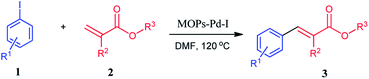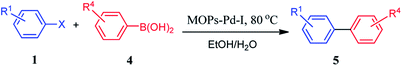Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Palladium catalyst immobilized on functionalized microporous organic polymers for C–C coupling reactions†
Wei Xu‡
,
Cijie Liu‡,
Dexuan Xiang *,
Qionglin Luo,
You Shu,
Hongwei Lin,
Yangjian Hu,
Zaixing Zhang and
Yuejun Ouyang*
*,
Qionglin Luo,
You Shu,
Hongwei Lin,
Yangjian Hu,
Zaixing Zhang and
Yuejun Ouyang*
Hunan Engineering Laboratory for Preparation Technology of Polyvinyl Alcohol (PVA) Fiber Material, Institute of Organic Synthesis, Huaihua University, Huaihua 418000, China. E-mail: dexuanxiang@126.com; oyyj0816@163.com
First published on 28th October 2019
Abstract
Two microporous organic polymer immobilized palladium (MOP-Pd) catalysts were prepared from benzene and 1,10-phenanthroline by Scholl coupling reaction and Friedel–Crafts reaction, respectively. The structure and composition of the catalyst were characterized by FT-IR, TGA, N2 sorption, SEM, TEM, ICP-AES and XPS. MOP-Pd catalysts were found to possess high specific surface areas, large pore volume and low skeletal bone density. Moreover, the immobilized catalyst also had advantages, such as readily available raw materials, chemical and thermal stability, and low synthetic cost. The Pd catalyst is an effective heterogeneous catalyst for carbon–carbon (C–C) coupling reactions, such as the Heck reaction and Suzuki–Miyaura reaction, affording good to high yields. In these reactions, the catalyst was easily recovered and reused five times without significant activity loss.
Carbon–carbon (C–C) coupling reactions have become one of the most versatile and utilized reactions for the selective construction of C–C bonds for the formation of functionalised aromatics,1 natural products,2 pharmaceuticals,3 polymers4 and advanced materials.5 Many transition metals have been used as catalysts in these reactions, aided by a great variety of ligands ranging from simple, commercial phosphines to complex custom-made molecules.6 Among these transition metals, palladium plays a significant role in various cross-coupling reactions, such as Suzuki,7 Heck,8 Sonogashira,9 Stille,10 and Ullmann coupling reactions,11 due to their strong electrical and chemical properties.12 Over the past decades, various homogeneous catalytic systems have been developed for organic transformations,13 which often progress smoothly under the inert atmosphere in organic solvents, for example, toluene or tetrahydrofuran in the presence of soluble palladium complexes as catalysts. However, most homogeneous palladium catalysts suffer from drawbacks such as high-cost of phosphine ligands, use of various additives, difficult separation, metal leaching, recovery, recyclability, and the toxicity of phosphine ligands.
Heterogeneous catalysis have attracted increasing attention as they have been proven to be useful for different organic reactions owning to their unique properties, such as high reactivity, stability, easy separation, purification and recyclability.14 Many active heterogeneous palladium catalysts have been developed and widely applied in the C–C coupling reactions.15 Palladium has been immobilized on various solid supporting materials, such as zeolite,16 silica,17 metal organic frameworks,18 and functionalized graphene oxide.19 However, a substantial decrease in activity and selectivity of the heterogeneous palladium catalysts is frequently observed because of their long diffusion pathway to catalytic sites and the difference of electron density on active sites. To address these problems, materials with larger interface and more active site are employed to support palladium as heterogeneous catalysts, such as palladium immobilized on hyper-crosslinked polymers were high activity in Suzuki–Miyaura coupling reaction.20
Microporous organic polymers (MOPs) consists of purely organic elements have recently emerged as versatile platforms for heterogeneous catalysts thanks to their unique properties, including superior chemical, thermal and hydrothermal stability, synthetic diversity, low skeletal density and high surface area.20,21 More importantly, the bottom–up approach of MOPs provides an opportunity for the design of polymer frameworks with a range of functionalities into the porous structure to use as catalysts or ligands.22 Recently, Kaskel reported the incorporation of a thermally fragile imidazolium moiety into MOPs resulted in a heterogeneous organocatalyst active in carbene-catalyzed Umpolung reaction.23 Wang designed photocatalysts with microporous via the copolymerization from pyrene and dibenzothiophene-S,S-dioxide building blocks and tested the effect of the photocatalytic hydrogen evolution.24 Xu described the synthesis of microporous with N-heterocyclic carbenes by an external cross-linking reaction and applied it in Suzuki reaction.25 Zhou demonstrated for the first time that the microporous structure has a positive effect on controlling selectivities in the hydrosilylation of alkynes.26 Recently, we also reported three pyridine-functionalized N-heterocyclic carbene–palladium complexes and its application in Suzuki–Miyaura coupling reactions.27
1,10-phenanthroline is an ideal candidate of ligands due to its structural features such as two N-atom placed in juxta position to provide binding sites for metal cations.28 To utilize the unique structure feature, we employed it in the construction of MOPs via Scholl and Friedel–Crafts reaction, respectively. Therefore, this paper presents our recent studies on the synthesis of two heterogeneous palladium catalysts supported on MOPs through a simple and low-cost procedure. These catalysts displayed remarkable catalytic activity in C–C coupling reactions, including Suzuki–Miyaura reaction and Heck coupling reaction. The properties of simple preparation, wide application of this catalyst and good performance in C–C coupling reactions and adaptability with various substrates make it perfect catalytic option for C–C coupling reactions.
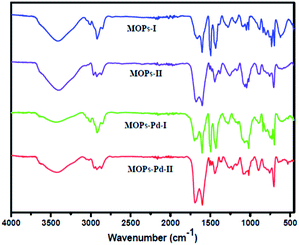
The microporous network with 1,10-phenanthroline functional groups and incorporation of Pd metal were confirmed by Fourier transform infrared (FT-IR) spectroscopy. The FT-IR spectra of MOPs and MOPs-Pd (Fig. 1) displayed a series of bands around 2800–3100 cm−1, which were assigned to the C–H stretching band and in-of-plane bending vibrations of the aryl rings. The bands around 1550–1750 cm−1 were attributed to the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N- stretching band. The bands around 1400–1450 and 850–700 cm−1 were corresponded to the benzene and 1,10-phenanthroline skeletal stretching and the C–H out-of-plane bending vibrations of the aryl rings, respectively. The bond around 1495 cm−1 in MOPs-I and MOPs-Pd-I is assigned to in-of-plane bending vibrations of CH2, which indicated that 1,10-phenanthroline and benzene were linked by CH2.
N- stretching band. The bands around 1400–1450 and 850–700 cm−1 were corresponded to the benzene and 1,10-phenanthroline skeletal stretching and the C–H out-of-plane bending vibrations of the aryl rings, respectively. The bond around 1495 cm−1 in MOPs-I and MOPs-Pd-I is assigned to in-of-plane bending vibrations of CH2, which indicated that 1,10-phenanthroline and benzene were linked by CH2.
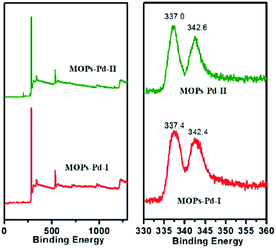
The X-ray photoelectron spectroscopy (XPS) analysis of the MOPs-Pd is performed to investigate the coordination states of palladium species (Fig. 2). In Fig. 2, the Pd 3d XPS spectra of the MOPs-Pd-I catalysts reveal that Pd is present in the +2 oxidation state rather than in the metallic state. This is corresponding to the binding energy (B.E.) of 337.4 eV and 342.4 eV, which are assigned to be Pd 3d5/2 and 3d3/2 of Pd (+2), respectively. Compared with the PdCl2 (337.9 eV and 343.1 eV), the Pd2+ binding energy in the MOPs-Pd-I catalyst shifts negatively by 0.5 eV and 0.7 eV. This can be attributed to the effect of the coordination with 1,10-phenanthroline in microporous networks. The results show that Pd2+ can be immobilized successfully on the MOPs by coordinating to 1,10-phenanthroline rather than by physical adsorption of Pd2+ on the surface. XPS graphs of MOPs-Pd-II also reveal that Pd2+ is immobilized on MOPs materials.
The surface area and pore structure of the MOPs and MOPs-Pd were investigated by nitrogen adsorption analyses at 77.3 K. In Fig. 3, the MOPs-Pd exhibits type I adsorption–desorption isotherms, which is similar to the isotherms exhibited by the parent MOPs polymers. The result implies that these microporous organic polymers and metalized polymers consist of both micropores and mesopores. The apparent Brunauer–Emmett–Teller surface areas (SBET) of MOPs-Pd are smaller than those of the non-metallized parent networks (Table 1), which can be attributed to both the partial pore filling with metal and simple increase in mass. However, the materials are still significantly microporous because of many micropore surface (SMicro) and micropore volume (VMicro) in these materials. The abundant micropores with a suitable size favored naturally the dispersion of the metal species. The presence of a spot of mesopore and macropore structure in the heterogeneous catalyst is also essential, because these structures enabled the frameworks to be highly soaked in a certain solvent. As a result, the accessibility of the catalytically active sites toward the substrates is maximized. The ICP-AES analysis indicates that the content of Pd in MOPs-Pd I and II are 2.5 and 2.4 wt% (Table 1), respectively.
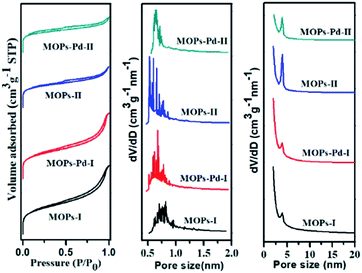 | ||
| Fig. 3 N2 adsorption–desorption isotherms and corresponding pore size distributions of MOPs and MOPs-Pd. | ||
| Sample | SBETa [m2 g−1] | SMicrob [m2 g−1] | VMicroc [m3 g−1] | [Pd]d [wt%] |
|---|---|---|---|---|
| a Surface area calculated from the nitrogen adsorption isotherm using the BET method.b The micropore volume derived using a t-plot method based on the Halsey thickness equation.c Total pore volume at P/P0 = 0.99.d Data were obtained by inductively coupled plasma mass spectrometry (ICP-AES). | ||||
| MOPs-I | 761 | 447 | 0.211 | — |
| MOPs-Pd-I | 744 | 422 | 0.199 | 2.5 |
| MOPs-II | 664 | 506 | 0.225 | — |
| MOPs-Pd-II | 623 | 502 | 0.225 | 2.4 |
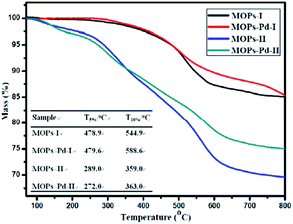
The thermal stability of the MOPs and MOPs-Pd was also assessed by TGA. The TGA traces obtained from MOPs and MOPs-Pd are shown in Fig. 4. The data analysis has been performed, and results are also shown in Fig. 4. These results show that MOPs and MOPs-Pd exhibit good thermal stability in nitrogen. It is obvious to see that the T5% and T10% of the MOPs-II and MOPs-Pd-II are lower compared with MOPs-I and MOPs-Pd-I. This is because of the large amount CH2 in MOPs-I and MOPs-Pd-I.
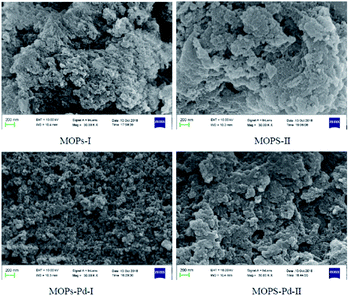
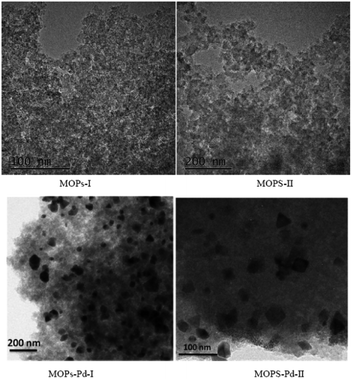
MOPs and MOPs-Pd were subjected to SEM and TEM analyses, and the results are shown in Fig. 5 and 6. We can see a large number of pores in MOPs and MOPs-Pd from the SEM imagines, and uniformly distributed Pd nanoparticles in MOPs-Pd from the TEM images. No remarkable change in terms of the morphology of the materials occurs after loading the palladium species. Then, scanning electron microscopy elemental mapping was employed to investigate the composition of MOPs-Pd. The results are shown in ESI† (Section IV). Obviously, the metal Pd in MOPs-Pd-I and II are distributed in the support with a high degree of dispersion. Meanwhile, C, N, Pd and Cl are observed from these images, implying those are the major elements to construct the MOPs-Pd catalyst.
Then, we investigated the activities of the MOPs-Pd catalysts to determine the potential relationships between the structure and catalyst activity. To check the catalytic activity of the MOPs-Pd in the Heck coupling reaction, iodobenzene 1a and ethyl acrylate 2a were taken as the model substrate in presence of MOPs-Pd catalyst for optimization of the reaction condition. First, the reaction of 1a with 2a was carried out in the present of Et3N with MOPs-Pd-I as catalysis in EtOH under reflex to afford 3a in 75% yield. Then, a series of experiments was carried out to screen the reaction conditions, including catalysis, base, solvent, and reaction temperature. The optimal results were obtained when the reaction of 1a with 2a was carried out in the present of Et3N with MOPs-Pd-I as catalysis in DMF at 120 °C for 1.5 h to afford 3a in 96% yield. Under the optimal conditions, we carried out a series of reactions of 1 with 2 aiming to determine its scope. As shown in Table 2, the MOPs-Pd-I catalyzed reaction proved to be suitable for a series of 1 and 2 bearing varied groups, affording the corresponding substituted 3a–o (entry 1–15) in very high yields.
| Entry | R1 | R2 | R3 | 3 | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (2.5 mmol), 2a (3.7 mmol), Et3N (3.7 mmol), MOPs-Pd-I (50 mg, 0.28 mol%), DMF (10 mL), 120 °C, 1.5 h.b Isolated yields. | |||||
| 1 | H | H | Et | 3a | 96 |
| 2 | 4-Me | H | Et | 3b | 98 |
| 3 | 4-MeO | H | Et | 3c | 97 |
| 4 | 4-Cl | H | Et | 3d | 95 |
| 5 | 4-NO2 | H | Et | 3e | 93 |
| 6 | 4-CN | H | Et | 3f | 93 |
| 7 | 3-Me | H | Et | 3g | 94 |
| 8 | 3,5-(Me)2 | H | Et | 3h | 97 |
| 9 | H | H | Me | 3i | 98 |
| 10 | 3-Me | H | Me | 3j | 95 |
| 11 | H | H | Bu | 3k | 97 |
| 12 | 3-Me | H | Bu | 3l | 94 |
| 13 | H | H | H | 3m | 94 |
| 14 | 4-Me | H | H | 3n | 95 |
| 15 | 4-MeO | Me | Me | 3o | 90 |
To determine the active catalyst, we did two experiments in the same condition as Table 2, entry 1. Those two reactions were quenched after the reaction carried out for 20 minutes. Then, we separated one of the reactions, where the yield of compound 3a is 61%. In another reaction, the heterogeneous catalyst was separated from the reaction mixture by filtering, and then the reaction liquid was allowed to react for another 70 minutes under the same conditions. No significant change in the yield of compound 3a, indicating that the catalyst for the reaction was not the dissolved Pd species leached from the heterogeneous catalyst.
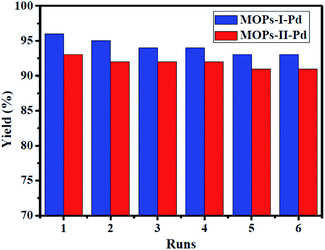
Recovery and recycling of solid heterogeneous catalysts is one of the major concerns in metal-supported catalysis, while supported metal catalyst usually undergo leaching of metal species to solution.29 Thus, we investigated the recycling performance in Heck reaction, and the results are shown in Fig. 7. The reaction was conducted in the present of Et3N with MOPs-Pd as catalysis in DMF at 120 °C for 1.5 h. Then, the catalyst was recovered by filtering, washing with water and ethyl acetate. Finally, the recovered catalyst was dried in an oven for 2.0 h. After six runs, the reused MOPs-Pd-I and II are still capable of catalyzing the reaction in 93% and 91% yield, respectively. This clearly reveals a slight decrease in catalytic activity and product yield. In addition, the morphology of recovered catalyst was analyzed by the SEM (see ESI,† Section IV), and the results show that there is no remarkable change in terms of the morphology of the materials. The contents of Pd in recovered MOPs-Pd-I and II were 2.3% and 2.1% by ICP-AES, implying a slight leaching of palladium species.
To extend the utility of MOPs-Pd in the carbon–carbon coupling reactions, we examined other organic reactions. Suzuki–Miyaura reaction is an important palladium-catalyzed cross coupling in organic synthesis. Therefore, we examined the MOPs-Pd catalysts in Suzuki–Miyaura reaction. First, the reaction of 1a with 4a was put together in the present of K3PO4 and MOPs-Pd-I in MeOH under reflex. As monitored by TLC, the reaction proceeded smoothly and the yield of 5a reached 91%. Then we investigated the optimization of the reaction conditions, including catalysis, base, solvent, and reaction temperature. A series of experiments revealed that EtOH/H2O (VEtOH/VH2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was effective for the synthesis of 5a. The yield of 5a reached 97% when the reaction of 1a with 4a was performed in the present of K3PO4 with MOPs-Pd-I as a catalyst at 80 °C for 1.0 hour. In this reaction, the MOPs-Pd-I catalyst also can be reused for 5 times with no significant decrease in activity and the Pd content of the recovered catalyst is 2.36% by ICP-AES.
1) was effective for the synthesis of 5a. The yield of 5a reached 97% when the reaction of 1a with 4a was performed in the present of K3PO4 with MOPs-Pd-I as a catalyst at 80 °C for 1.0 hour. In this reaction, the MOPs-Pd-I catalyst also can be reused for 5 times with no significant decrease in activity and the Pd content of the recovered catalyst is 2.36% by ICP-AES.
Under the optimal conditions, we carried out a series of reactions of 1 with 4 aiming to determine its scope. In Table 3, a range of halogenated benzene 1 and aryl boronic acid 4 with electron-donating group and electron-withdrawing group were applied to the conditions in parallel, affording the corresponding substituted biphenyl 5b–i in high yields. It is worth noting that the cross-coupling reactions can proceed smoothly when aryl bromide was used in the reaction (entry 12).
| Entry | R1 | X | R4 | 5 | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (2.5 mmol), 4a (3.0 mmol), K3PO4 (5.0 mmol), MOPs-Pd-I (50 mg, 0.28 mol%), EtOH/H2O (10 mL), 80 °C, 1.0 h.b Isolated yields.c The reaction time was 3.0 h. | |||||
| 1 | H | I | H | 5a | 97 |
| 2 | H | I | 2-Me | 5b | 96 |
| 3 | H | I | 3-Me | 5c | 99 |
| 4 | H | I | 4-Me | 5d | 98 |
| 5 | H | I | 2 F | 5e | 96 |
| 6 | H | I | 3 F | 5f | 95 |
| 7 | H | I | 4 F | 5g | 97 |
| 8 | H | I | 4-CN | 5h | 95 |
| 9 | 4-Me | I | H | 5d | 98 |
| 10 | 4-OMe | I | H | 5i | 99 |
| 11 | 4-CN | I | H | 5h | 94 |
| 12c | H | Br | H | 5a | 92 |
In summary, a simple and low-cost method for synthesis of palladium complexes supported on microporous organic polymers was described. The MOPs-Pd catalysts were constructed based on highly stable microporous material, and characterized by FT-IR, TGA, SEM, TEM, N2 sorption, XPS and ICP. These heterogeneous catalysts displayed outstanding catalytic activities in Heck reaction and Suzuki coupling reaction. In these reactions, the MOPs-Pd catalyst was easily recovered and reused without loss of catalytic activity. The potential utilization and application of these heterogeneous catalysts are currently under investigation in our laboratory.
Conflicts of interest
The authors declare that there are no conflicts of interests.Acknowledgements
This research was financial supported by the Natural Science Foundation of Hunan Province (no. 2018JJ3409), Scientific Research Fund of Hunan Provincial Education Department (no. 17B207), Huaihua Science and Technology Plan Project (2018G2201) and the construct program of the key discipline in Huaihua University.Notes and references
- V. J. Ram, M. Nath, P. Srivastava, S. Sarkhel and P. R. Maulik, J. Chem. Soc., Perkin Trans. 1, 2000, 3719–3723 RSC.
- S. R. Chemler, D. Trauner and S. J. Danishefsky, Angew. Chem., Int. Ed., 2001, 40, 4544–4568 CrossRef CAS.
- (a) J. Magano and J. R. Dunetz, Chem. Rev., 2011, 111, 2177–2250 CrossRef CAS; (b) C. Torborg and M. Beller, Adv. Synth. Catal., 2009, 351, 3027–3043 CrossRef CAS.
- A. Yokoyama, H. Suzuki, Y. Kubota, K. Ohuchi, H. Higashimura and T. Yokozawa, J. Am. Chem. Soc., 2007, 129, 7236–7237 CrossRef CAS.
- (a) G. Javier and H. María Antonia, Nanoscale, 2010, 2, 1390–1400 RSC; (b) S. Xu, E. H. Kim, A. Wei and E. Negishi, Sci. Technol. Adv. Mater., 2014, 15, 044201 CrossRef.
- (a) A. Dhakshinamoorthy, A. M. Asiri and H. Garcia, Chem. Soc. Rev., 2015, 44, 1922–1947 RSC; (b) A. H. Cherney, N. T. Kadunce and S. E. Reisman, Chem. Rev., 2015, 115, 9587–9652 CrossRef CAS; (c) M. Henrion, V. Ritleng and M. J. Chetcuti, ACS Catal., 2015, 5, 1283–1302 CrossRef CAS; (d) Y. Xia, D. Qiu and J. Wang, Chem. Rev., 2017, 117, 13810–13889 CrossRef CAS; (e) S.-S. Li, L. Qin and L. Dong, Org. Biomol. Chem., 2016, 14, 4554–4570 RSC; (f) G. Chelucci, Coord. Chem. Rev., 2017, 331, 1–36 CrossRef CAS.
- (a) B. H. Ridgway and K. A. Woerpel, J. Org. Chem., 2016, 63, 458–460 CrossRef; (b) A. I. Moncada, S. Manne, J. M. Tanski and L. M. Slaughter, Organometallics, 2016, 25, 491–505 CrossRef.
- (a) M. Larhed and A. Hallberg, J. Org. Chem., 2016, 62, 7858–7862 CrossRef; (b) S. Raoufmoghaddam, S. Mannathan, A. J. Minnaard, J. G. De Vries and J. N. H. Reek, Chem.–Eur. J., 2016, 21, 18811–18820 CrossRef.
- (a) K. T. Neumann, S. R. Laursen, A. T. Lindhardt, B. Bang-Andersen and T. Skrydstrup, Org. Lett., 2014, 16, 2216–2219 CrossRef CAS; (b) X. Qi, L. B. Jiang, C. L. Li, R. Li and X. F. Wu, Chem.–Asian J., 2016, 47, 1870–1873 Search PubMed.
- (a) A. Skhiri, R. B. Salem, J. F. Soulé and H. Doucet, Chemcatchem, 2017, 9, 2895–2913 CrossRef CAS; (b) M. Hervé, G. Lefèvre, E. A. Mitchell, B. U. Maes and A. Jutand, Chem.–Eur. J., 2016, 21, 18401–18406 CrossRef.
- (a) Q. Yan, E. Gin, M. Wasinska-Kalwa, M. G. Banwell and P. D. Carr, J. Org. Chem., 2017, 82, 4148–4159 CrossRef CAS PubMed; (b) F. Khan, M. Dlugosch, X. Liu, M. Khan, M. Banwell, J. Ward and P. Carr, Org. Lett., 2018, 20, 2770–2773 CrossRef CAS PubMed.
- A. Balanta, C. Godard and C. Claver, Chem. Soc. Rev., 2011, 40, 4973–4985 RSC.
- (a) Y. X. Jia, R. R. Liu, Y. G. Wang, Y. L. Li, B. B. Huang and R. X. Liang, Angew. Chem., Int. Ed., 2017, 56 Search PubMed; (b) U. K. Das, R. Clément, C. W. Johannes, E. G. Robins, H. Jong and R. T. Baker, Catal. Sci. Technol., 2017, 7 Search PubMed; (c) M. J. Mphahlele, T. J. Makhafola and M. M. Mmonwa, Bioorg. Med. Chem., 2016, 24, 4576–4586 CrossRef CAS; (d) A. D. Sonawane, D. R. Garud, T. Udagawa and M. Koketsu, Org. Biomol. Chem., 2017, 16, 245–255 RSC; (e) A. Mondal, P. Kundu, M. Jash and C. Chowdhury, Org. Biomol. Chem., 2018, 16, 963–980 RSC; (f) G. Z. Wang, R. Shang and Y. Fu, Org. Lett., 2018, 20, 888–891 CrossRef CAS.
- (a) L. Chen, D. Leslie, M. G. Coleman and J. Mack, Chem. Sci., 2018, 9, 4650–4661 RSC; (b) T. Ichikawa, M. Mizuno, S. Ueda, N. Ohneda, H. Odajima, Y. Sawama, Y. Monguchi and H. Sajiki, Tetrahedron, 2018, 74, 1810–1816 CrossRef CAS; (c) Q. X. Liu, Z. L. Hu, S. C. Yu, Z. X. Zhao, D. C. Wei and H. L. Li, ACS Omega, 2018, 3, 4035–4047 CrossRef CAS PubMed; (d) A. Elhage, B. Wang, N. Marina, M. L. Marin, M. Cruz, A. E. Lanterna and J. C. Scaiano, Chem. Sci., 2018, 9, 6844–6852 RSC.
- (a) L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133–173 CrossRef CAS; (b) Y. Zhao, R. Tang and R. Huang, Catal. Lett., 2015, 145, 1961–1971 CrossRef CAS; (c) S. Sadjadi, Appl. Organomet. Chem., 2018, 32, e4211 CrossRef; (d) M. Khajehzadeh and M. Moghadam, J. Organomet. Chem., 2018, 863, 60–69 CrossRef CAS; (e) R. Zhong, A. C. Lindhorst, F. J. Groche and F. E. Kuhn, Chem. Rev., 2017, 117, 1970–2058 CrossRef CAS PubMed.
- (a) J. Zhang, L. Wang, Y. Shao, Y. Wang, B. Gates and F. S. Xiao, Angew. Chem., Int. Ed., 2017, 129, 9879–9883 CrossRef; (b) M. Sharma, B. Das, B. K. Deka, Y. B. Park, S. K. Bhargava and K. K. Bania, ACS Appl. Mater. Interfaces, 2017, 9, 35453–35462 CrossRef CAS PubMed.
- M. Braun, U. D. Thach, B. Prelot, P. Hesemann and D. Esposito, J. Chem. Technol. Biotechnol., 2017, 92, 2229–2235 CrossRef CAS.
- (a) M. Vico Solano, G. González Miera, V. Pascanu and A. K. Inge, Chemcatchem, 2018, 10, 1089–1095 CrossRef CAS; (b) C. I. Ezugwu, B. Mousavi, M. A. Asrafa, A. Mehta, H. Vardhan and F. Verpoort, Catal. Sci. Technol., 2016, 6, 2050–2054 RSC.
- (a) M. Sarvestani and R. Azadi, Appl. Organomet. Chem., 2017, 32, e3906 CrossRef; (b) M. Nasrollahzadeh, S. M. Sajadi, A. Rostamivartooni, M. Alizadeh and M. Bagherzadeh, J. Colloid Interface Sci., 2016, 466, 360–368 CrossRef CAS.
- B. Li, Z. Guan, W. Wang, X. Yang, J. Hu, B. Tan and T. Li, Adv. Mater., 2012, 24, 3390–3395 CrossRef CAS.
- (a) J. G. Kim, C. C. Min, J. Lee, T. Choi and Y. C. Ji, ACS Appl. Mater. Interfaces, 2017, 9, 38081–38088 CrossRef CAS PubMed; (b) Z. Jia, K. Wang, B. Tan and Y. Gu, ACS Catal., 2017, 7, 3693–3702 CrossRef CAS; (c) K. Song, Z. Zou, D. Wang, B. Tan, J. Wang, C. Jian and L. Tao, J. Phys. Chem. C, 2016, 120, 2187–2197 CrossRef CAS.
- (a) D. Xu, F. Wang, G. Yu, H. Zhao, J. Yang, M. Yuan, X. Zhang and Z. Dong, Chemcatchem, 2018, 10, 4569–4577 CrossRef CAS; (b) K. Wang, Z. Jia, X. Yang, W. Ling, Y. Gu and B. Tan, J. Catal., 2017, 348, 168–176 CrossRef CAS; (c) M. Liu, B. Zhou, Z. Lei, X. Zhen, L. Shen and C. Long, J. Mater. Chem. A, 2018, 6, 9860–9865 RSC.
- E. Troschke, K. D. Nguyen, S. Paasch, J. Schmidt, G. Nickerl, I. Senkovska, E. Brunner and S. Kaskel, Chem.–Eur. J., 2018, 24, 18629–18633 CrossRef CAS PubMed.
- Y. Zhao, W. Ma, Y. Xu, C. Zhang, Q. Wang, T. Yang, X. Gao, F. Wang, C. Yan and J.-X. Jiang, Macromolecules, 2018, 51, 9502–9508 CrossRef CAS.
- S. Xu, K. Song, T. Li and B. Tan, J. Mater. Chem. A, 2015, 3, 1272–1278 RSC.
- Y. Zhou, Z. Liu, X. Fan, R. Li, G. Zhang, L. Chen, Y. Pan, H. Tang, J. Zeng and Z. Zhan, Org. Lett., 2018, 20, 7748–7752 CrossRef CAS PubMed.
- X. Liu, W. Xu, D. Xiang, Z. Zhang, D. Chen, Y. Hu, Y. Li, Y. Ouyang and H. Lin, New J. Chem., 2019, 43(31), 12206–12210 RSC.
- C. A. Wang, K. Nie, G. D. Song, Y. W. Li and Y. F. Han, RSC Adv., 2019, 9, 8239–8245 RSC.
- D. B. Eremin and V. P. Ananikov, Coord. Chem. Rev., 2017, 346, 2–19 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, spectral characterization data and supplementary figures. See DOI: 10.1039/c9ra07303e |
| ‡ Wei Xu and Cijie Liu contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |