CrossRef CAS.
- H.-U. Kim and K.-H. Lim, Colloids Surf., A, 2004, 235, 121 CrossRef CAS.
- A. Beesley, D. F. Evans and R. G. Laughlin, J. Phys. Chem., 1988, 92, 791 CrossRef CAS.
- B. Bergenstaahl and P. Stenius, J. Phys. Chem., 1987, 91, 5944 CrossRef CAS.
- L. Qin and X.-H. Wang, RSC Adv., 2017, 7, 51426 RSC.
- D. Attwood and A. T. Florence, Surfactant Systems, Chapman and Hall London, New York, 1985 Search PubMed.
- P. Taboada, P. M. Landeira, J. M. Ruso, M. Garcia and V. Mosquera, Colloids Surf., A, 2002, 197, 95 CrossRef CAS.
- J. W. Comeau, A. A. McLachlan and D. G. Marangoni, J. Dispersion Sci. Technol., 2009, 30, 1288 CrossRef CAS.
- E. Pramauro and E. Pelizzetti, Surfactants in Analytical Chemistry: Applications of Organized Media, in Comprehensive Analytical Chemistry, ed. S. G. Weber, Elsevier, Amsterdam, 1996 Search PubMed.
- A. Beesley, D. F. Evans and R. G. Laughlin, J. Phys. Chem., 1988, 92, 791 CrossRef CAS.
- V. Bhardwaja, P. Sharma, M. S. Chauhan and S. Chauhan, J. Saudi Chem. Soc., 2016, 20, S109 CrossRef.
- S. K. Shivaji and A. K. Rakshit, J. Surfactants Deterg., 2004, 7, 305 CrossRef.
- M. R. Amin, S. Mahbub, M. R. Molla, M. M. Alam, M. F. Hossain, S. Rana, M. A. Rub, M. A. Hoque and D. Kumar, J. Chem. Eng. Data, 2019, 64, 2750 CrossRef CAS.
- L. J. Chen, S. Y. Linand and C. C. Huang, J. Phys. Chem., 1998, 102, 4350 CrossRef CAS.
- S. Mahbub, M. A. Rub and M. A. Hoque, J. Chem. Eng. Data, 2019, 64, 4181 CrossRef CAS.
- G. Sugihara and M. Hisatomi, J. Colloid Interface Sci., 1999, 219, 31 CrossRef CAS PubMed.
- M. A. Hoque, M. O. F. Patoary, M. R. Molla, M. A. Halim, M. A. Khan and M. A. Rub, J. Dispersion Sci. Technol., 2017, 38, 1578 CrossRef CAS.
- R. Lumry and S. Rajender, Biopolymers, 1970, 9, 1125 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra00213e |
| This journal is © The Royal Society of Chemistry 2020 |