Open Access Article
Open Access ArticleVisible-light-induced aerobic C3–H fluoroalkoxylation of quinoxalin-2(1H)-ones with fluoroalkyl alcohols†
Xiaobo Xuab,
Chengcai Xia *a,
Xiaojun Lic,
Jian Suna and
Liqiang Haoa
*a,
Xiaojun Lic,
Jian Suna and
Liqiang Haoa
aPharmacy College, Shandong First Medical University, Shandong Academy of Medical Sciences, Taian 271000, China. E-mail: xiachc@163.com
bShanghai Synmedia Chemical Co., Ltd, Shanghai 201201, China
cDepartment of Fundamental Medicine, Xinyu University, Xinyu 338004, China
First published on 9th January 2020
Abstract
A novel and efficient method of visible-light-induced C3–H fluoroalkoxylation of quinoxalin-2(1H)-ones with fluoroalkyl alcohols is developed. This approach uses readily available fluoroalkyl alcohols as fluoroalkoxylation reagents and displays a wide substrate scope, providing the fluoroalkoxylated products in moderate to good yields. Compared with the previous method, such a transformation uses oxygen as an oxidant, which avoids the utilization of plenty of PhI(TFA)2. In addition, this strategy also gives a practical tool for the rapid synthesis of histamine-4 receptor antagonist and new N-containing bidentate ligands. A radical mechanism was suggested according to the results of control experiments.
Introduction
As a class of fluorine-containing molecules, fluoroalkoxyl aryl ethers are widely used in agricultural agents, advanced materials and pharmaceuticals because of the unique impact of the fluorine atoms on physicochemical and biological properties.1 The conventional methods to prepare such compounds are nucleophilic aromatic substitution reactions (SNAr), which usually suffer from high temperature and low reactivity.2 Consequently, the development of more practical and efficient approaches to access fluoroalkoxyl aryl ethers has recently received considerable attention.In recent years, transition-metal-catalyzed reactions have been regarded as reliable strategies for the preparation of fluoroalkoxyl aryl ethers.3 For example, Weng,3a,b Ji,3c Crousse,3d and Qing3e reported novel methods for the construction of fluoroalkoxyl aryl ethers through copper-catalyzed C–O cross couplings of aryl halides or aryl boronic acids with fluoroalkyl alcohols. In addition, the Singh group achieved a palladium-catalyzed dehalogenated coupling of aryl halides with fluoroalkyl alcohols for the synthesis of fluoroalkoxyl aryl ethers.3f Alternatively, palladium-catalyzed amide-directed C–H trifluoroethoxylation provided another route to fluoroalkoxyl aryl ethers.3g Despite the utilities, the pre-functionalization of the starting materials in above methods limited their applications. In addition, the metal catalysts are toxic, and trace amounts of metal residues in final products are quite difficult to remove, which is a crucial issue in the pharmaceutical industry.4
As an important class of heterocyclic units, quinoxalin-2(1H)-ones widely exist in natural products, organic intermediates and pharmaceuticals.5 Therefore, great efforts have been devoted to the development of new methods for the synthesis of quinoxalin-2(1H)-ones and its derivatives.6 Thanks to the development of C–H functionalization, lots of transformations so far have been achieved,7 such as phosphorization,8 amination,9 alkylation,10 arylation,11 acylation,12 trifluoromethylation,13 difluoromethylation,14 alkoxylation15 and thiolation.16 In sharp contrast, C–H fluoroalkoxylation of quinoxalin-2(1H)-ones was rarely reported. Very recently, the first example of C3–H fluoroalkoxylation of quinoxalin-2(1H)-ones with fluoroalkyl alcohols was reported by Li and Zhang (Scheme 1a).17 However, such reaction suffers from plenty of PhI(TFA)2, which failed to meet the requirements of green chemistry.18
As a clean and sustainable approach, photocatalyzed C–H functionalization has become an important strategy to introduce fluorine-containing functional groups into organic molecules.19 For a long time, we are always working to develop photocatalyzed C–H functionalization of N-heterocycle.20 Recently, our group reported photocatalyzed aerobic C3–H perfluoroalkylation of quinoxalin-2(1H)-ones with sodium perfluoroalkanesulfinates.13a As a further work, herein, we demonstrated a visible light induced aerobic C3–H fluoroalkoxylation of quinoxalin-2(1H)-ones with fluoroalkyl alcohols, providing a green and efficient method to introduce fluoroalkoxy into quinoxalin-2(1H)-ones molecules (Scheme 1b). During the preparation of our manuscript, Wang and Li groups developed an useful approach for alkoxylation of quinoxalin-2(1H)-ones respectively, but it only gave one trifluoroethoxylated example.15a,b Therefore, the development of simple and widely applicable approaches for the C3–H fluoroalkoxylation of quinoxalin-2(1H)-ones is still meaningful. In addition, many further transformations have been done in our work, which clearly demonstrated the application value of the reaction.
Results and discussion
Initially, the coupling reaction of quinoxalin-2(1H)-ones with trifluoroethanol was performed in air by using 5 mol% of rose bengal as a photocatalyst under the radiation of 18 W blue LED. The desired product (2) was obtained in 25% yield (Table 1, entry 1). Encouraged by this result, we next screened a series of photocatalysts such as Acr+–Mes ClO4−, fluorescein, eosin Y, methylene blue, erythrosine, Ru(bpy)3Cl2 and Ir(ppy)3. It was found that eosin Y was the best photocatalyst, which provided the product in 70% yield (Table 1, entries 2–8). No desired product (2) was generated without photocatalyst (Table 1, entry 9). To further improve the product yield, some additives and solvents were explored, but no better result was gained (Table 1, entries 10–18). To our delight, the yield was enhanced to 85% when the reaction was performed under O2 atmosphere (Table 1, entry 19). No desired product was generated when the transformation was carried out under N2 atmosphere (Table 1, entry 20). These results implied that O2 played a key role in this transformation. Further investigations on the light sources revealed that blue light was the best choice for the reaction (Table 1, entries 21–23).| Entry | Photocatalyst | Additive | Solvent | Yieldb [%] |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), photocatalyst (5 mol%), additive (1.5 equiv.), CF3CH2OH (0.5 mL), blue LED (18 W), rt, under air atmosphere, 24 h.b Isolated yields.c CF3CH2OH (5.0 equiv.), solvent (0.5 mL).d Under O2 atmosphere.e Under N2 atmosphere.f Green LED (18 W).g White LED (18 W).h Without light. | ||||
| 1 | Rose bengal | — | — | 25 |
| 2 | Acr+–Mes ClO4− | — | — | 65 |
| 3 | Fluorescein | — | — | Trace |
| 4 | Eosin Y | — | — | 70 |
| 5 | Methylene blue | — | — | Trace |
| 6 | Erythrosine | — | — | Trace |
| 7 | Ru(bpy)3Cl2 | — | — | 28 |
| 8 | Ir(ppy)3 | — | — | Trace |
| 9 | — | — | — | 0 |
| 10 | Eosin Y | TFA | — | 66 |
| 11 | Eosin Y | H3PO4 | — | 43 |
| 12 | Eosin Y | Na2CO3 | — | 20 |
| 13 | Eosin Y | AcONa | — | 34 |
| 14 | Eosin Y | Bu4NBr | — | 48 |
| 15c | Eosin Y | — | CH3CN | 52 |
| 16c | Eosin Y | — | DCE | Trace |
| 17c | Eosin Y | — | DMF | 24 |
| 18c | Eosin Y | — | H2O | Trace |
| 19d | Eosin Y | — | — | 85 |
| 20e | Eosin Y | — | — | 0 |
| 21d,f | Eosin Y | — | — | 15 |
| 22d,g | Eosin Y | — | — | 27 |
| 23d,h | Eosin Y | — | — | 0 |
Having established the best reaction conditions, we subsequently explored the substrate scope of quinoxalin-2(1H)-ones (Table 2). Generally, the C–H fluoroalkoxylation reaction showed good substituent group tolerance. Quinoxalin-2(1H)-ones bearing different of N-protecting groups such as methyl (–CH3), ethyl (–C2H5), butyl (–nC4H9), isoamyl (–iC5H11), cyclopropylmethyl, cyclohexylmethyl and esteryl (–CH2CO2R) were well tolerant, giving the corresponding products (2–9) in 70–87% yields. The N-benzyl groups (–CH2Ar) also were compatible, affording the target products in good yields (10–19). The molecular structure of product 18 was confirmed by X-ray diffraction studies (see ESI†). In addition, quinoxalin-2(1H)-ones with substituents on the benzene ring also could undergo this transformation smoothly, providing the trifluoroethoxylated products (20–27) in 45–82% yields. Furthermore, quinoxalin-2(1H)-one and 2H-benzo[b][1,4]oxazin-2-one were also tolerant under standard reaction conditions, yielding the corresponding products (28 and 29) in 75% and 78% yields, responsively.
After that, the efforts were further focused on the exploration of fluoroalkyl alcohols (Table 3). It was found that fluoroalkyl alcohols such as pentafluoro-1-propanol, heptafluoro-1-butanol, tetrafluoro-1-propanol, trifluorobutan-1-butanol, difluoro-1-ethanol and fluoro-1-ethanol could also undergo this reaction successfully, producing the corresponding products (30–41) in good yields. Regrettably, the longer-chain and bulky fluoroalkyl alcohols such as octafluoropentyl alcohol, hexafluoroisopropanol and nonafluoro-tert-butanol could not be converted into corresponding products (42–44) since the effect of large steric hindrance. We also tried to extend the method to the modification of other important N-containing molecules, but failed (see ESI, Scheme S1†).
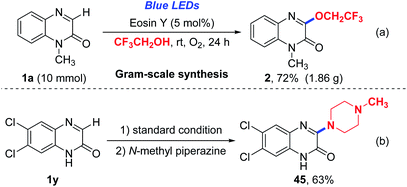
To demonstrate the application value of the reaction, we firstly performed a gram-scale reaction. To our delight, the product (2) could be isolated in 72% yield (Scheme 2a). In addition, the histamine-4 receptor antagonist 45 could be obtained in 63% yield through the C–H trifluoroethoxylation, followed by nucleophilic substitution (Scheme 2b).21 Furthermore, the N-containing bidentate ligand 46 also could be synthesized in 50% yield by using product 28 as building block (Scheme 3). We further applied this ligand to catalyse traditional C–N cross-couplings to examine its reactivity. It was found that compare to other ligands that were reported in the literature,22 the ligand 46 has relatively good reactivity, providing the product in moderate yield.
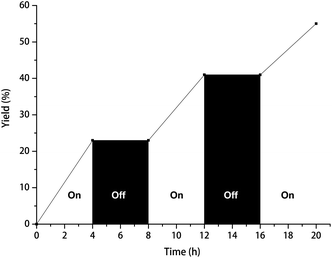
To obtain more details of the C–H fluoroalkoxylation reaction, the mechanism investigations were started. Firstly, the C–H fluoroalkoxylation reaction was dramatically suppressed when two equivalents of TEMPO (2,2,6,6-tetramethylpiperidinooxy) or DPE (1,1-diphenylethlene) was added respectively (Scheme 4). These results implied that a radical pathway might be involved in this reaction. The visible light irradiation on/off experiments revealed that the visible light played a crucial role in this reaction (Fig. 1). In addition, the measurement result of potential indicated that the generation of trifluoroethoxy radical from trifluoroethanol is very difficult (Scheme S2†).
Based on the results of mechanism studies and previous works,7–17 we proposed a reasonable mechanism for this visible-light-induced C–H fluoroalkoxylation reaction (Scheme 5). Initially, the excited eosin Y* was generated under the irradiation of blue LEDs. A subsequent single-electron-transfer (SET) process took place between eosin Y* and N-methyl-quinoxalin-2(1H)-one 1a to form the eosin Y˙− species and intermediate A. The intermediate A was further trapped by trifluoroethanol to generate intermediate B. Meanwhile, the second single-electron-transfer (SET) process happened between eosin Y˙− species and O2 to give O2˙− species and eosin Y. The final product was obtained through oxidation and proton transfer process with the release of H2O2, which was detected by a starch potassium iodide test paper.
Conclusions
We have reported a visible-light-induced aerobic C3–H fluoroalkoxylation of quinoxalin-2(1H)-ones with fluoroalkyl alcohols. This approach uses readily available fluoroalkyl alcohols as fluoroalkoxylation reagents and substrates with various functional groups were tolerant, providing the fluoroalkoxylated products in moderate to good yields. The control experiments results demonstrated that a radical pathway was answerable for this reaction.Experimental section
General information
The starting materials, solvents and other chemicals used in experiments were purchased from Energy Chemical without further purification. All products were isolated by short chromatography on a silica gel (200–300 mesh) column using petroleum ether (60–90 °C) and ethyl acetate. 1H, 13C and 19F NMR spectra were recorded on Bruker Avance DRX-500 spectrometers at ambient temperature with CDCl3 as solvent and tetramethylsilane (TMS) as the internal standard. All chemical shift values are quoted in ppm and coupling constants quoted in Hz. Compounds for HRMS were analyzed by positive mode electrospray ionization (ESI) using Agilent 6530 QTOF mass spectrometer.General procedure for the synthesis of fluoroalkoxylated quinoxalin-2(1H)-ones (2–41)
Quinoxalin-2(1H)-ones derivatives 1 (0.2 mmol), eosin Y (5 mol%), RfOH (0.5 mL) were combined in a 15 mL tube. The mixture was then stirred for 24 hours under O2 atmosphere by the radiation of 18 W blue LED. After the conversion was completed as indicated by TLC, to the residue was added water (10 mL) and extracted with ethyl acetate (5 mL × 3). The collected organic layer was washed with brine, dried with MgSO4, filtered and concentrated in vacuo. The residue was purified directly by flash column chromatography.General procedure for the gram-scale synthesis of fluoroalkoxylated quinoxalin-2(1H)-one (2)
Quinoxalin-2(1H)-ones derivatives 1a (10.0 mmol), eosin Y (5 mol%), RfOH (20 mL) were combined in a 100 mL flask. The mixture was then stirred for 24 hours under O2 atmosphere by the radiation of 18 W blue LED. After the conversion was completed as indicated by TLC, to the residue was added water (10 mL) and extracted with ethyl acetate (50 mL × 3). The collected organic layer was washed with brine, dried with MgSO4, filtered and concentrated in vacuo. The residue was purified directly by flash column chromatography.General procedure for the synthesis of histamine-4 receptor antagonist (45)
Quinoxalin-2(1H)-ones derivative 1y (0.5 mmol), eosin Y (5 mol%), RfOH (1.0 mL) were combined in a 15 mL tube. The mixture was then stirred for 24 hours under O2 atmosphere by the radiation of 18 W blue LED. After the conversion was completed as indicated by TLC, to the residue was added water (10 mL) and extracted with ethyl acetate (10 mL × 3). The collected organic layer was washed with brine, dried with MgSO4, filtered and concentrated in vacuo. After the solvent was removed, DMSO (1.5 mL), Et3N (1.5 equiv.) and N-methylpiperazine (1.5 equiv.) were added to the residue, and the mixture was stirred at 120 °C for 12 hours. After the conversion was completed as indicated by TLC, to the residue was added water (10 mL) and extracted with ethyl acetate (10 mL × 3). The collected organic layer was washed with brine, dried with MgSO4, filtered and concentrated in vacuo. The residue was purified directly by flash column chromatography.General procedure for the synthesis of 3,3′-bis(2,2,2-trifluoroethoxy)-2,2′-biquinoxaline (46)
Fluoroalkoxylated quinoxalin-2(1H)-one 28 (2.0 mmol), POCl3 (1.2 equiv.) and pyridine (1.0 equiv.) were combined in a 15 mL tube. The mixture was then stirred at 160 °C for 30 min. After the conversion was completed as indicated by TLC, to the residue was added saturated NaHCO3 solution (15 mL) and extracted with ethyl acetate (20 mL × 3). The collected organic layer was washed with brine, dried with MgSO4, filtered and concentrated in vacuo. After the solvent was removed, NiCl2·6H2O (5 mol%), LiCl (1.0 equiv.), zinc dust (1.2 equiv.) and DMF (10.0 mL) in a 50 mL flask was heated to 50 °C, then, a grain of iodine crystal and two drops of acetic acid were added to the mixture. The mixture was stirred at 60 °C for 2 hours. After the conversion was completed as indicated by TLC, to the residue was added water (15 mL) and extracted with ethyl acetate (20 mL × 3). The collected organic layer was washed with brine, dried with MgSO4, filtered and concentrated in vacuo. The residue was purified directly by flash column chromatography.General procedure for the synthesis of 1-(p-tolyl)-1H-indole (49)
K3PO4 (2.2 mmol) was added to a Schlenk tube equipped with a stirring bar and the tube was dried under vacuum and then filled with an argon. CuI (0.1 mmol) and ligand (0.1 mmol) and DMF (4.0 mL) were added and the mixture was stirred at 50 °C for 1 h, 1-iodo-4-methylbenzene (2.0 mmol) and indole (3.0 mmol) were added, and then the mixture was stirred at 110 °C for 24 h. After the completion of the reaction, the mixture was cooled, then the precipitate was removed by filtration and the product was extracted with ethyl acetate (20 mL × 3). The collected organic layer was washed with brine, dried with MgSO4, filtered and concentrated in vacuo. The residue was purified directly by flash column chromatography.1-Methyl-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (2)
White solid (85% yield), mp 154–155 °C. 1H NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 8.0, 1.3 Hz, 1H), 7.51–7.45 (m, 1H), 7.36–7.32 (m, 1H), 7.30 (d, J = 8.3 Hz, 1H), 4.90 (q, J = 8.3 Hz, 2H), 3.74 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 151.03, 149.21, 131.10, 128.96, 127.14, 126.86, 123.21, 122.10 (q, J = 278.5 Hz), 112.79, 61.78 (q, J = 37.8 Hz), 28.60. 19F NMR (471 MHz, CDCl3) δ −73.02. HRMS (ESI): calculated for C11H9F3N2O2+: 259.0689 [M + H]+, found: 259.0686.1-Ethyl-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (3)
White solid (82% yield), mp 126–127 °C. 1H NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 8.2, 1.5 Hz, 1H), 7.50–7.45 (m, 1H), 7.35–7.30 (m, 2H), 4.90 (q, J = 8.3 Hz, 2H), 4.36 (q, J = 7.2 Hz, 2H), 1.40 (t, J = 7.2 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 152.03, 149.71, 130.99, 130.32, 128.16, 128.14, 124.03, 123.15 (q, J = 278.5 Hz), 113.67, 62.81 (q, J = 37.8 Hz), 37.87, 12.36. 19F NMR (471 MHz, CDCl3) δ −73.00. HRMS (ESI): calculated for C12H11F3N2O2+: 273.0846 [M + H]+, found: 273.0849.1-Butyl-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (4)
White solid (80% yield), mp 118–119 °C. 1H NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 7.9, 1.3 Hz, 1H), 7.50–7.44 (m, 1H), 7.31 (dd, J = 13.1, 8.1 Hz, 2H), 4.89 (q, J = 8.3 Hz, 2H), 4.33–4.26 (m, 2H), 1.79–1.72 (m, 2H), 1.49 (dd, J = 15.1, 7.5 Hz, 2H), 1.00 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 152.04, 149.94, 131.28, 130.29, 128.12, 128.05, 123.99, 123.15 (q, J = 278.5 Hz), 113.84, 62.82 (q, J = 37.8 Hz), 42.62, 29.25, 20.22, 13.76. 19F NMR (471 MHz, CDCl3) δ −73.01. HRMS (ESI): calculated for C14H15F3N2O2+: 301.1159 [M + H]+, found: 301.1154.1-Isopentyl-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (5)
White solid (87% yield), mp 123–124 °C. 1H NMR (500 MHz, CDCl3) δ 7.65 (d, J = 7.8 Hz, 1H), 7.50–7.45 (m, 1H), 7.34–7.27 (m, 2H), 4.88 (q, J = 8.3 Hz, 2H), 4.32–4.26 (m, 2H), 1.78 (dd, J = 13.3, 6.6 Hz, 1H), 1.67–1.62 (m, 2H), 1.04 (d, J = 6.6 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 152.01, 149.86, 131.22, 130.32, 128.13, 128.09, 124.00, 123.15 (q, J = 278.5 Hz), 113.75, 62.83 (q, J = 37.8 Hz), 41.44, 35.82, 26.44, 22.47. 19F NMR (471 MHz, CDCl3) δ −72.56. HRMS (ESI): calculated for C15H17F3N2O2+: 315.1315 [M + H]+, found: 315.1318.1-(Cyclopropylmethyl)-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (6)
White solid (81% yield), mp 136–137 °C. 1H NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 7.9, 1.0 Hz, 1H), 7.51–7.45 (m, 1H), 7.43 (d, J = 8.0 Hz, 1H), 7.33 (t, J = 7.5 Hz, 1H), 4.90 (q, J = 8.3 Hz, 2H), 4.23 (d, J = 7.0 Hz, 2H), 1.31–1.25 (m, 1H), 0.62–0.53 (m, 4H). 13C NMR (126 MHz, CDCl3) δ 152.18, 150.28, 131.50, 130.22, 128.09, 128.05, 124.02, 123.17 (q, J = 278.5 Hz), 114.09, 62.86 (q, J = 37.8 Hz), 46.74, 9.57, 4.13. 19F NMR (471 MHz, CDCl3) δ −72.97. HRMS (ESI): calculated for C14H13F3N2O2+: 299.1002 [M + H]+, found: 299.1006.1-(Cyclohexylmethyl)-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (7)
White solid (84% yield), mp 145–146 °C. 1H NMR (500 MHz, CDCl3) δ 7.57 (dd, J = 7.9, 1.4 Hz, 1H), 7.40–7.36 (m, 1H), 7.26–7.20 (m, 2H), 4.81 (q, J = 8.3 Hz, 2H), 4.10 (d, J = 7.3 Hz, 2H), 1.88–1.80 (m, 1H), 1.68–1.56 (m, 5H), 1.12 (t, J = 9.9 Hz, 5H). 13C NMR (126 MHz, CDCl3) δ 151.05, 149.37, 130.67, 129.20, 127.07, 126.89, 122.92, 122.12 (q, J = 278.5 Hz), 113.28, 61.83 (q, J = 37.8 Hz), 47.53, 35.46, 29.83, 25.11, 24.73. 19F NMR (471 MHz, CDCl3) δ −72.98. HRMS (ESI): calculated for C17H19F3N2O2+: 341.1472 [M + H]+, found: 341.1476.Methyl-2-(2-oxo-3-(2,2,2-trifluoroethoxy)quinoxalin-1(2H)-yl)acetate (8)
White solid (73% yield), mp 133–134 °C. 1H NMR (500 MHz, CDCl3) δ 7.67 (dd, J = 8.0, 1.4 Hz, 1H), 7.47–7.41 (m, 1H), 7.37–7.31 (m, 1H), 7.06 (dd, J = 8.3, 0.7 Hz, 1H), 5.07 (s, 2H), 4.90 (q, J = 8.3 Hz, 2H), 3.78 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 167.30, 151.79, 149.97, 131.26, 130.04, 128.39, 128.23, 124.57, 123.09 (q, J = 278.5 Hz), 113.24, 62.98 (q, J = 37.8 Hz), 52.95, 43.75. 19F NMR (471 MHz, CDCl3) δ −73.00. HRMS (ESI): calculated for C13H11F3N2O4+: 317.0744 [M + H]+, found: 317.0746.tert-Butyl-2-(2-oxo-3-(2,2,2-trifluoroethoxy)quinoxalin-1(2H)-yl)acetate (9)
White solid (70% yield), mp 127–128 °C. 1H NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 8.0, 1.4 Hz, 1H), 7.47–7.41 (m, 1H), 7.35–7.29 (m, 1H), 7.05 (dd, J = 8.3, 0.7 Hz, 1H), 4.97 (s, 2H), 4.90 (q, J = 8.3 Hz, 2H), 1.46 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 165.76, 151.84, 149.96, 131.39, 130.00, 128.21, 128.16, 124.40, 123.09 (q, J = 278.5 Hz), 113.32, 83.41, 62.93 (q, J = 37.8 Hz), 44.54, 27.96. 19F NMR (471 MHz, CDCl3) δ −73.01. HRMS (ESI): calculated for C16H17F3N2O4+: 359.1213 [M + H]+, found: 359.1216.1-Benzyl-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (10)
White solid (80% yield), mp 126–127 °C. 1H NMR (500 MHz, CDCl3) δ 7.65 (d, J = 7.8 Hz, 1H), 7.31 (ddd, J = 22.1, 13.2, 5.1 Hz, 8H), 5.52 (s, 2H), 4.92 (q, J = 8.2 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 152.11, 150.45, 134.91, 131.44, 130.22, 128.98, 128.15, 128.00, 127.87, 127.03, 124.27, 122.05 (q, J = 278.5 Hz), 114.67, 62.98 (q, J = 37.8 Hz), 46.42. 19F NMR (471 MHz, CDCl3) δ −72.94. HRMS (ESI): calculated for C17H13F3N2O2+: 335.1002 [M + H]+, found: 335.1008.1-(2-Fluorobenzyl)-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (11)
White solid (70% yield), mp 141–142 °C. 1H NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 7.9, 1.4 Hz, 1H), 7.40–7.35 (m, 1H), 7.31–7.28 (m, 1H), 7.26–7.23 (m, 2H), 7.15–7.07 (m, 2H), 7.05–7.00 (m, 1H), 5.58 (s, 2H), 4.93 (q, J = 8.3 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 160.30 (d, J = 245.7 Hz), 152.02, 150.62, 131.15, 130.17, 129.65 (d, J = 7.6 Hz), 128.72 (d, J = 3.8 Hz), 128.36, 128.03, 124.76 (d, J = 3.8 Hz), 124.43, 123.15 (q, J = 278.5 Hz), 122.00 (d, J = 13.8 Hz), 115.59 (d, J = 21.4 Hz), 114.26 (d, J = 2.5 Hz), 63.00 (q, J = 37.8 Hz), 39.83 (d, J = 5.0 Hz). 19F NMR (471 MHz, CDCl3) δ −72.95, −118.26. HRMS (ESI): calculated for C17H12F4N2O2+: 353.0908 [M + H]+, found: 353.0905.1-(2-Chlorobenzyl)-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (12)
White solid (69% yield), mp 150–151 °C. 1H NMR (500 MHz, CDCl3) δ 7.67 (dd, J = 7.8, 1.6 Hz, 1H), 7.45 (dd, J = 8.0, 0.9 Hz, 1H), 7.36–7.32 (m, 1H), 7.30 (td, J = 7.6, 1.3 Hz, 1H), 7.22 (td, J = 7.9, 1.2 Hz, 1H), 7.13–7.08 (m, 1H), 7.04 (dd, J = 8.2, 1.1 Hz, 1H), 6.81 (d, J = 7.7 Hz, 1H), 5.61 (s, 2H), 4.94 (q, J = 8.3 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 152.02, 150.47, 132.63, 131.92, 131.15, 130.18, 129.82, 128.99, 128.39, 128.00, 127.39, 127.00, 124.49, 123.12 (q, J = 278.5 Hz), 114.58, 63.02 (q, J = 37.8 Hz), 44.00. 19F NMR (471 MHz, CDCl3) δ −72.92. HRMS (ESI): calculated for C17H12ClF3N2O2+: 369.0612 [M + H]+, found: 369.0615.1-(3-Methylbenzyl)-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (13)
White solid (78% yield), mp 127–128 °C. 1H NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 7.9, 1.4 Hz, 1H), 7.37–7.33 (m, 1H), 7.30–7.26 (m, 2H), 7.23–7.18 (m, 1H), 7.07 (d, J = 5.6 Hz, 3H), 5.49 (s, 2H), 4.93 (q, J = 8.3 Hz, 2H), 2.30 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 152.13, 150.47, 138.82, 134.83, 131.51, 130.22, 128.81, 128.65, 128.14, 127.95, 127.60, 124.22, 124.06, 123.15 (q, J = 278.5 Hz), 114.73, 62.98 (q, J = 37.8 Hz), 46.47, 21.41. 19F NMR (471 MHz, CDCl3) δ −72.94. HRMS (ESI): calculated for C18H15F3N2O2+: 349.1159 [M + H]+, found: 349.1155.1-(3-Chlorobenzyl)-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (14)
White solid (72% yield), mp 149–150 °C. 1H NMR (500 MHz, CDCl3) δ 7.67 (dd, J = 7.9, 1.4 Hz, 1H), 7.40–7.35 (m, 1H), 7.31 (td, J = 7.8, 1.2 Hz, 1H), 7.26 (d, J = 4.7 Hz, 3H), 7.19 (dd, J = 8.3, 0.8 Hz, 1H), 7.17–7.10 (m, 1H), 5.49 (s, 2H), 4.93 (q, J = 8.3 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 152.03, 150.38, 136.92, 134.96, 131.23, 130.28, 130.23, 128.28, 128.20, 128.15, 127.11, 125.19, 124.47, 123.11 (q, J = 278.5 Hz), 114.38, 63.04 (q, J = 37.8 Hz), 45.91. 19F NMR (471 MHz, CDCl3) δ −72.94. HRMS (ESI): calculated for C17H12ClF3N2O2+: 369.0612 [M + H]+, found: 369.0615.1-(3-Bromobenzyl)-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (15)
White solid (75% yield), mp 154–155 °C. 1H NMR (500 MHz, CDCl3) δ 7.67 (dd, J = 7.9, 1.5 Hz, 1H), 7.44–7.35 (m, 3H), 7.33–7.28 (m, 1H), 7.22–7.17 (m, 3H), 5.48 (s, 2H), 4.93 (q, J = 8.3 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 152.02, 150.37, 137.19, 131.22, 131.15, 130.54, 130.22, 129.98, 128.30, 128.15, 125.66, 124.48, 123.11 (q, J = 278.5 Hz), 123.10, 114.37, 63.04 (q, J = 37.8 Hz), 45.84. 19F NMR (471 MHz, CDCl3) δ −72.94. HRMS (ESI): calculated for C17H12BrF3N2O2+: 413.0107 [M + H]+, found: 413.0109.1-(4-Methylbenzyl)-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (16)
White solid (83% yield), mp 171–172 °C. 1H NMR (500 MHz, CDCl3) δ 7.63 (dd, J = 8.2, 1.5 Hz, 1H), 7.36–7.32 (m, 1H), 7.29–7.25 (m, 2H), 7.17 (d, J = 8.1 Hz, 2H), 7.11 (d, J = 8.0 Hz, 2H), 5.47 (s, 2H), 4.91 (q, J = 8.3 Hz, 2H), 2.30 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 152.11, 150.44, 137.63, 131.91, 131.45, 130.21, 129.62, 128.11, 127.95, 127.07, 124.19, 123.16 (q, J = 278.5 Hz), 114.69, 62.95 (q, J = 37.8 Hz), 46.20, 21.08. 19F NMR (471 MHz, CDCl3) δ −72.94. HRMS (ESI): calculated for C18H15F3N2O2+: 349.1159 [M + H]+, found: 349.1157.1-(4-Chlorobenzyl)-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (17)
White solid (77% yield), mp 184–185 °C. 1H NMR (500 MHz, CDCl3) δ 7.66 (d, J = 7.9 Hz, 1H), 7.39–7.34 (m, 1H), 7.32–7.27 (m, 3H), 7.21 (dd, J = 11.8, 8.3 Hz, 3H), 5.49 (s, 2H), 4.92 (q, J = 8.2 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 152.05, 150.39, 133.82, 133.41, 131.22, 130.24, 129.18, 128.51, 128.22, 128.15, 124.43, 123.10 (q, J = 278.5 Hz), 114.39, 63.01 (q, J = 37.8 Hz), 45.80. 19F NMR (471 MHz, CDCl3) δ −72.96. HRMS (ESI): calculated for C17H12ClF3N2O2+: 369.0612 [M + H]+, found: 369.0617.1-(4-Bromobenzyl)-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (18)
White solid (80% yield), mp 197–198 °C. 1H NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 7.9, 1.5 Hz, 1H), 7.45 (d, J = 8.5 Hz, 2H), 7.39–7.34 (m, 1H), 7.30 (td, J = 7.7, 1.3 Hz, 1H), 7.19 (dd, J = 8.3, 1.1 Hz, 1H), 7.16 (d, J = 8.5 Hz, 2H), 5.47 (s, 2H), 4.92 (q, J = 8.3 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 152.04, 150.40, 133.94, 132.13, 131.20, 130.24, 128.82, 128.23, 128.15, 124.45, 121.99 (q, J = 278.5 Hz), 121.88, 114.39, 63.01 (q, J = 37.8 Hz), 45.86. 19F NMR (471 MHz, CDCl3) δ −72.97. HRMS (ESI): calculated for C17H12BrF3N2O2+: 413.0107 [M + H]+, found: 413.0106.1-(4-Nitrobenzyl)-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (19)
White solid (64% yield), mp 193–194 °C. 1H NMR (500 MHz, CDCl3) δ 8.19 (d, J = 8.7 Hz, 2H), 7.69 (dd, J = 7.8, 1.6 Hz, 1H), 7.44 (d, J = 8.7 Hz, 2H), 7.38 (td, J = 7.9, 1.6 Hz, 1H), 7.33 (td, J = 7.6, 1.2 Hz, 1H), 7.12 (dd, J = 8.2, 1.0 Hz, 1H), 5.62 (s, 2H), 4.94 (q, J = 8.3 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 151.97, 150.35, 147.66, 142.18, 131.00, 130.27, 128.41, 128.39, 127.87, 124.76, 124.28, 123.06 (q, J = 278.5 Hz), 114.04, 63.09 (q, J = 37.8 Hz), 45.85. 19F NMR (471 MHz, CDCl3) δ −72.96. HRMS (ESI): calculated for C17H12F3N3O4+: 380.0853 [M + H]+, found: 380.0856.1,6-Dimethyl-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (20)
White solid (80% yield), mp 164–165 °C. 1H NMR (500 MHz, CDCl3) δ 7.52 (d, J = 8.1 Hz, 1H), 7.15 (d, J = 8.2 Hz, 1H), 7.09 (s, 1H), 4.88 (q, J = 8.4 Hz, 2H), 3.73 (s, 3H), 2.50 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 151.43, 150.32, 138.62, 131.93, 127.86, 127.53, 125.37, 123.15 (q, J = 278.5 Hz), 114.03, 62.70 (q, J = 37.8 Hz), 29.53, 21.86. 19F NMR (471 MHz, CDCl3) δ −73.04. HRMS (ESI): calculated for C12H11F3N2O2+: 273.0846 [M + H]+, found: 273.0848.6-Methoxy-1-methyl-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (21)
White solid (82% yield), mp 169–170 °C. 1H NMR (500 MHz, CDCl3) δ 7.20 (d, J = 9.1 Hz, 1H), 7.13 (d, J = 2.8 Hz, 1H), 7.08 (dd, J = 9.1, 2.9 Hz, 1H), 4.90 (q, J = 8.3 Hz, 2H), 3.88 (s, 3H), 3.72 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 156.47, 152.57, 149.75, 130.82, 126.20, 123.12 (q, J = 278.5 Hz), 116.53, 114.65, 110.27, 62.79 (q, J = 37.8 Hz), 55.75, 29.73. 19F NMR (471 MHz, CDCl3) δ −73.12. HRMS (ESI): calculated for C12H11F3N2O3+: 289.0795 [M + H]+, found: 289.0794.6-Chloro-1-methyl-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (22)
White solid (72% yield), mp 145–146 °C. 1H NMR (500 MHz, CDCl3) δ 7.58–7.55 (m, 1H), 7.32–7.29 (m, 1H), 7.29 (s, 1H), 4.88 (q, J = 8.3 Hz, 2H), 3.71 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 152.05, 149.95, 133.96, 132.95, 128.86, 128.52, 124.54, 121.15 (q, J = 278.5 Hz), 113.95, 62.93 (q, J = 37.8 Hz), 29.76. 19F NMR (471 MHz, CDCl3) δ −73.02. HRMS (ESI): calculated for C11H8ClF3N2O2+: 293.0299 [M + H]+, found: 293.0296.6-Bromo-1-methyl-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (23)
White solid (75% yield), mp 151–152 °C. 1H NMR (500 MHz, CDCl3) δ 7.50 (dd, J = 7.7, 1.2 Hz, 1H), 7.45–7.42 (m, 2H), 4.88 (q, J = 8.3 Hz, 2H), 3.71 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 152.20, 149.89, 133.15, 129.07, 128.91, 127.43, 121.15 (q, J = 278.5 Hz), 121.76, 116.90, 62.94 (q, J = 37.8 Hz), 29.76. 19F NMR (471 MHz, CDCl3) δ −73.02. HRMS (ESI): calculated for C11H8BrF3N2O2+: 336.9794 [M + H]+, found: 336.9798.6-Benzoyl-1-methyl-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (24)
White solid (58% yield), mp 167–168 °C. 1H NMR (500 MHz, CDCl3) δ 8.08 (d, J = 1.9 Hz, 1H), 8.00 (dd, J = 8.7, 1.9 Hz, 1H), 7.81 (d, J = 7.7 Hz, 2H), 7.64 (t, J = 7.5 Hz, 1H), 7.53 (t, J = 7.7 Hz, 2H), 7.41 (d, J = 8.7 Hz, 1H), 4.90 (q, J = 8.3 Hz, 2H), 3.80 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 194.98, 152.62, 150.16, 137.36, 135.12, 133.39, 132.66, 130.25, 129.91, 129.61, 129.31, 128.50, 122.97 (q, J = 278.5 Hz), 114.01, 63.01 (q, J = 37.8 Hz), 29.96. 19F NMR (471 MHz, CDCl3) δ −72.93. HRMS (ESI): calculated for C18H13F3N2O3+: 363.0951 [M + H]+, found: 363.0953.1-Methyl-6-nitro-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (25)
Yellow solid (45% yield), mp 172–173 °C. 1H NMR (500 MHz, CDCl3) δ 7.92 (d, J = 2.4 Hz, 1H), 7.61 (dd, J = 9.0, 2.4 Hz, 1H), 7.27 (d, J = 9.0 Hz, 1H), 4.90 (q, J = 8.3 Hz, 2H), 3.67 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 151.23, 149.38, 133.57, 133.29, 131.31, 130.89, 130.02, 123.15 (q, J = 278.5 Hz), 115.23, 63.33 (q, J = 37.8 Hz), 29.40. 19F NMR (471 MHz, CDCl3) δ −73.12. HRMS (ESI): calculated for C11H8F3N3O4+: 304.0540 [M + H]+, found: 304.0543.1,6,7-Trimethyl-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (26)
White solid (80% yield), mp 137–138 °C. 1H NMR (500 MHz, CDCl3) δ 7.40 (s, 1H), 7.04 (s, 1H), 4.87 (q, J = 8.4 Hz, 2H), 3.70 (s, 3H), 2.39 (s, 3H), 2.33 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 151.53, 150.24, 137.59, 133.13, 129.99, 128.09, 128.01, 123.21 (q, J = 278.5 Hz), 114.47, 62.66 (q, J = 37.8 Hz), 29.53, 20.32, 19.19. 19F NMR (471 MHz, CDCl3) δ −73.02. HRMS (ESI): calculated for C13H13F3N2O2+: 287.1002 [M + H]+, found: 287.1004.6,7-Dichloro-1-methyl-3-(2,2,2-trifluoroethoxy)quinoxalin-2(1H)-one (27)
White solid (70% yield), mp 160–161 °C. 1H NMR (500 MHz, CDCl3) δ 7.74 (s, 1H), 7.38 (s, 1H), 4.87 (q, J = 8.2 Hz, 2H), 3.70 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 152.80, 149.63, 132.13, 131.52, 129.23, 128.71, 128.02, 122.89 (q, J = 278.5 Hz), 115.32, 63.09 (q, J = 37.8 Hz), 29.92. 19F NMR (471 MHz, CDCl3) δ −73.00. HRMS (ESI): calculated for C11H7Cl2F3N2O2+: 326.9910 [M + H]+, found: 326.9915.3-(2,2,2-Trifluoroethoxy)quinoxalin-2(1H)-one (28)
Brown solid (75% yield), mp 195–196 °C. 1H NMR (500 MHz, DMSO) δ 12.63 (s, 1H), 7.64–7.60 (m, 1H), 7.49–7.44 (m, 1H), 7.36–7.30 (m, 2H), 5.11 (q, J = 8.9 Hz, 2H). 13C NMR (126 MHz, DMSO) δ 153.28, 150.26, 131.31, 129.74, 128.20, 126.78, 124.23 (q, J = 278.5 Hz), 123.97, 115.66, 62.71 (q, J = 37.8 Hz). 19F NMR (471 MHz, CDCl3) δ −72.87. HRMS (ESI): calculated for C10H7F3N2O2+: 245.0533 [M + H]+, found: 245.0536.3-(2,2,2-Trifluoroethoxy)-2H-benzo[b][1,4]oxazin-2-one (29)
White solid (78% yield), mp 157–158 °C. 1H NMR (500 MHz, CDCl3) δ 7.56 (dd, J = 7.9, 1.5 Hz, 1H), 7.45–7.40 (m, 1H), 7.37–7.30 (m, 2H), 4.87 (q, J = 8.2 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 149.02, 148.54, 145.60, 129.02, 128.84, 127.12, 125.95, 122.73 (q, J = 278.5 Hz), 116.35, 63.40 (q, J = 37.8 Hz). 19F NMR (471 MHz, CDCl3) δ −73.17. HRMS (ESI): calculated for C10H6F3NO3+: 246.0373 [M + H]+, found: 246.0373.1-Methyl-3-(2,2,3,3,3-pentafluoropropoxy)quinoxalin-2(1H)-one (30)
White solid (83% yield), mp 110–111 °C. 1H NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 8.0, 1.4 Hz, 1H), 7.49 (ddd, J = 8.7, 7.4, 1.5 Hz, 1H), 7.36–7.32 (m, 1H), 7.30 (dd, J = 8.4, 1.0 Hz, 1H), 4.97 (td, J = 12.9, 0.9 Hz, 2H), 3.74 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 152.07, 150.13, 132.15, 129.92, 128.21, 127.88, 124.24, 119.72–117.15 (m), 113.82, 61.73 (t, J = 27.7 Hz), 29.59. 19F NMR (471 MHz, CDCl3) δ −83.67, −122.90. HRMS (ESI): calculated for C12H9F5N2O2+: 309.2153 [M + H]+, found: 309.2155.3-(2,2,3,3,4,4,4-Heptafluorobutoxy)-1-methylquinoxalin-2(1H)-one (31)
White solid (79% yield), mp 135–136 °C. 1H NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 8.0, 1.4 Hz, 1H), 7.50–7.46 (m, 1H), 7.36–7.32 (m, 1H), 7.30 (d, J = 8.3 Hz, 1H), 5.01 (t, J = 13.5 Hz, 2H), 3.74 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 152.08, 150.11, 132.13, 129.90, 128.18, 127.86, 124.21, 119.72–117.15 (m), 113.80, 61.81 (t, J = 27.7 Hz), 29.57. 19F NMR (471 MHz, CDCl3) δ −80.74, −119.93, −127.51. HRMS (ESI): calculated for C13H9F7N2O2+: 359.0625 [M + H]+, found: 359.0627.1-Methyl-3-(2,2,3,3-tetrafluoropropoxy)quinoxalin-2(1H)-one (32)
White solid (72% yield), mp 131–132 °C. 1H NMR (500 MHz, CDCl3) δ 7.59 (dd, J = 8.0, 1.3 Hz, 1H), 7.43–7.38 (m, 1H), 7.29–7.25 (m, 1H), 7.23 (d, J = 8.3 Hz, 1H), 6.08 (tt, J = 53.0, 5.0 Hz, 1H), 4.78 (t, J = 12.2 Hz, 2H), 3.67 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 152.11, 150.18, 132.04, 130.06, 128.13, 127.89, 124.25, 113.79, 114.59–106.74 (m), 62.85 (t, J = 27.7 Hz), 29.55. 19F NMR (471 MHz, CDCl3) δ −124.57, −139.29. HRMS (ESI): calculated for C12H10F4N2O2+: 291.0751 [M + H]+, found: 291.0752.1-Methyl-3-(4,4,4-trifluorobutoxy)quinoxalin-2(1H)-one (33)
White solid (78% yield), mp 108–109 °C. 1H NMR (500 MHz, CDCl3) δ 7.55 (dd, J = 7.9, 1.4 Hz, 1H), 7.38–7.32 (m, 1H), 7.23 (td, J = 7.8, 1.2 Hz, 1H), 7.21–7.18 (m, 1H), 4.47 (t, J = 6.3 Hz, 2H), 3.65 (s, 3H), 2.32–2.23 (m, 2H), 2.09 (dt, J = 13.5, 6.3 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 153.56, 150.92, 131.64, 130.91, 127.60, 127.26, 127.02 (q, J = 275.9 Hz), 124.02, 113.65, 65.60, 30.74 (q, J = 29.0 Hz), 29.46, 21.46 (q, J = 2.5 Hz). 19F NMR (471 MHz, CDCl3) δ −66.31. HRMS (ESI): calculated for C13H13F3N2O2+: 287.1002 [M + H]+, found: 287.1006.3-(2,2-Difluoroethoxy)-1-methylquinoxalin-2(1H)-one (34)
White solid (73% yield), mp 128–129 °C. 1H NMR (500 MHz, CDCl3) δ 7.58 (dd, J = 7.9, 1.4 Hz, 1H), 7.42–7.37 (m, 1H), 7.26 (td, J = 8.0, 1.2 Hz, 1H), 7.22 (dd, J = 8.4, 0.8 Hz, 1H), 6.19 (tt, J = 55.4, 4.4 Hz, 1H), 4.61 (td, J = 13.0, 4.4 Hz, 2H), 3.67 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 152.66, 150.49, 131.97, 130.29, 127.89, 127.82, 124.19, 113.76, 112.91 (t, J = 241.9 Hz), 65.34 (t, J = 31.5 Hz), 29.58. 19F NMR (471 MHz, CDCl3) δ −125.03. HRMS (ESI): calculated for C11H10F2N2O2+: 241.0783 [M + H]+, found: 241.0787.3-(2-Fluoroethoxy)-1-methylquinoxalin-2(1H)-one (35)
White solid (80% yield), mp 136–137 °C. 1H NMR (500 MHz, CDCl3) δ 7.54 (d, J = 7.9 Hz, 1H), 7.35 (t, J = 7.8 Hz, 1H), 7.23 (t, J = 7.6 Hz, 1H), 7.20–7.17 (m, 1H), 4.83–4.80 (m, 1H), 4.74–4.71 (m, 1H), 4.70–4.67 (m, 1H), 4.66–4.61 (m, 1H), 3.65 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 153.42, 150.78, 131.72, 130.68, 127.59, 127.36, 123.97, 113.65, 80.96 (d, J = 171.4 Hz), 66.03 (d, J = 21.4 Hz), 29.48. 19F NMR (471 MHz, CDCl3) δ −224.30. HRMS (ESI): calculated for C11H11FN2O2+: 223.0878 [M + H]+, found: 223.0879.3-(2,2-Difluoroethoxy)-1-ethylquinoxalin-2(1H)-one (36)
White solid (70% yield), mp 102–103 °C. 1H NMR (500 MHz, CDCl3) δ 7.59 (dd, J = 8.4, 1.5 Hz, 1H), 7.45–7.34 (m, 1H), 7.25 (t, J = 7.4 Hz, 2H), 6.19 (tt, J = 55.4, 4.4 Hz, 1H), 4.61 (td, J = 13.0, 4.4 Hz, 2H), 4.29 (q, J = 7.2 Hz, 2H), 1.32 (t, J = 7.2 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 152.65, 149.96, 130.82, 130.63, 128.11, 127.87, 123.98, 113.62, 112.94 (t, J = 241.9 Hz), 65.32 (t, J = 31.5 Hz), 37.79, 12.36. 19F NMR (471 MHz, CDCl3) δ −124.95. HRMS (ESI): calculated for C12H12F2N2O2+: 255.0940 [M + H]+, found: 255.0946.1-Isopentyl-3-(2,2,3,3-tetrafluoropropoxy)quinoxalin-2(1H)-one (37)
White solid (75% yield), mp 119–120 °C. 1H NMR (500 MHz, CDCl3) δ 7.58 (dd, J = 8.0, 1.3 Hz, 1H), 7.40 (t, J = 7.8 Hz, 1H), 7.25 (t, J = 7.6 Hz, 1H), 7.21 (d, J = 8.4 Hz, 1H), 6.10 (tt, J = 53.0, 5.0 Hz, 1H), 4.75 (t, J = 12.1 Hz, 2H), 4.22–4.17 (m, 2H), 1.71–1.69 (m, 1H), 1.59–1.54 (m, 2H), 0.97 (d, J = 6.6 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 152.06, 149.86, 131.14, 130.43, 128.17, 128.10, 124.07, 113.75, 114.43–107.04 (m), 62.94 (t, J = 27.7 Hz), 41.45, 35.81, 26.45, 22.45. 19F NMR (471 MHz, CDCl3) δ −124.65, −139.38. HRMS (ESI): calculated for C16H18F4N2O2+: 347.1377 [M + H]+, found: 347.1379.1-Butyl-3-(2,2,3,3,4,4,4-heptafluorobutoxy)quinoxalin-2(1H)-one (38)
White solid (74% yield), mp 129–130 °C. 1H NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 7.9, 1.3 Hz, 1H), 7.49–7.45 (m, 1H), 7.31 (dd, J = 13.9, 7.6 Hz, 2H), 5.00 (t, J = 13.5 Hz, 2H), 4.31–4.25 (m, 2H), 1.80–1.72 (m, 2H), 1.49 (dd, J = 15.1, 7.5 Hz, 2H), 1.00 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 152.10, 149.84, 131.31, 130.23, 128.13, 128.10, 123.99, 119.72–117.15 (m), 113.85, 61.88 (t, J = 27.7 Hz), 42.65, 29.24, 20.23, 13.76. 19F NMR (471 MHz, CDCl3) δ −80.74, −119.93, −127.51. HRMS (ESI): calculated for C16H15F7N2O2+: 401.1095 [M + H]+, found: 401.1098.6-Bromo-3-(2-fluoroethoxy)-1-methylquinoxalin-2(1H)-one (39)
White solid (78% yield), mp 172–173 °C. 1H NMR (500 MHz, CDCl3) δ 7.78 (d, J = 2.2 Hz, 1H), 7.52 (dd, J = 8.8, 2.3 Hz, 1H), 7.14 (d, J = 8.9 Hz, 1H), 4.90–4.87 (m, 1H), 4.79 (dd, J = 5.0, 3.6 Hz, 1H), 4.77–4.74 (m, 1H), 4.70 (dd, J = 5.0, 3.6 Hz, 1H), 3.71 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 154.16, 150.49, 131.80, 130.93, 130.14, 130.00, 116.64, 115.05, 80.87 (d, J = 171.4 Hz), 66.38 (d, J = 21.4 Hz), 29.71. 19F NMR (471 MHz, CDCl3) δ −224.13. HRMS (ESI): calculated for C11H10BrFN2O2+: 300.9983 [M + H]+, found: 300.9989.6-Bromo-1-methyl-3-(4,4,4-trifluorobutoxy)quinoxalin-2(1H)-one (40)
White solid (70% yield), mp 119–120 °C. 1H NMR (500 MHz, CDCl3) δ 7.78 (d, J = 2.2 Hz, 1H), 7.52 (dd, J = 8.8, 2.2 Hz, 1H), 7.13 (d, J = 8.8 Hz, 1H), 4.53 (t, J = 6.3 Hz, 2H), 3.70 (s, 3H), 2.38–2.30 (m, 2H), 2.17 (dd, J = 15.3, 6.6 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 154.24, 150.59, 132.00, 130.82, 130.11, 130.02, 126.98 (q, J = 277.2 Hz), 116.66, 115.03, 65.96, 30.72 (q, J = 29.0 Hz), 29.67, 21.44 (q, J = 3.8 Hz). 19F NMR (471 MHz, CDCl3) δ −66.21. HRMS (ESI): calculated for C13H12BrF3N2O2+: 365.0107 [M + H]+, found: 365.0108.1,6,7-Trimethyl-3-(2,2,3,3,3-pentafluoropropoxy)quinoxalin-2(1H)-one (41)
White solid (74% yield), mp 132–133 °C. 1H NMR (500 MHz, CDCl3) δ 7.41 (s, 1H), 7.05 (s, 1H), 4.94 (td, J = 13.0, 0.7 Hz, 2H), 3.71 (s, 3H), 2.40 (s, 3H), 2.34 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 151.58, 150.15, 137.61, 133.10, 130.04, 128.10, 127.96, 119.72–117.15 (m), 114.47, 61.57 (t, J = 27.7 Hz), 29.50, 20.33, 19.19. 19F NMR (471 MHz, CDCl3) δ −83.70, −122.92. HRMS (ESI): calculated for C14H13F5N2O2+: 337.0970 [M + H]+, found: 337.0972.6,7-Dichloro-3-(4-methylpiperazin-1-yl)quinoxalin-2(1H)-one (45)
Yellow solid (63% yield), mp 234–235 °C. 1H NMR (500 MHz, DMSO) δ 7.46 (s, 1H), 7.39 (s, 1H), 3.92 (s, 4H), 2.45–2.37 (m, 4H), 2.19 (s, 3H). 13C NMR (126 MHz, DMSO) δ 151.78, 151.27, 132.56, 129.34, 125.49, 125.40, 124.63, 115.39, 54.62, 45.98, 45.68. HRMS (ESI): calculated for C13H14Cl2N4O+: 313.0618 [M + H]+, found: 313.0615.3,3′-Bis(2,2,2-trifluoroethoxy)-2,2′-biquinoxaline (46)
White solid (50% yield), mp 213–214 °C. 1H NMR (500 MHz, CDCl3) δ 8.24–8.19 (m, 1H), 7.99–7.95 (m, 1H), 7.84–7.78 (m, 1H), 7.74–7.69 (m, 1H), 4.93 (q, J = 8.3 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 153.67, 142.47, 140.16, 139.50, 131.49, 129.71, 128.09, 127.19, 123.16 (q, J = 278.5 Hz), 62.62 (q, J = 37.8 Hz). 19F NMR (471 MHz, CDCl3) δ −72.93. HRMS (ESI): calculated for C20H12F6N4O2+: 455.0937 [M + H]+, found: 455.0939.1-(p-Tolyl)-1H-indole (49)
Yellow liquid (71% yield), 1H NMR (500 MHz, CDCl3) δ 7.67 (d, J = 7.7 Hz, 1H), 7.52 (dd, J = 8.2, 0.6 Hz, 1H), 7.37 (d, J = 8.3 Hz, 2H), 7.31–7.27 (m, 3H), 7.22–7.18 (m, 1H), 7.17–7.13 (m, 1H), 6.65 (dd, J = 3.2, 0.6 Hz, 1H), 2.42 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 137.34, 136.36, 136.02, 130.19, 129.22, 128.11, 124.37, 122.24, 121.10, 120.22, 110.55, 103.23, 21.09.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Natural Science Foundation of Shandong Province (ZR2017LB006).Notes and references
- (a) J. Irurre, J. Casas and A. Messeguer, Bioorg. Med. Chem. Lett., 1993, 3, 179 CrossRef CAS; (b) J. Legros, J. R. Dehli and C. Bolm, Adv. Synth. Catal., 2005, 347, 19 CrossRef CAS; (c) M. R. Reddy, N. Shibata, Y. Kondo, S. Nakamura and T. Toru, Angew. Chem., Int. Ed., 2006, 45, 8163 CrossRef CAS PubMed; (d) J.-P. Bégué and D. Bonnet-Delpon, Bioorganic and Medicinal Chemistry of Fluorine, Wiley, Hoboken, 2008 CrossRef.
- (a) T. Nakai, K. Tanaka and N. Ishikawa, J. Fluorine Chem., 1977, 9, 89 CrossRef CAS; (b) J. P. Idoux, J. T. Gupton, C. K. McCurry, A. D. Crews, C. D. Jurss, C. Colon and R. C. Rampi, J. Org. Chem., 1983, 48, 3771 CrossRef CAS; (c) A. Kamal, T. B. Pratap, K. V. Ramana, A. V. Ramana and A. H. Babu, Tetrahedron Lett., 2002, 43, 7353 CrossRef CAS; (d) X. Shen, C. N. Neumann, C. Kleinlein, N. W. Goldberg and T. Ritter, Angew. Chem., Int. Ed., 2015, 54, 5662 CrossRef CAS PubMed.
- (a) R. Huang, Y. Huang, X. Lin, M. Rong and Z. Weng, Angew. Chem., Int. Ed., 2015, 54, 5736 CrossRef CAS PubMed; (b) Y. Huang, R. Huang and Z. Weng, Synlett, 2015, 26, 2327 CrossRef CAS; (c) Y. Guo, Y.-D. Li, C. Chen, J.-H. Zhao, H.-W. Liu, D.-H. Liao and Y.-F. Ji, Res. Chem. Intermed., 2016, 42, 2525 CrossRef CAS; (d) D. Vuluga, J. Legros, B. Crousse and D. Bonnet-Delpon, Eur. J. Org. Chem., 2009, 3513 CrossRef CAS; (e) K. Zhang, X.-H. Xu and F.-L. Qing, J. Fluorine Chem., 2017, 196, 24 CrossRef CAS; (f) T. M. Rangarajan, R. Singh, R. Brahma, K. Devi, R. P. Singh, R. P. Singh and A. K. Prasad, Chem.–Eur. J., 2014, 20, 14218 CrossRef CAS PubMed; (g) L. Yang, S. Li, L. Cai, Y. Ding, L. Fu, Z. Cai, H. Ji and G. Li, Org. Lett., 2017, 19, 2746 CrossRef CAS PubMed.
- (a) The European Agency for the Evaluation of Medicinal Products, Committee for Proprietary Medicinal Products, London, 2002 Search PubMed; (b) D. Nair, J. Scarpello, L. White, L. Freista dos Santos, I. Vankelecom and A. Livingston, Tetrahedron Lett., 2001, 42, 8219 CrossRef CAS; (c) C. Garett and K. Prasad, Adv. Synth. Catal., 2004, 346, 889 CrossRef.
- (a) L.-L. Shi, H. Zhou, J.-F. Wu and X. Li, Mini-Rev. Org. Chem., 2015, 12, 96 CrossRef CAS; (b) L. Shi, W. Hu, J. Wu, H. Zhou, H. Zhou and X. Li, Mini-Rev. Med. Chem., 2018, 18, 392 CrossRef CAS PubMed; (c) A. Carta, S. Piras, G. Loriga and G. Paglietti, Mini-Rev. Med. Chem., 2006, 6, 1179 CrossRef CAS PubMed; (d) X. Li, K. Yang, W. Li and W. Xu, Drugs Future, 2006, 31, 979 CrossRef CAS; (e) T. Ishi-i, K. Yaguma, R. Kuwahara, Y. Taguri and S. Mataka, Org. Lett., 2006, 8, 585 CrossRef CAS PubMed.
- (a) F. Wang, B.-L. Hu, L. Liu, H.-Y. Tu and X.-G. Zhang, J. Org. Chem., 2017, 82, 11247 CrossRef CAS PubMed; (b) H. Mtiraoui, K. Renault, M. Sanselme, M. Msaddek, P.-Y. Renard and C. Sabot, Org. Biomol. Chem., 2017, 15, 3060 RSC; (c) R. Klemme, C. Bentz, T. Zukowski, L. Schefzig, D. Lentz, H.-U. Reissig and R. Zimmer, Synthesis, 2016, 48, 1491 CrossRef CAS; (d) T. Yang, H. Zhu and W. Yu, Org. Biomol. Chem., 2016, 14, 3376 RSC; (e) D. Li, H. Ma and W. Yu, Adv. Synth. Catal., 2015, 357, 3696 CrossRef CAS; (f) H. Jiang, X. An, K. Tong, T. Zheng, Y. Zhan and S. Yu, Angew. Chem., Int. Ed., 2015, 54, 4055 CrossRef CAS PubMed; (g) X.-D. An and S. Yu, Org. Lett., 2015, 17, 2692 CrossRef CAS PubMed.
- (a) Q. Ke, G. Yan, J. Yu and X. Wu, Org. Biomol. Chem., 2019, 17, 5863 RSC; (b) P. Mao, J. Zhu, J. Yuan, L. Yang, Y. Xiao and C. Zhang, Chin. J. Org. Chem., 2019, 39, 1529 CrossRef.
- (a) M. Gao, Y. Li, L. Xie, R. Chauvin and X. Cui, Chem. Commun., 2016, 52, 2846 RSC; (b) J. Wang, J. Li, Y. Wei, J. Yang and C. Huo, Org. Chem. Front., 2018, 5, 3534 RSC; (c) Y. Kim and D. Y. Kim, Tetrahedron Lett., 2018, 59, 2443 CrossRef CAS; (d) W.-P. Mai, J.-W. Yuan, J.-L. Zhu, Q.-Q. Li, L. R. Yang, Y.-M. Xiao, P. Mao and L.-B. Qu, ChemistrySelect, 2019, 4, 11066 CrossRef CAS.
- (a) A. Gupta, M. S. Deshmukh and N. Jain, J. Org. Chem., 2017, 82, 4784 CrossRef CAS PubMed; (b) T. T. Hoang, T. A. To, V. T. T. Cao, A. T. Nguyen, T. T. Nguyen and N. T. S. Phan, Catal. Commun., 2017, 101, 20 CrossRef CAS; (c) W. Wei, L. Wang, P. Bao, Y. Shao, H. Yue, D. Yang, X. Yang, X. Zhao and H. Wang, Org. Lett., 2018, 20, 7125 CrossRef CAS PubMed; (d) Q. Yang, Y. Zhang, Q. Sun, K. Shang, H.-Y. Zhang and J. Zhao, Adv. Synth. Catal., 2018, 360, 4509 CrossRef CAS; (e) Q. Yang, Z. Yang, Y. Tan, J. Zhao, Q. Sun, H.-Y. Zhang and Y. Zhang, Adv. Synth. Catal., 2019, 361, 1662 CrossRef CAS; (f) J.-W. Yuan, J.-L. Zhu, B. Li, L.-Y. Yang, P. Mao, S.-R. Zhang, Y.-C. Li and L.-B. Qu, Org. Biomol. Chem., 2019, 17, 10178–10187 RSC.
- (a) W. Wei, L. Wang, H. Yue, P. Bao, W. Liu, C. Hu, D. Yang and H. Wang, ACS Sustainable Chem. Eng., 2018, 6, 17252 CrossRef CAS; (b) J. Fu, J. Yuan, Y. Zhang, Y. Xiao, P. Mao, X. Diao and L. Qu, Org. Chem. Front., 2018, 5, 3382 RSC; (c) L. Hu, J. Yuan, J. Fu, T. Zhang, L. Gao, Y. Xiao, P. Mao and L. Qu, Eur. J. Org. Chem., 2018, 4113 CrossRef CAS; (d) S. Liu, Y. Huang, F.-L. Qing and X.-H. Xu, Org. Lett., 2018, 20, 5497 CrossRef CAS PubMed; (e) J. Yuan, J. Fu, J. Yin, Z. Dong, Y. Xiao, P. Mao and L. Qu, Org. Chem. Front., 2018, 5, 2820 RSC; (f) W. Zhang, Y.-L. Pan, C. Yang, L. Chen, X. Li and J.-P. Cheng, J. Org. Chem., 2019, 84, 7786 CrossRef CAS PubMed; (g) W. Xue, Y. Su, K.-H. Wang, R. Zhang, Y. Feng, L. Cao, D. Huang and Y. Hu, Org. Biomol. Chem., 2019, 17, 6654 RSC; (h) L. X. Liu, N. Pan, W. Sheng, L. Su, L. Liu, J. Y. Dong, Y. B. Zhou and S. F. Yin, Adv. Synth. Catal., 2019, 361, 4126 CrossRef CAS; (i) Z. Yan, B. Sun, X. Zhang, X. Zhuang, J. Yang, W. Su and C. Jin, Chem.–Asian J., 2019, 14, 3344 CrossRef CAS PubMed; (j) L.-Y. Xie, L.-L. Jiang, J.-X. Tan, Y. Wang, X.-Q. Xu, B. Zhang, Z. Cao and W.-M. He, ACS Sustainable Chem. Eng., 2019, 7, 14153 CrossRef CAS.
- (a) K. Yin and R. Zhang, Org. Lett., 2017, 19, 1530 CrossRef CAS PubMed; (b) J. Yuan, S. Liu and L. Qu, Adv. Synth. Catal., 2017, 359, 4197 CrossRef CAS; (c) K. Yin and R. Zhang, Synlett, 2018, 29, 597 CrossRef CAS; (d) M. Noikham, T. Kittikool and S. Yotphan, Synthesis, 2018, 50, 2337 CrossRef CAS; (e) B. Ramesh, C. R. Reddy, G. R. Kumar and B. V. S. Reddy, Tetrahedron Lett., 2018, 59, 628 CrossRef CAS.
- (a) X. Zeng, C. Liu, X. Wang, J. Zhang, X. Wang and Y. Hu, Org. Biomol. Chem., 2017, 15, 8929 RSC; (b) J.-W. Yuan, J.-H. Fu, S.-N. Liu, Y.-M. Xiao, P. Mao and L.-B. Qu, Org. Biomol. Chem., 2018, 16, 3203 RSC; (c) L.-Y. Xie, S. Peng, T.-G. Fan, Y.-F. Liu, M. Sun, L.-L. Jiang, X.-X. Wang, Z. Cao and W.-M. He, Sci. China: Chem., 2019, 62, 460 CrossRef CAS.
- (a) Z. Wei, S. Qi, Y. Xu, H. Liu, J. Wu, H.-S. Li, C. Xia and G. Duan, Adv. Synth. Catal., 2019, 361, 5490 CrossRef CAS; (b) J. Wang, B. Sun, L. Zhang, T. Xu, Y. Xie and C. Jin, Asian J. Org. Chem., 2019, 8, 1942 CrossRef CAS; (c) L. Wang, Y. Zhang, F. Li, X. Hao, H.-Y. Zhang and J. Zhao, Adv. Synth. Catal., 2018, 360, 3969 CrossRef CAS; (d) W. Xue, Y. Su, K.-H. Wang, L. Cao, Y. Feng, W. Zhang, D. Huang and Y. Hu, Asian J. Org. Chem., 2019, 8, 887 CrossRef CAS.
- (a) G. Hong, J. Yuan, J. Fu, G. Pan, Z. Wang, L. Yang, Y. Xiao, P. Mao and X. Zhang, Org. Chem. Front., 2019, 6, 1173 RSC; (b) L. Wang, H. Liu, F. Li, J. Zhao, H.-Y. Zhang and Y. Zhang, Adv. Synth. Catal., 2019, 361, 2354 CrossRef CAS; (c) C. Jin, X. Zhuang, B. Sun, D. Li and R. Zhu, Asian J. Org. Chem., 2019, 8, 1490 CrossRef CAS.
- (a) L. Zhao, L. Wang, Y. Gao, Z. Wang and P. Li, Adv. Synth. Catal., 2019, 361, 5363 CrossRef CAS; (b) J. Zhou, P. Zhou, T. Zhao, Q. Ren and J. Li, Adv. Synth. Catal., 2019, 361, 5371 CrossRef CAS; (c) Q. Yang, X. Han, J. Zhao, H.-Y. Zhang and Y. Zhang, J. Org. Chem., 2019, 84, 11417 CrossRef CAS PubMed.
- (a) Q.-H. Teng, Y. Yao, W.-X. Wei, H.-T. Tang, J.-R. Li and Y.-M. Pan, Green Chem., 2019, 21, 6241 RSC; (b) L.-Y. Xie, Y.-L. Chen, L. Qin, Y. Wen, J.-W. Xie, J.-X. Tan, Y. Huang, Z. Cao and W.-M. He, Org. Chem. Front., 2019, 6, 3950 RSC.
- J. Xu, H. Yang, H. Cai, H. Bao, W. Li and P. Zhang, Org. Lett., 2019, 21, 4698 CrossRef CAS PubMed.
- (a) K.-J. Liu, Z.-H. Duan, X.-L. Zeng, M. Sun, Z. Tang, S. Jiang, Z. Cao and W.-M. He, ACS Sustainable Chem. Eng., 2019, 7, 10293 CrossRef CAS; (b) L.-Y. Xie, T.-G. Fang, J.-X. Tan, B. Zhang, Z. Cao, L.-H. Yang and W.-M. He, Green Chem., 2019, 21, 3858 RSC; (c) L.-Y. Xie, J. Qu, S. Peng, K.-J. Liu, Z. Wang, M.-H. Ding, Y. Wang, Z. Cao and W.-M. He, Green Chem., 2018, 20, 760 RSC; (d) B. Liu, F. Jin, T. Wang, X. Yuan and W. Han, Angew. Chem., Int. Ed., 2017, 56, 12712 CrossRef CAS PubMed; (e) B. Yang and Z. Lu, ACS Catal., 2017, 7, 8362 CrossRef CAS; (f) S. Yang, P. Li, Z. Wang and L. Wang, Org. Lett., 2017, 19, 3386 CrossRef CAS PubMed; (g) L.-Y. Xie, Y.-J. Li, J. Qu, Y. Duan, J. Hu, K.-J. Liu, Z. Cao and W.-M. He, Green Chem., 2017, 19, 5642 RSC; (h) X. Zhu, P. Li, Q. Shi and L. Wang, Green Chem., 2016, 18, 6373 RSC; (i) X. Wang, C. Wang, Y. Liu and J. Xiao, Green Chem., 2016, 18, 4605 RSC; (j) X. Cheng, B. Yang, X. Hu, Q. Xu and Z. Lu, Chem.–Eur. J., 2016, 22, 17566 CrossRef CAS PubMed.
- (a) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC; (b) X. Pan, H. Xia and J. Wu, Org. Chem. Front., 2016, 3, 1163 RSC; (c) D. A. Nagib and D. W. C. MacMillan, Nature, 2011, 480, 224 CrossRef CAS PubMed; (d) P. V. Pham, D. A. Nagib and D. W. C. MacMillan, Angew. Chem., Int. Ed., 2011, 50, 6119 CrossRef CAS PubMed; (e) T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527 CrossRef CAS PubMed; (f) Y. Ye and M. S. Sanford, J. Am. Chem. Soc., 2012, 134, 9034 CrossRef CAS PubMed; (g) L. Li, X. Mu, W. Liu, Y. Wang, Z. Mi and C.-J. Li, J. Am. Chem. Soc., 2016, 138, 5809 CrossRef CAS PubMed; (h) X.-L. Yu, J.-R. Chen, D.-Z. Chen and W.-J. Xiao, Chem. Commun., 2016, 52, 8275 RSC; (i) Y. Xiang, Y. Kuang and J. Wu, Org. Chem. Front., 2016, 3, 901 RSC; (j) H. Xiang, Q. Zhao, Z. Tang, J. Xiao, P. Xia, C. Wang, C. Yang, X. Chen and H. Yang, Org. Lett., 2017, 19, 146 CrossRef CAS PubMed; (k) H.-T. Qin, S.-W. Wu, J.-L. Liu and F. Liu, Chem. Commun., 2017, 53, 1696 RSC; (l) P. Dai, X. Yu, P. Teng, W.-H. Zhang and C. Deng, Org. Lett., 2018, 20, 6901 CrossRef CAS PubMed; (m) T. Rawner, E. Lutsker, C. Kaiser and O. Reiser, ACS Catal., 2018, 8, 3950 CrossRef CAS; (n) L. Zou, P. Li, B. Wang and L. Wang, Chem. Commun., 2019, 55, 3737 RSC; (o) L. Zhao, P. Li, H. Zhang and L. Wang, Org. Chem. Front., 2019, 6, 87 RSC; (p) Y. Liu, X.-L. Chen, K. Sun, X.-Y. Li, F.-L. Zeng, X.-C. Liu, L.-B. Qu, Y.-F. Zhao and B. Yu, Org. Lett., 2019, 21, 4019 CrossRef CAS PubMed; (q) F.-L. Zeng, K. Sun, X.-L. Chen, X.-Y. Yuan, S.-Q. He, Y. Liu, Y.-Y. Peng, L.-B. Qu, Q.-Y. Lv and B. Yu, Adv. Synth. Catal., 2019, 361, 5176 CrossRef CAS.
- (a) C. Xia, K. Wang, G. Wang and G. Duan, Org. Biomol. Chem., 2018, 16, 2214 RSC; (b) J. Han, G. Wang, J. Sun, H. Li, G. Duan, F. Li and C. Xia, Catal. Commun., 2019, 118, 81 CrossRef CAS; (c) G. You, K. Wang, X. Wang, G. Wang, J. Sun, G. Duan and C. Xia, Org. Lett., 2018, 20, 4005 CrossRef CAS PubMed.
- J. P. Edwards and J. D. Venable, Quinoxaline compounds. US 20050070527A1, March 2005, Chem. Abstr., 2005, 142, 355284 Search PubMed.
- S. Haneda, Y. Adachi and M. Hayashi, Tetrahedron, 2009, 65, 10459 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and analytical data. CCDC 1958968. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra10194b |
| This journal is © The Royal Society of Chemistry 2020 |