Open Access Article
Open Access ArticleSynthesis of hierarchical Sn/SnO nanosheets assembled by carbon-coated hollow nanospheres as anode materials for lithium/sodium ion batteries
Fengrong Hea,
Qi Xu*b,
Baoping Zhenga,
Jun Zhangc,
Zhenguo Wu b,
Yanjun Zhong
b,
Yanjun Zhong b,
Yanxiao Chen
b,
Yanxiao Chen b,
Wei Xiangd,
Benhe Zhongb and
Xiaodong Guo
b,
Wei Xiangd,
Benhe Zhongb and
Xiaodong Guo *be
*be
aDong guan Hec Technology Research Corporation, Dongguan, Guangdong 523871, P. R. China
bSchool of Chemical Engineering, Sichuan University, Chengdu 610065, P. R. China. E-mail: xiaodong2009@scu.edu.cn
cRuyuan Dongyangguang Magnetic Material Limited Company, Shaoguan, Guangdong 512710, P. R. China
dCollege of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059, P. R. China
eInstitute for Superconducting and Electronic Materials, University of Wollongong, Wollongong, NSW 2522, Australia
First published on 6th February 2020
Abstract
Tin-based anode materials have aroused interest due to their high capacities. Nevertheless, the volume expansion problem during lithium insertion/extraction processes has severely hindered their practical application. In particular, nano–micro hierarchical structure is attractive with the integrated advantages of nano-effect and high thermal stability of the microstructure. Herein, hierarchical Sn/SnO nanosheets assembled by carbon-coated hollow nanospheres were successfully synthesized by a facile glucose-assisted hydrothermal method, in which the glucose served as both morphology-control agent and carbon source. The hierarchical Sn/SnO nanosheets exhibit excellent electrochemical performances owing to the unique configuration and carbon coating. Specifically, a reversible high capacity of 2072.2 mA h g−1 was observed at 100 mA g−1. Further, 964.1 mA h g−1 after 100 cycles at 100 mA g−1 and 820.4 mA h g−1 at 1000 mA g−1 after 300 cycles could be obtained. Encouragingly, the Sn/SnO also presents certain sodium ion storage properties. This facile synthetic strategy may provide new insight into fabricating high-performance Sn-based anode materials combining the advantages of both structure and carbon coating.
1. Introduction
Currently, considerable attention has been paid to lithium ion batteries (LIBs) for their promising application in hybrid electric vehicles (HEVs), electric vehicles (EVs) and large-scale renewable energy storage systems owing to their high energy density, long life span and relatively low environmental impacts.1–4 Although graphite is extensively employed as an anode, its limited theoretical density (372 mA h g−1) prevents it from meeting the ever-increasing requirements for higher power and energy density.5 Thus, tremendous efforts have been devoted to develop new anodes with higher specific capacity and excellent cycle stability for next-generation LIBs.6–10 Among them, tin-based anode materials have been regarded as potential candidates with high theoretical capacity (Sn: 994 mA h g−1; SnO: 875 mA h g−1; SnO2: 783 mA h g−1), natural abundance and environmental benignity.11,12 Unfortunately, Sn-based anodes inevitably suffer from pulverization and aggregation problems caused by the large volume variation (>300%) during lithiation–delithiation processes, which would lead to the loss of interparticle contact and consequently rapid capacity fading during cycling processes.13,14Substantial significant research studies have been focused on overcoming these problems. One effective strategy is to design various nanostructures to alleviate the mechanical strain and shorten the electron and ion diffusion lengths. A variety of novel nanostructures including nanowires,15,16 nanosheets,17,18 nanoboxes,19 nanotubes20,21 and hollow nanospheres22–24 have been explored. Particularly, hierarchically porous nanostructures such as hollow nanospheres have drawn extensive interest due to their peculiar hollow interior, which can provide large contact area and short diffusion path as well as sustain the huge volume change, thereby improving the cycling stability. For example, Gurunathan et al.25 have synthesized hierarchically porous SnO2 hollow microspheres (HMS) by employing the resorcinol–formaldehyde (RF) gel method. An initial discharge capacity of 800 mA h g−1 and 79% capacity retention could be delivered at 1C rate after 100 cycles when using Na-alginate as binder. Another available method is to introduce a carbon layer, which can not only buffer the volume expansion but also improve the electrical conductivity. For instance, Li and co-workers26 have designed SnO2 nanospheres with oxygen vacancies encapsulated in the N-doped graphene hierarchical network by an electrostatic adsorption-induced self-assembled together with a thermal reduction process. The as-prepared sample exhibits excellent lithium storage properties with a high reversible capacity of 912 mA h g−1 at the end of 200 cycles at 0.5 A g−1 and 652 mA h g−1 at the end of 200 cycles at 1 A g−1. However, the synthesis of hollow structures is generally complicated and difficult to realize the structure design and carbon coating in a one-step process. Therefore, it is highly desirable to develop more efficient methods to fabricate materials integrating the advantages of both structure and carbon coating.
Although LIBs have dominated the portable electronics market in the past two decades, the high cost and limited lithium reserves potentially hinder their large-scale application from a long-time prospective.27,28 In this context, sodium ion batteries (SIBs) have emerged as promising alternatives owing to the advantage of natural abundance, even distribution, and lower cost of Na resources.29,30 Despite the similarity between Li-ion and Na-ion intercalation chemistry, graphite, which is commercially applied as an anode material for LIBs, is not a suitable option for SIBs due to sodium's unique characteristics.31,32 According to some previous reports, Sn-based materials are considered to be prospective SIB anodes.33,34 Zhang and co-workers35 have prepared SnO nanosheets with controlled number of atomic layers and lateral size. A high reversible capacity of 665 mA h g−1 after 100 cycles at 0.1 A g−1 and 452 mA h g−1 after 1000 cycles at a high current density of 1.0 A g−1 was delivered, with superior rate capability. Qin et al.36 obtained tetrahedral SnO microflowers by means of an ultrafast ionic liquid-assisted microwave method. The as-prepared SnO anode exhibits high average coulombic efficiency (CE) (98.5% for 50 cycles at a specific current of 100 mA g−1) together with an unprecedented capacity contribution in the low potential region (5 mV to 0.5 V vs. Na/Na+).
In this work, integrating the two typical strategies mentioned above, we successfully designed a hierarchical structure of Sn/SnO nanosheets composed of carbon-coated hollow nanospheres by a facile glucose-assisted hydrothermal method. The as-prepared Sn/SnO sample exhibits outstanding lithium-storage properties due to the unique configuration and carbon coating layer. A high reversible capacity of 964.1 mA h g−1 after 100 cycles at 100 mA g−1 and 820.4 mA h g−1 at 1000 mA g−1 after 300 cycles could be obtained. Surprisingly, the as-prepared sample also presents certain sodium ion battery properties. It's expected that this facile synthetic strategy could open a new sight into fabricating high-performance anode materials combining the advantages of both structure and carbon coating.
2. Experimental section
2.1. Synthesis of hierarchical Sn/SnO nanosheets
All chemicals were analytically pure and used directly without further purification. The hierarchical Sn/SnO nanosheets were prepared via a simple glucose-assisted hydrothermal treatment of commercial Sn nanoparticles (Nanjing Emperor Nano Material Co., Ltd, Nanjing, China). In a typical procedure, 2 g Sn nanoparticles and 6 g glucose (Sinopharm Chemical Reagent Co. Ltd, Shanghai, China) were mixed in 60 ml deionized water under vigorous stirring. The resultant hybrid was transferred to an oven and then maintained at 180 °C for 4 h. After cooled down to room temperature, the product was obtained by centrifugation, washed several times with water and ethanol, and then dried at 80 °C in a vacuum oven for 12 h.2.2. Material characterization
The crystalline phase was identified by powder X-ray diffraction (XRD, Philips X'pert Pro Super X-ray diffractometer, Cu Kα radiation). The semi-quantitative XRD analysis is done by pattern simulation using Jade software. The morphology was investigated by field emission scanning electron microscopy (SEM, HITACHI S-4800) and transmission electron microscopy (TEM, JEM 2100). Nitrogen adsorption/desorption measurement was conducted on a Micromeritics Tristar 3000 system and the specific surface area was evaluated using the Brunauer–Emmett–Teller (BET) method. X-ray photoelectron spectroscopy (XPS) experiments were performed on a PHI QUANTUM 2000 instrument to determine the chemical composition of the samples.2.3. Electrochemical measurements
The electrodes were prepared by spread the slurry of 75 wt% Sn/SnO active material, 15 wt% acetylene black and 10 wt% LA 132 (an acrylonitrile copolymer-based binder, Chengdu Indigo Power Source Co. Ltd.) onto copper foil current collectors, followed by drying at 80 °C overnight in a vacuum oven. The mass loading of the electrode is about 2.0 mg cm−2. CR2025-type coin cells were employed to test the electrochemical properties of the electrodes. For lithium half-cells, lithium foil was served as the counter electrode and a polypropylene (PP) film (Celgard 2400) as the separator. The electrolyte consists of 1 M LiPF6 and a mixture of ethylene carbonate (EC)/dimethyl carbonate (DMC)/diethyl carbonate (DEC) (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 2 wt% vinylene carbonate (VC) as the additive (Tinci Materials Technology Co., Ltd, Guangzhou, China). The sodium half-cells were assembled in a similar way. Metallic sodium and glass fiber (GF/A, Whatman) were utilized as the counter electrode and the separator, respectively. The electrolyte is composed of 1 M NaPF6 and a mixture of EC/DEC (volume ratio 1
1) with 2 wt% vinylene carbonate (VC) as the additive (Tinci Materials Technology Co., Ltd, Guangzhou, China). The sodium half-cells were assembled in a similar way. Metallic sodium and glass fiber (GF/A, Whatman) were utilized as the counter electrode and the separator, respectively. The electrolyte is composed of 1 M NaPF6 and a mixture of EC/DEC (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fosai New Materials Co., Ltd, Jiangsu, China). Galvanostatic charge–discharge tests were performed on a LAND-2001A battery test system (Land Electronic Co., Ltd, Wuhan, China). Cyclic voltammetry (CV) measurement was conducted on a CHI 660D electrochemical workstation (CH Instruments Co., Ltd, Shanghai, China).
1) (Fosai New Materials Co., Ltd, Jiangsu, China). Galvanostatic charge–discharge tests were performed on a LAND-2001A battery test system (Land Electronic Co., Ltd, Wuhan, China). Cyclic voltammetry (CV) measurement was conducted on a CHI 660D electrochemical workstation (CH Instruments Co., Ltd, Shanghai, China).
3. Results and discussion
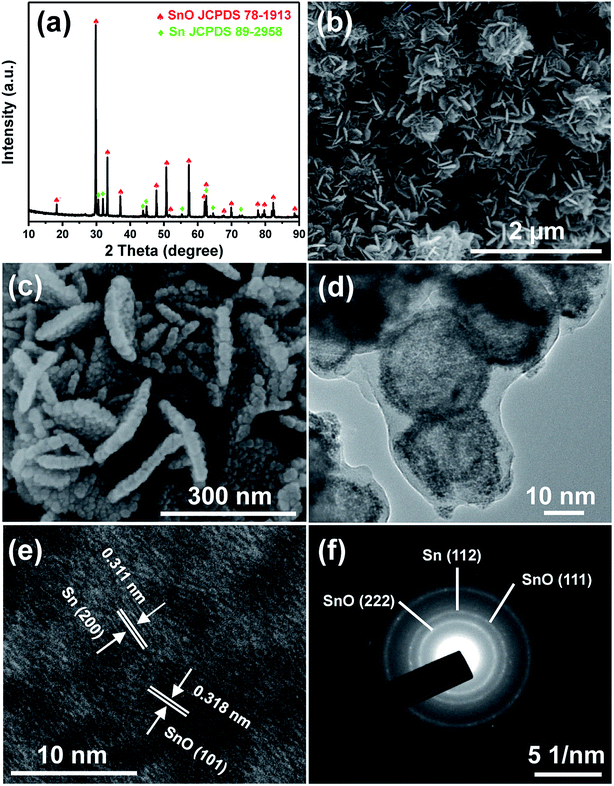
The crystalline structure and phase composition of the as-prepared sample were characterized by XRD pattern. As shown in Fig. 1a, the diffraction peaks can be indexed to tetragonal SnO with a space group of (129) P4/nmm (JCPDS card no. 78-1913) and tetragonal Sn with the space group (141) I41/amd (JCPDS card no. 89-2958), indicating the presence of metallic Sn and SnO in the composite, while no peaks of other impurities were detected. In addition, the strong intensity and sharpness of the diffraction peaks manifest good crystallinity of the sample. By means of semi-quantitative analysis of the XRD pattern, the mass ratio of SnO and Sn is determined to be about 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, indicating the dominance of SnO in the composite. The morphology and structure of the as-prepared Sn/SnO sample was investigated by SEM and TEM images. As depicted in Fig. 1b, the Sn/SnO composite consists of densely stacked nanosheets with a diameter of about 200 to 300 nm. A higher magnification image of the nanosheets (Fig. 1c) clearly presents a hierarchical structure composed of intimately interconnected nanospheres. TEM images display more details about the nanospheres. As can be seen from Fig. 1d, the Sn/SnO nanospheres have a hollow structure, with a diameter of about 30 nm. Additionally, a carbon shell with about 2 nm thickness can also be observed coating on the surface of nanospheres. In the representative HRTEM image (Fig. 1e), distinct crystal lattice with interplanar spacing of 0.311 nm and 0.318 nm are detected, corresponding to the (200) plane of Sn and (101) plane of SnO, respectively, further demonstrating the coexist of metallic Sn and SnO in the composite. The selected area electron diffraction (SAED) pattern (Fig. 1f) indicates the polycrystalline nature of the Sn/SnO sample. The several lattice fringes can be assigned to the (222) and (111) plane of SnO, and the (112) plane of Sn.
11, indicating the dominance of SnO in the composite. The morphology and structure of the as-prepared Sn/SnO sample was investigated by SEM and TEM images. As depicted in Fig. 1b, the Sn/SnO composite consists of densely stacked nanosheets with a diameter of about 200 to 300 nm. A higher magnification image of the nanosheets (Fig. 1c) clearly presents a hierarchical structure composed of intimately interconnected nanospheres. TEM images display more details about the nanospheres. As can be seen from Fig. 1d, the Sn/SnO nanospheres have a hollow structure, with a diameter of about 30 nm. Additionally, a carbon shell with about 2 nm thickness can also be observed coating on the surface of nanospheres. In the representative HRTEM image (Fig. 1e), distinct crystal lattice with interplanar spacing of 0.311 nm and 0.318 nm are detected, corresponding to the (200) plane of Sn and (101) plane of SnO, respectively, further demonstrating the coexist of metallic Sn and SnO in the composite. The selected area electron diffraction (SAED) pattern (Fig. 1f) indicates the polycrystalline nature of the Sn/SnO sample. The several lattice fringes can be assigned to the (222) and (111) plane of SnO, and the (112) plane of Sn.
 | ||
| Fig. 1 (a) XRD pattern, (b and c) SEM images, (d) TEM image, (e) HRTEM image and (f) SAED pattern of the Sn/SnO sample. | ||
N2 adsorption–desorption measurement was employed to estimate the specific surface area and pore characteristics of the as-prepared Sn/SnO sample. As revealed in Fig. 2, the N2 adsorption–desorption isotherm of the Sn/SnO nanosheets appears as a type-IV curve with an evident type H3 hysteresis loop, suggesting the formation of mesoporous structure. On the basis of the Brunauer–Emmett–Teller (BET) method, the as-prepared Sn/SnO sample delivers a specific surface area of 9.25 m2 g−1. The mean pore size and cumulative pore volume of the sample are about 7.18 nm and 0.035 nm, respectively, as calculated from the pore size distribution curve (the inset of Fig. 2) by Barrett–Joyner–Halendar (BJH) method. Such porous structure with internal void can not only provide large contact area for electrode and electrolyte but also accommodate the volume change of Sn/SnO nanosheet and facilitate the electrons and ions diffusion.24,37
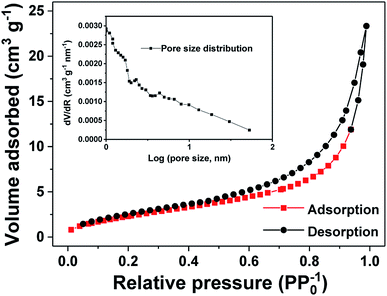 | ||
| Fig. 2 N2 adsorption–desorption isotherms and corresponding pore size distribution curve (the inset) of the Sn/SnO sample. | ||
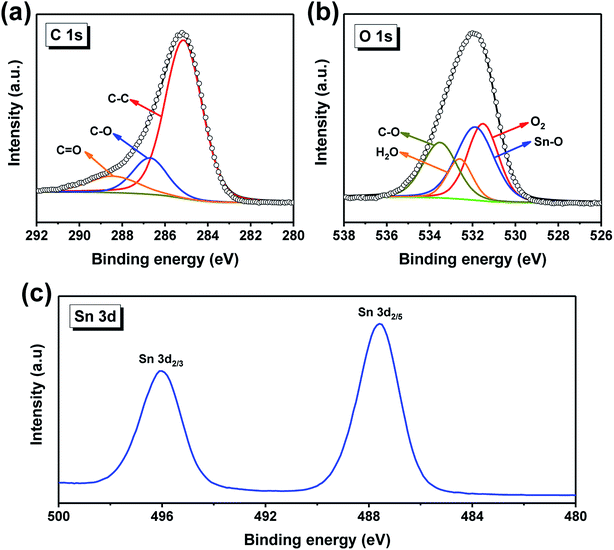
XPS spectra were applied to study the chemical state on the surface of the as-prepared sample. The high-resolution spectrum of C 1s is depicted in Fig. 3a, which can be fitted into three peaks centered at 285.1, 286.7 and 288.4 eV, corresponding to C–C, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups,38 respectively. Fig. 3b displays the typical high-resolution spectrum of O 1s, there are four peaks located at 531.5, 531.9, 532.6 and 533.5 eV, which are assigned to O2, Sn–O, H2O and C–O, separately.39 The Sn 3d spectrum (Fig. 3c) shows two peaks appearing at 487.6 eV (Sn 3d5/2) and 496.0 eV (Sn 3d3/2), which means that Sn exists principally in the form of tin oxide.40,41
O groups,38 respectively. Fig. 3b displays the typical high-resolution spectrum of O 1s, there are four peaks located at 531.5, 531.9, 532.6 and 533.5 eV, which are assigned to O2, Sn–O, H2O and C–O, separately.39 The Sn 3d spectrum (Fig. 3c) shows two peaks appearing at 487.6 eV (Sn 3d5/2) and 496.0 eV (Sn 3d3/2), which means that Sn exists principally in the form of tin oxide.40,41
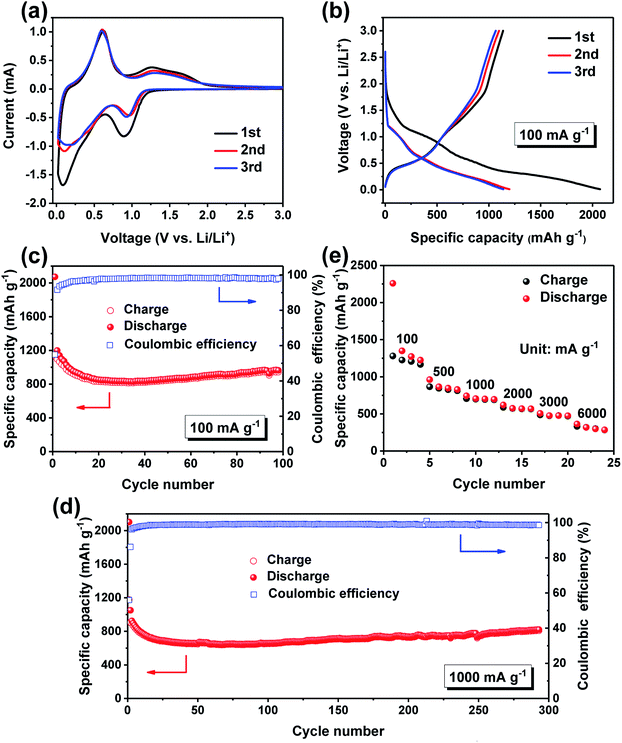
The electrochemical performances of the Sn/SnO sample as anode material for LIBs are investigated. Fig. 4a shows the CV curves of the Sn/SnO sample in the potential window of 0–3.0 V (vs. Li/Li+) at a scanning rate of 0.1 mV s−1 for the first three cycles. An irreversible reduction peak at 0.9 V can be observed during the first cathodic sweep, which is associated with the reduction of SnO to metallic Sn and the formation of a solid electrolyte interface (SEI) layer on the electrode surface.42,43 The pronounced cathodic peak at the voltage close to 0.0 V can be ascribed to the alloying reaction of metallic Sn with lithium to form LixSn. In the following anodic scan, a strong peak at 0.6 V and a broad peak at 1.27 V can be detected, corresponding to the dealloying reaction of LixSn and partial reversible formation of SnO, respectively, consistent with the Sn-based anodes reported previously.44,45 Besides, the CV curves almost overlap in the subsequent cycles, indicating the formation of stable SEI film and good reversibility of the electrochemical reactions in the electrode.
Fig. 4b shows the galvanostatic charge–discharge profiles of the Sn/SnO nanosheets electrode for the first three cycles at a current density of 100 mA g−1 in a voltage window of 0.01–3 V (vs. Li+/Li). The initial charge and discharge capacities are 1137.2 mA h g−1 and 2072.2 mA h g−1, corresponding to an initial coulombic efficiency (ICE) of 54.9%. Note that the specific capacity was calculated on the basis of the total mass of the Sn/SnO composite, including the negligible capacity supported by carbon. The large initial irreversible capacity loss (45.1%) could be attributed to the formation of SEI layer on the electrode surface and the decomposition of the electrolyte.11,26 After the first cycle, the CE climbs to 91.6% and 93.6% in the 2nd and 3rd cycles, respectively, indicating the formation of a stable SEI film.
The cycling performance of the as-prepared Sn/SnO sample at a current density of 100 and 1000 mA g−1 are presented in Fig. 4c and d, respectively. As can be seen, the Sn/SnO electrode delivers a reversible discharge capacity as high as 964.1 mA h g−1 after 100 cycles at 100 mA g−1 (Fig. 4c). Furthermore, after the first few cycles, the CE quickly increases and stabilizes at around 98% for the subsequent cycles, demonstrating the superior reversibility and cycling stability. When the current density increases to 1000 mA g−1, a discharge capacity of 820.4 mA h g−1 can still be obtained after 300 cycles, which is around 89% retention of the discharge capacity in the second cycle, revealing the excellent stability of the Sn/SnO electrode (Fig. 4d). Interestingly, the capacity fluctuation phenomenon can be obviously observed during the cycling test, which has already been reported for many metal oxide anodes.11,46–48 This phenomenon may be mainly ascribed to the reversible formation/decomposition of an organic polymeric gel-like film on the electrode surface derived from the electrolyte decomposition, which could provide excess lithium ions storage sites. Fig. 4e depicts the rate capability of the Sn/SnO nanosheets at different current densities ranging from 100 to 6000 mA g−1. It can be seen that the Sn/SnO nanosheets electrode exhibits outstanding rate capability with reversible discharge capacity of 1349.2, 961.8, 743.9, 620.0, 507.1 and 363.7 mA h g−1 at a current density of 100, 500, 1000, 2000, 3000 and 6000 mA g−1, respectively.
The morphology changes of the electrode before cycling and after 100 cycles at 100 mA g−1 are presented in Fig. 5. It can be seen that the active material still remains homogenously distributed on the current collector with only some cracks appeared (Fig. 5a and b). Although the hollow spheres are hard to be observed, the Sn/SnO sample still preserves the sheet-like structure after long-time cycling (Fig. 5c).
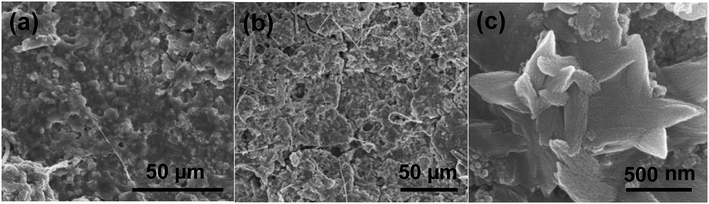 | ||
| Fig. 5 SEM images of the Sn/SnO electrode (a) before cycling and (b and c) after 100 cycles at a current density of 100 mA g−1. | ||
The superior electrochemical performances of the Sn/SnO nanosheets can be attributed to the following factors. First, the peculiar hierarchical structure of nanosheets composed of hollow nanospheres can provide more lithium storage sites and shorter lithium electrons and ions transport length. Second, the hierarchical structure could help relieve the volume change. Third, the carbon layer leads to enhanced electrical conductivity and better structure stability.
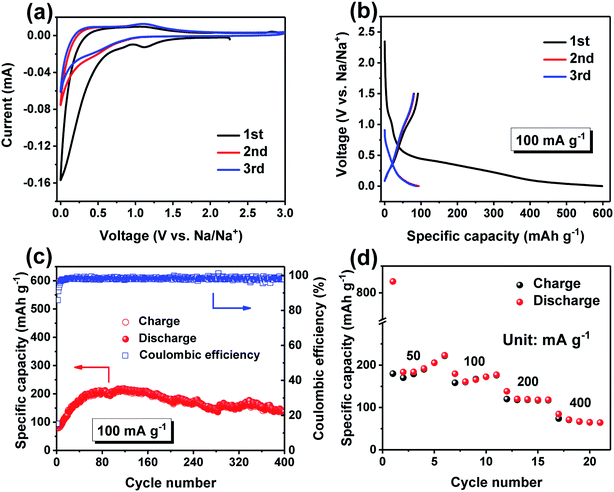
The sodium storage properties of Sn/SnO anode were also investigated. Fig. 6a shows the CV curves of the Sn/SnO electrode for the first three cycles at a scan rate of 0.1 mV s−1 between 0.0 and 3.0 V. During the first cathodic scan, an irreversible peak at 1.1 V appeared, corresponding to the insertion of Na+ into SnO and the formation of SEI film. The weak oxidation peaks in the charge process can be assigned to the reversible dealloying of NaxSn.49–51 From the second cycle, all the redox peaks have few changes, implying the high electrochemical reversibility of the Sn/SnO electrode. The representative charge–discharge curves for the initial three cycles of the Sn/SnO sample at 100 mA g−1 are depicted in Fig. 6b. The initial charge and discharge capacity are 246.9 and 831.1 mA h g−1, respectively, corresponding to an ICE of 29.7%. The irreversible capacity loss could also be ascribed to the irreversible electrolyte decomposition and formation of SEI on the surface of electrode.35,36 In the subsequent second and third cycles, the CE quickly increases to 97.5% and 92.2%, respectively. The corresponding cycling performance is presented in Fig. 6c. As can be seen, a reversible discharge capacity of 137.0 mA h g−1 could be delivered after 400 cycles. Specifically, after an evident increase for about 120 cycles, the specific capacity decreases slowly during the following cycles, indicating the material underwent an activated process. Such capacity increase has also been reported by Lu et al.52 Fig. 6d illustrates the rate performances of the Sn/SnO electrode at different current densities from 50 to 400 mA g−1. As can be seen, the electrode delivers a discharge capacity of 223.1, 179.6, 138.2 and 84.4 mA h g−1 at 50, 100, 200 and 400 mA g−1, respectively. These results demonstrate the inspiring electrochemical performances of the Sn/SnO sample in SIBs.
4. Conclusions
In summary, we have successfully developed a facile hydrothermal method to synthesize hierarchical Sn/SnO nanosheets assembled by carbon-coated hollow nanospheres. When evaluated as an anode material for LIBs, the Sn/SnO sample exhibits excellent electrochemical properties. Specifically, a high reversible capacity of 2072.2 mA h g−1 was observed at 100 mA g−1 with 964.1 mA h g−1 retained after 100 cycles. Moreover, 820.4 mA h g−1 can be maintained during a long-term cycling at 1000 mA g−1 after 300 cycles along with a remarkable rate capability of 363.7 mA h g−1 at 6000 mA g−1. Furthermore, the Sn/SnO sample also presents attractive sodium ion storage properties. Such superior electrochemical properties could be attributed to the unique architecture. On one hand, the peculiar hierarchical structure can not only provide more lithium storage sites, shorter lithium electrons and ions transport length but also help alleviate the volume variation as well as facilitate the electrolyte penetration. On the other hand, the carbon layer leads to enhanced electrical conductivity and better structure stability.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21878195, 21805198, 21805018), Distinguished Young Scholars of Sichuan University (2017SCU04A08) and Huohua Ku Project of Sichuan University (2018SCUH0094). Research Foundation for the Postdoctoral Program of Sichuan University (No. 2017SCU12018 and 2018SCU12045). Research Foundation for the Sichuan University and Zigong City Joint Research Project (2018CDZG-16). Thanks Dr Zhuo Zheng and for the help of data analysis.References
- N. Nitta, F. X. Wu, J. T. Lee and G. Yushin, Mater. Today, 2015, 18, 252–264 CrossRef CAS.
- J. B. Goodenough and K. S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- B. Scrosati, J. Hassoun and Y. K. Sun, Energy Environ. Sci., 2011, 4, 3287–3295 RSC.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- M. Winter, J. O. Besenhard, M. E. Spahr and P. Novák, Adv. Mater., 2010, 10, 725–763 CrossRef.
- L. L. Wang, Q. F. Zhang, J. Y. Zhu, X. D. Duan, Z. Xu, Y. T. Liu, H. G. Yang and B. A. Lu, Energy Storage Mater., 2019, 16, 37–45 CrossRef.
- T. Chen, Z. G. Wu, W. Xiang, E. H. Wang, T. R. Chen, X. D. Guo, Y. X. Chen and B. H. Zhong, Electrochim. Acta, 2017, 246, 931–940 CrossRef CAS.
- L. Yu, H. B. Wu and X. W. Lou, Adv. Mater., 2013, 25, 2296–2300 CrossRef CAS PubMed.
- Z. G. Wu, Y. J. Zhong, J. Liu, J. H. Wu, X. D. Guo, B. H. Zhong and Z. Zhang, J. Mater. Chem. A, 2015, 3, 10092–10099 RSC.
- Z. S. Wu, W. C. Ren, L. Wen, L. B. Gao, J. P. Zhao, Z. P. Chen, G. M. Zhou, F. Li and H. M. Cheng, ACS Nano, 2010, 4, 3187–3194 CrossRef CAS PubMed.
- C. Zhu, D. Wei, Y. Wu, Z. Zhang, G. Zhang, J. Duan, L. Li, H. Zhu, Z. Zhu and Z. Chen, J. Alloys Compd., 2019, 778, 731–740 CrossRef CAS.
- M. Sahoo and S. Ramaprabhu, Carbon, 2018, 127, 627–635 CrossRef CAS.
- Y. Zhao, X. F. Li, B. Yan, D. B. Xiong, D. J. Li, S. Lawes and X. L. Sun, Adv. Energy Mater., 2016, 6, 19 Search PubMed.
- D. Saikia, J. R. Deka, C. J. Chou, H. M. Kao and Y. C. Yang, J. Alloys Compd., 2019, 791, 892–904 CrossRef CAS.
- C. Guan, X. Wang, Q. Zhang, Z. Fan, H. Zhang and H. J. Fan, Nano Lett., 2014, 14, 4852–4858 CrossRef CAS PubMed.
- W. Ren, C. Wang, L. Lu, D. Li, C. Cheng and J. Liu, J. Mater. Chem. A, 2013, 1, 13433–13438 RSC.
- Y. Q. Zhu, H. Z. Guo, H. Z. Zhai and C. B. Cao, ACS Appl. Mater. Interfaces, 2015, 7, 2745–2753 CrossRef CAS PubMed.
- W. Su, Y. Liang and Y. Tang, J. Alloys Compd., 2019, 801, 402–408 CrossRef CAS.
- Z. Wang, D. Luan, F. Y. Boey and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 4738 CrossRef CAS PubMed.
- C. H. Han, B. X. Zhang, K. N. Zhao, J. S. Meng, Q. He, P. He, W. Yang, Q. Li and L. Q. Mai, Chem. Commun., 2017, 53, 9542–9545 RSC.
- Y. Wang, H. C. Zeng and J. Y. Lee, Adv. Mater., 2006, 18, 645–649 CrossRef CAS.
- H. Woo, S. Wi, J. Kim, J. Kim, S. Lee, T. Hwang, J. Kang, J. Kim, K. Park and B. Gil, Carbon, 2018, 129, 342–348 CrossRef CAS.
- W. S. Kim, Y. Hwa, J. H. Jeun, H. J. Sohn and S. H. Hong, J. Power Sources, 2013, 225, 108–112 CrossRef CAS.
- B. K. Cao, Z. Q. Liu, C. Y. Xu, J. T. Huang, H. T. Fang and Y. Chen, J. Power Sources, 2019, 414, 233–241 CrossRef CAS.
- P. Gurunathan, P. M. Ette and K. Ramesha, ACS Appl. Mater. Interfaces, 2014, 6, 16556–16564 CrossRef CAS PubMed.
- N. T. Wu, W. Z. Du, X. Gao, L. Zhao, G. L. Liu, X. M. Liu, H. Wu and Y. B. He, Nanoscale, 2018, 10, 11460–11466 RSC.
- S. Y. Hong, Y. Kim, Y. Park, A. Choi, N.-S. Choi and K. T. Lee, Energy Environ. Sci., 2013, 6, 2067–2081 RSC.
- P. K. Nayak, L. Yang, W. Brehm and P. Adelhelm, Angew. Chem., Int. Ed., 2018, 57, 102–120 CrossRef CAS PubMed.
- J. Y. Hwang, S. T. Myung and Y. K. Sun, Chem. Soc. Rev., 2017, 46, 3529–3614 RSC.
- N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Chem. Rev., 2014, 114, 11636–11682 CrossRef CAS PubMed.
- S. W. Kim, D. H. Seo, X. H. Ma, G. Ceder and K. Kang, Adv. Energy Mater., 2012, 2, 710–721 CrossRef CAS.
- V. L. Chevrier and G. Ceder, J. Electrochem. Soc., 2011, 158, A1011–A1014 CrossRef CAS.
- Z. Li, J. Feng, H. Hu, Y. Dong, H. Ren, W. Wu, Z. Hu and M. Wu, J. Mater. Chem. A, 2018, 6, 18920–18927 RSC.
- Z. Li, J. Ding and D. Mitlin, Acc. Chem. Res., 2015, 48, 1657–1665 CrossRef CAS PubMed.
- F. Zhang, J. Zhu, D. Zhang, U. Schwingenschlögl and H. N. Alshareef, Nano Lett., 2017, 17, 1302–1311 CrossRef CAS PubMed.
- B. Qin, H. Zhang, T. Diemant, D. Geiger, R. Raccichini, R. J. Behm, U. Kaiser, A. Varzi and S. Passerini, ACS Appl. Mater. Interfaces, 2017, 9, 26797–26804 CrossRef CAS PubMed.
- P. Y. Chang and R. A. Doong, J. Alloys Compd., 2019, 775, 214–224 CrossRef CAS.
- J. Guo, P. Li, L. Chai, Y. Su, J. Diao and X. Guo, RSC Adv., 2017, 7, 30070–30079 RSC.
- Y. Lee, M. R. Jo, K. Song, K. M. Nam, J. T. Park and Y.-M. Kang, ACS Appl. Mater. Interfaces, 2012, 4, 3459–3464 CrossRef CAS PubMed.
- F. H. Du, Y. S. Liu, J. Long, Q. C. Zhu, K. X. Wang, X. Wei and J. S. Chen, Chem. Commun., 2014, 50, 9961–9964 RSC.
- J. F. Wang and D. N. He, Dalton Trans., 2018, 47, 15307–15311 RSC.
- J. Liang, X. Y. Yu, H. Zhou, H. B. Wu, S. Ding and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 12803–12807 CrossRef CAS PubMed.
- Y. Cheng, Q. Li, C. Wang, L. Sun, Z. Yi and L. Wang, Small, 2017, 13, 1701993 CrossRef PubMed.
- Z. G. Wu, J. T. Li, Y. J. Zhong, J. Liu, X. D. Guo, L. Huang, B. H. Zhong and S. G. Sun, J. Alloys Compd., 2015, 620, 407–412 CrossRef CAS.
- J. F. Ye, H. J. Zhang, R. Yang, X. G. Li and L. M. Qi, Small, 2010, 6, 296–306 CrossRef CAS PubMed.
- J. Liu, J. Xiao, X. Zeng, P. Dong, J. Zhao, Y. Zhang and X. Li, J. Alloys Compd., 2017, 699, 401–407 CrossRef CAS.
- G. Gao, S. Lu, B. Dong, Y. Xiang, K. Xi and S. Ding, J. Mater. Chem. A, 2016, 4, 6264–6270 RSC.
- C. J. Wu, Z. G. Wu, X. B. Zhang, R. Rajagopalan, B. H. Zhong, W. Xiang, M. Z. Chen, H. T. Li, T. R. Chen, E. H. Wang, Z. G. Yang and X. D. Guo, ACS Appl. Mater. Interfaces, 2017, 9, 43596–43602 CrossRef CAS PubMed.
- X. Zhao, Z. Zhang, F. Yang, Y. Fu, Y. Lai and J. Li, RSC Adv., 2015, 5, 31465–31471 RSC.
- Y. Wang, D. Su, C. Wang and G. Wang, Electrochem. Commun., 2013, 29, 8–11 CrossRef CAS.
- Y. X. Wang, Y. G. Lim, M. S. Park, S. L. Chou, J. H. Kim, H. K. Liu, S. X. Dou and Y. J. Kim, J. Mater. Chem. A, 2014, 2, 529–534 RSC.
- Y. C. Lu, C. Ma, J. Alvarado, T. Kidera, N. Dimov, Y. S. Meng and S. Okada, J. Power Sources, 2015, 284, 287–295 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |