Open Access Article
Open Access ArticleThe aggression behavior study of Cl− on the defect structure of passive films on copper
Aoni Xu ab,
Chaofang Dong*a,
Xin Weia,
Xiaogang Lia and
Digby D. Macdonaldb
ab,
Chaofang Dong*a,
Xin Weia,
Xiaogang Lia and
Digby D. Macdonaldb
aCorrosion and Protection Center, Key Laboratory for Corrosion and Protection (MOE), University of Science and Technology Beijing, Beijing 100083, China. E-mail: cfdong@ustb.edu.cn; Fax: +86-10-62334005; Tel: +86-10-62333931 extn 518
bDepartment of Material Science and Engineering, The University of California at Berkeley, Berkeley, CA 94720, USA
First published on 21st May 2019
Abstract
The destructive role of chloride ions on the defect structure of barrier layers (bl) is vitally important for understanding the initial breakdown of passive films on metals. Here photo-electrochemical and density functional theory (DFT) were applied to investigate the influence of chloride on the defect structure of the bl in passive films. The results show a bl with a narrow band gap, in which the valence band maximum (VBM) increased upon introducing chloride into the electrolyte. DFT calculations indicate that an increase in the copper vacancy concentration, due to cation extraction at the bl/solution interface could increase the VBM while oxygen vacancy generation results in a decrease in the conduction band minimum (CBM). The combination of these results verifies the aggressive role of chloride as proposed by the Point Defect Model (PDM) where an enhancement of the cation vacancy concentration across the bl occurs in response to the absorption of Cl− into oxygen vacancies on the bl.
1. Introduction
Copper generally exhibits excellent chemical stability under oxidizing conditions in contact with aqueous solutions owing to the formation of a thin oxide film that is only a few nanometers thick. Unfortunately, in spite of having this self-protective function, copper still suffers serious damage in some highly aggressive environments.1–3 Early investigations4–7 established the susceptibility of Cu to pitting corrosion resulting from the rapid breakdown of the protective film in aqueous halide solutions, especially in chloride-containing solutions. The overall reaction can be written as: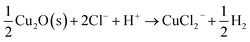 8 However, the role of highly defective structure of the passive film should not be neglected when we discuss the detailed mechanism of chloride ion-induced breakdown. Our previous work9 has demonstrated that the breakdown of passive films on copper in chloride solution is due to the adsorption behavior rather than the penetration of Cl− into the barrier oxide layer (bl) by both experiment and Density Functional Theory (DFT) calculations. A further investigation should be performed aimed at the detailed process of initial breakdown of the passive film induced by adsorbed chloride ions in solution. It is evident that the point defect structure of the bl is vitally important in understanding the physico-electrochemistry of passivity breakdown on metals and alloys.10 Thus, how the chloride ions impact the defect structure of passive film would be crucial to understand the initial breakdown. Not surprisingly, this has led many workers to examine the defect structure of bls using a variety of techniques.11–13 However, the defect structure of a barrier layer is difficult to define directly using conventional methods, such as photoluminescence excitation14 and positron annihilation spectroscopy (PAS),15 since the bl of a passive film is generally so thin (1–10 nm) that it is impossible to separate its response from that of the metal substrate. In past studies, researchers usually measured the carrier concentration in passive film using Mott–Schottky analysis (MSA) and used the results to reflect upon the variation of the point defect concentration.16–19 Nevertheless, the value of point defect concentration in the bl, as determined by MSA, is problematic, as noted elsewhere,20 and the relationship between point defect and carrier (electron and hole) concentrations is not well-defined because a lack of confluence between Mott–Schottky theory and the bl, which is not a simple p–n junction. Fortunately, the variation of the band structure also could reflect the change in the point defect concentration, since the point defects act as the dopants. Taking cuprous oxide (Cu2O) as an example, Paul et al.21 pointed out that the presence of copper vacancy could introduce hole states located on about 0.45 eV above the valence band maximum (VBM) in the band structure.
8 However, the role of highly defective structure of the passive film should not be neglected when we discuss the detailed mechanism of chloride ion-induced breakdown. Our previous work9 has demonstrated that the breakdown of passive films on copper in chloride solution is due to the adsorption behavior rather than the penetration of Cl− into the barrier oxide layer (bl) by both experiment and Density Functional Theory (DFT) calculations. A further investigation should be performed aimed at the detailed process of initial breakdown of the passive film induced by adsorbed chloride ions in solution. It is evident that the point defect structure of the bl is vitally important in understanding the physico-electrochemistry of passivity breakdown on metals and alloys.10 Thus, how the chloride ions impact the defect structure of passive film would be crucial to understand the initial breakdown. Not surprisingly, this has led many workers to examine the defect structure of bls using a variety of techniques.11–13 However, the defect structure of a barrier layer is difficult to define directly using conventional methods, such as photoluminescence excitation14 and positron annihilation spectroscopy (PAS),15 since the bl of a passive film is generally so thin (1–10 nm) that it is impossible to separate its response from that of the metal substrate. In past studies, researchers usually measured the carrier concentration in passive film using Mott–Schottky analysis (MSA) and used the results to reflect upon the variation of the point defect concentration.16–19 Nevertheless, the value of point defect concentration in the bl, as determined by MSA, is problematic, as noted elsewhere,20 and the relationship between point defect and carrier (electron and hole) concentrations is not well-defined because a lack of confluence between Mott–Schottky theory and the bl, which is not a simple p–n junction. Fortunately, the variation of the band structure also could reflect the change in the point defect concentration, since the point defects act as the dopants. Taking cuprous oxide (Cu2O) as an example, Paul et al.21 pointed out that the presence of copper vacancy could introduce hole states located on about 0.45 eV above the valence band maximum (VBM) in the band structure.
Furthermore, exploring the relationship between point defects and electronic structure of material by first principle calculations to explain reaction mechanism or guide material design has been generally accepted and has made significant progress in recent years. Raebiger et al.22 studied the formation enthalpies of copper vacancy (VCu) and oxygen interstice (Oi) using DFT and found that the cation deficiency of Cu2O is accommodated mostly by Cu vacancies VCu rather than by oxygen interstitials Oi (that is, the stoichiometry of the bl is Cu2−xO rather than Cu2O1+y, both of which give Cu/O < 2). Thus, in this paper, we used photo-electrochemical spectra to measure the band gap and ultraviolet photoelectron spectroscopy (UPS) to assess the position of VBM relative to the Fermi level. In this way, a simplified band structure of passive film could be obtained. Furthermore, defective Cu2O slab models were employed to simulate the barrier layer on copper. The characteristic band structures were identified for various kinds of point defects using HSE functional. Finally, a rational, theoretical explanation combined with experimental observation, was proposed to explain the effect of chloride on the defect structure of bl and hence for the occurrence of initial passivity breakdown.
2. Methods and material
2.1 Experiments
Electrochemical experiments were performed at ambient temperature (22 ± 2 °C) in a special, three-electrode cell containing two quartz windows for optical access. The working electrode, fabricated from a pure copper rod (99.99%), was mounted in epoxy resin with a 0.3 cm2 area exposed to the electrolyte. A saturated calomel electrode (SCE) and a Pt plate were employed as the reference electrode (RE) and the counter electrode (CE), respectively. A solution of pH = 7.6 ± 0.02 was established with borate buffer (Na2B4O7·10H2O 99.5% and H2BO3 99.5% assay) reagent-grade sodium chloride (NaCl 99.5%). During the entire experimental time, continuous nitrogen sparging into electrochemical cells were performed to avoid contamination from atmospheric oxygen.Potentiodynamic polarizations (PDP) were commenced in the negative-to-positive direction at a scan rate of 0.1667 mV s−1 after pre-performed cathodically polarization at −0.6 V vs. SCE. Subsequently, anodic polarization at 0.2 V in passivity range was performed for 24 h to generate a steady-state passive film. Then, the work functions of passive films were measured using a Thermo Scientific ESCALab 250Xi system employing UPS. The gas discharge lamp was used with admitted helium gas and the HeI (21.22 eV) emission line employed. In the meantime, we performed photo-electrochemical measurements using a 150 W xenon lamp and a 1200/mm grating monochromator just after anodic polarization. The photocurrent spectra were obtained by scanning the light wavelength in steps of 10 nm from 800 to 300 nm. All of the electrochemical and photo-electrochemical experiments were achieved on a Solartron Analytical Modulab photo-electrochemistry workstation.
2.2 Computational studies
HSE06 functional23 was applied in all calculations to obtain more accurate descriptions of electronic properties, in which the value of the exact nonlocal exchange correlation was set as 0.325 according to our previous work.24 Similarly, norm-conserving pseudopotentials (NCPP) were used to describe the interaction between electron and ion. For a cubic Cu2O supercell, the plane-wave energy cutoff and k-point meshes were set as 520 eV and 4 × 4 × 4 respectively. Extensive tests have been carried out to ensure convergence when the total force on each ion was reduced to less than 0.01 eV Å−1. Then, three 5-layer (3 × 3) Cu2O unit cells with a 15 Å vacuum region were constructed for modeling Cu2O (100), (110) and (111) surfaces as ultrathin passive film on copper. The surface energies and electronic properties of three different facets models were calculated after structure optimization. To estimate the contribution of point defects to the electronic structure, vacancies were created by removing Cu/O ions individually from Cu2O slab, followed by structure optimization. Meanwhile, configurations with chloride ions inserting into surface oxygen vacancies were employed to investigate the breakdown effect of chloride ions (negative chloride ions have same charge with cation vacancies, thus, it's unreasonable that anions interact with cation vacancy). Finally, band structures and density of states (DOS) calculations were performed for these defective models using PWmat software.25,263. Results
3.1 Experimental results
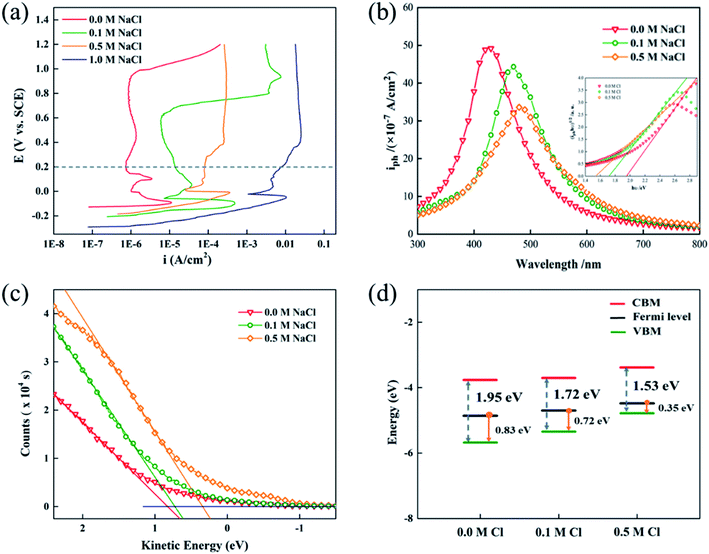
Fig. 1(a) shows the potentiodynamic polarization curves of Cu in pH = 7.64 buffer solution containing chloride. Clearly, the passive range of copper in neutral solution was gradually narrowed as the chloride concentration increased. Even no passivity, as evidenced by the passive current higher than ∼10−5 A cm−2, was observed in 1 M NaCl. Then 0.2 V, which lies within the passivity range of copper in all electrolytes, was chose to form the passive film. Photocurrent spectra relating to anodic passive films grown in various solutions were measured and are shown in Fig. 1(b). The photo-absorption coefficient, α, depends on the photon energy related to the band gap of samples:27| α = A(hν − Eg)n/hν | (1) |
| Experimental results | Calculation results | ||
|---|---|---|---|
| Electrolyte | Work function/eV | Orientation | Surface energy/eV Å−2 |
| 0.0 M NaCl | 4.86 | Cu2O (100) | 0.10 |
| 0.1 M NaCl | 4.70 | Cu2O (110) | 0.08 |
| 0.5 M NaCl | 4.48 | Cu2O (111) | 0.04 |
Taking the results of band gap, position of the VBM, and work function into consideration, the band structures of the bl can be represented as Fig. 1(d). The bl of the passive film grown in 0.5 M NaCl had the narrowest band gap and the highest Fermi level (lowest work function), while the band gaps and Fermi levels were similar for the bls formed in two other solutions. Combining with the introducing of doping states by point defect, this change in band structure of passive film demonstrated that the chloride ions in electrolyte play a significant role in determining the defect structure of the bl by creating fresh vacancies. A similar increasing tendency of carrier density was also reported by Fattah-Alhosseini33 by Mott–Schottky analysis. However, the interaction between chloride ions and the bl still lacks detail. Oxygen vacancies 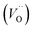 and cation vacancies
and cation vacancies 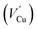 in the bl, and even chloride absorbed into surface oxygen vacancies
in the bl, and even chloride absorbed into surface oxygen vacancies 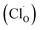 in bl, can all generate electronic doping states in the band gap, which renders the process difficult to be analyzed only from experiment. Thus, a more detailed analysis is warranted by using first-principles calculations.
in bl, can all generate electronic doping states in the band gap, which renders the process difficult to be analyzed only from experiment. Thus, a more detailed analysis is warranted by using first-principles calculations.
3.2 Calculational results
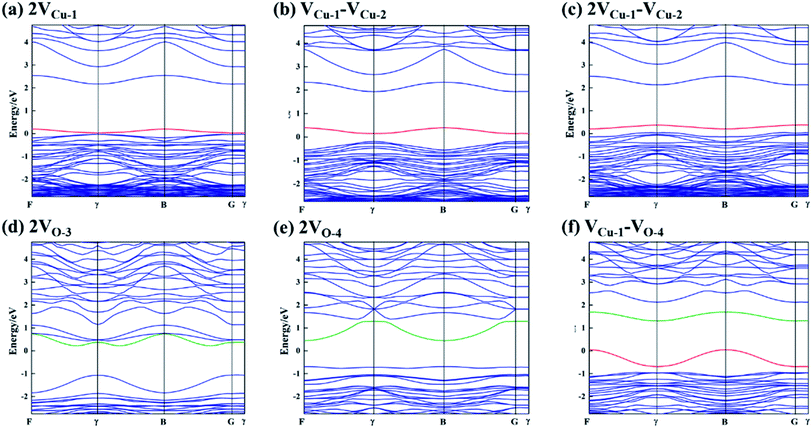
In this section, the most stable facet with lowest surface energy (Table 1), Cu2O (111), which is also observed to be similar to that of the barrier layer of the passive film on copper by scanning tunneling microscope,34 was used to study in detail the breakdown mechanism of chloride ions on the aspect of electronic properties.On the most stable O-terminated Cu2O (111) surface, 4 kinds of ions exist, named as Cu-1, Cu-2, O-3 and O-4 according to their coordination numbers [Fig. 2(g)]. We first investigated the surface work functions for complete and defective surface. For a perfect Cu2O (111) surface, the work function equals to 4.87 eV which is very similar with our experimental result in Table 1. However, when we introduce surface point defect on our slab model, the work function values all decreased obviously where 4.77 and 4.75 eV for 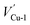 and
and 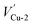 , 4.73 and 4.70 eV for
, 4.73 and 4.70 eV for 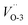 and
and 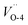 , respectively. We also calculated the work function for the adsorption model of chloride, where 5.04 and 5.35 eV for the configurations of Cl absorbed on Cu-1 and Cu-2. Therefore, a reasonable explanation for the decreasing work functions with increase in molarity of Cl observed in experiments was proposed “The barrier layer formed in a dense Cl-containing solution has a more defective structure”. Furthermore, Fig. 2 displays the band structures and DOS of Cu2O (111) with a copper or oxygen vacancies or an oxygen vacancy occupied by chloride. We have discussed the effect of cation vacancies on electronic properties and magnetic moments in detail in our previous work,24 where O 2p and Cu 3d states dominate an additional acceptor state above VBM in band gap. On the contrary, for oxygen vacancies, especially Cl-occupied oxygen vacancies, a clear shift down of CBM was observed by introducing a donor state in the band gap [Fig. 2e and f]. Similarly, 3d states of copper atoms surrounding oxygen vacancy dominate the extension of the conduction band owing to the unsaturated dangling bonds appearing around the vacancy. The donor state introduced by Cl-occupied oxygen vacancy never changes the direct gap feature of stoichiometric Cu2O compared with an unoccupied oxygen vacancy, which shifts the direct transition point in the Brillouin zone from Point γ- to Point B point for
, respectively. We also calculated the work function for the adsorption model of chloride, where 5.04 and 5.35 eV for the configurations of Cl absorbed on Cu-1 and Cu-2. Therefore, a reasonable explanation for the decreasing work functions with increase in molarity of Cl observed in experiments was proposed “The barrier layer formed in a dense Cl-containing solution has a more defective structure”. Furthermore, Fig. 2 displays the band structures and DOS of Cu2O (111) with a copper or oxygen vacancies or an oxygen vacancy occupied by chloride. We have discussed the effect of cation vacancies on electronic properties and magnetic moments in detail in our previous work,24 where O 2p and Cu 3d states dominate an additional acceptor state above VBM in band gap. On the contrary, for oxygen vacancies, especially Cl-occupied oxygen vacancies, a clear shift down of CBM was observed by introducing a donor state in the band gap [Fig. 2e and f]. Similarly, 3d states of copper atoms surrounding oxygen vacancy dominate the extension of the conduction band owing to the unsaturated dangling bonds appearing around the vacancy. The donor state introduced by Cl-occupied oxygen vacancy never changes the direct gap feature of stoichiometric Cu2O compared with an unoccupied oxygen vacancy, which shifts the direct transition point in the Brillouin zone from Point γ- to Point B point for 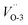 and introduces an indirect gap for
and introduces an indirect gap for 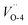 .
.
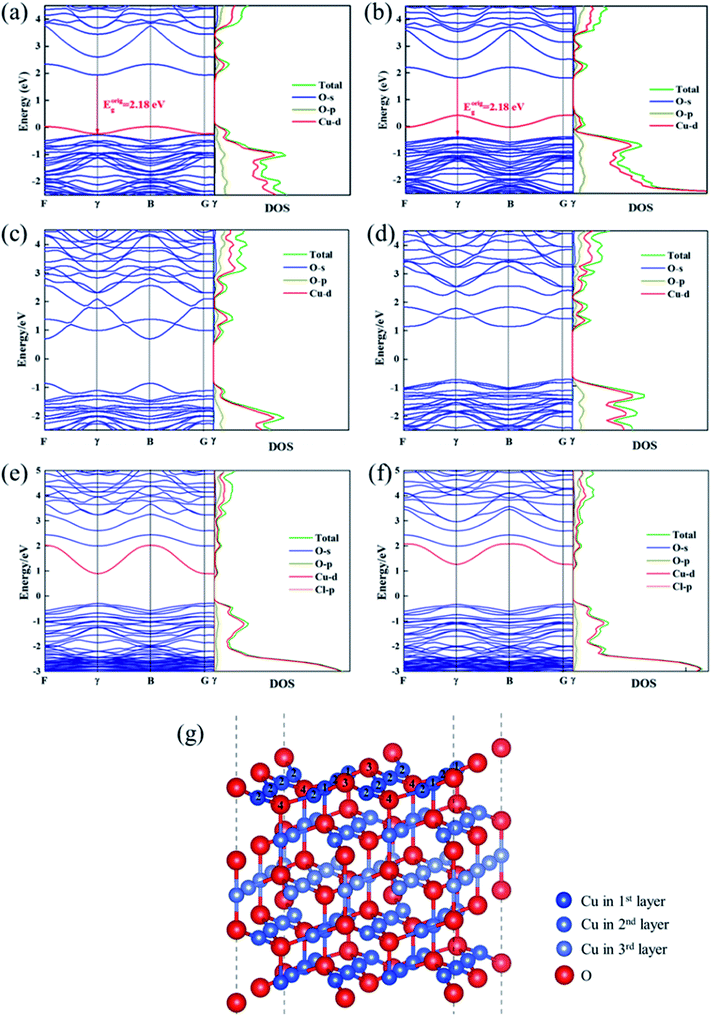 | ||
Fig. 2 Band structures and DOS of Cu2O (111) with (a) 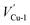 ; (b) ; (b) 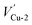 ; (c) ; (c) 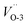 ; (d) ; (d) 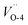 ; (e) ; (e) 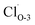 ; (f) ; (f) 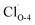 ; (g) side view of Cu2O (111) slab model. ; (g) side view of Cu2O (111) slab model. | ||
It should be noted that the real bl is inhomogeneous with gradients of the electric field and of the distribution of vacancies. Naturally, the field penetration and band bending must exist across the bls on metals owing the variational phase environment. However, it's almost impossible to create a model with the same distribution of vacancies and also electric field as a real passive film which is still a mystery on characterization experiments. Similarly, we have to be honest to say we cannot calculate the exact band structure of bl. But, we can apply the calculated electronic structure of cuprous oxide in a specific defective structure to analyze the effective role of a specific defect on electronic properties for bl by comparing calculation results of defective model and complete one. That's because the effective role of a specific vacancy for a semiconductor whether perfect or defective remains unchanged. Taking copper vacancy as an example, copper vacancy always acts as acceptor introducing hole states over valence band in band structure for CuxO (1 < x < 2).22,35,36 To demonstrate that in detail, we also supplemented many electronic structure calculations under electric field of various values and with different defect concentrations.
Fig. 3 shows the band structures of Cu2O (111) slab model with one 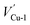 under 0, 1, 3 and 5 × 106 V cm−1 electric field. Meanwhile, the direction of applied electric field is parallel to z axis and the vacuum layer. As seen, the copper vacancy VCu-1 always acts as acceptor and introduce a shallow hole state above valence band no matter in which electric field. The electronic structures of cuprous oxide with different defect concentration were calculated to indicate the effective role of a specific defect on band structure for oxide is not going to change due to varying concentration. The results were shown in Fig. 4. We improved the vacancy concentration by removing more cations or oxygen ions from Cu2O (111) model. All of the band structures were obtained after relaxation calculations. From Fig. 4, it's clearly that the copper vacancy always can increase valence band maximum by introducing acceptor states in band gap no matter with any concentration of itself or oxygen vacancies. Similarly, the oxygen vacancy always cannot increase valence band maximum in any conditions. Thus, transplanting these results to passive film, only copper vacancy in bl could increase the VBM, while oxygen vacancy and chloride-occupied oxygen vacancy just result in a decrease in CBM.
under 0, 1, 3 and 5 × 106 V cm−1 electric field. Meanwhile, the direction of applied electric field is parallel to z axis and the vacuum layer. As seen, the copper vacancy VCu-1 always acts as acceptor and introduce a shallow hole state above valence band no matter in which electric field. The electronic structures of cuprous oxide with different defect concentration were calculated to indicate the effective role of a specific defect on band structure for oxide is not going to change due to varying concentration. The results were shown in Fig. 4. We improved the vacancy concentration by removing more cations or oxygen ions from Cu2O (111) model. All of the band structures were obtained after relaxation calculations. From Fig. 4, it's clearly that the copper vacancy always can increase valence band maximum by introducing acceptor states in band gap no matter with any concentration of itself or oxygen vacancies. Similarly, the oxygen vacancy always cannot increase valence band maximum in any conditions. Thus, transplanting these results to passive film, only copper vacancy in bl could increase the VBM, while oxygen vacancy and chloride-occupied oxygen vacancy just result in a decrease in CBM.
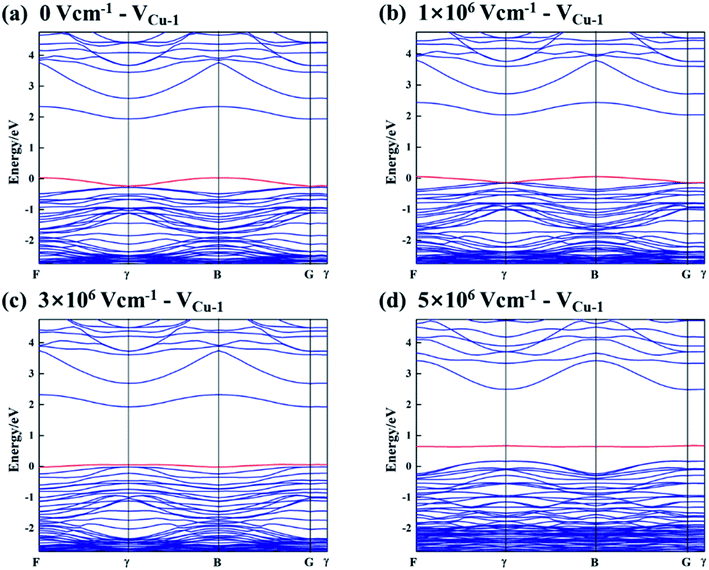 | ||
| Fig. 3 Band structures of Cu2O (111) with one VCu-1 under (a) 0, (b) 1, (c) 3 and (d) 5 × 106 V cm−1 electric field. | ||
 | ||
| Fig. 4 Band structures of Cu2O (111) with different concentrations of copper vacancies or oxygen vacancies. | ||
Combining with experimental results where VBM increased significantly with introducing chloride into the electrolyte, a reasonable speculation could be concluded that an increase of copper vacancy concentration in passive film would be substantial indeed owing to the participation of chloride ions. This conclusion is coincidentally in agreement with the assumption of Point Defect Model (PDM). In PDM, the aggression process of chloride ions for passive film was described as chloride absorbs into surface oxygen vacancies at bl/outer layer (ol) interface and extract a cation in an autocatalytic process that leads to the continual generation of cation vacancies that are then migrate across the bl to condense at the metal/bl interface to separate the bl from substrate.
4. Summary and conclusions
In this paper, we investigated the band structure of the barrier layer of the passive film on copper in Cl-containing solutions by photo-electrochemical measurements and DFT calculations. The results were showed as follows:(1) The band gap of passive film narrows with introducing chloride into the electrolyte, where VBM increased significantly from photo-electrochemical and UPS measurements.
(2) DFT calculations indicated that only copper vacancy in the oxide model could increase the VBM, while oxygen vacancy and chloride-occupied oxygen vacancy just result in a decrease in CBM.
(3) The combining results verified that an increase of copper vacancy concentration in passive film would be substantial indeed owing to the participation of chloride ions, which is consistent with the description of aggression role of Cl− on the initial breakdown of passive film in PDM where Cl− in solution could extract cation vacancy in the barrier layer of the passive film by interacting with a surface oxygen vacancy.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2017YFB0702300) and the National Natural Science Foundation of China (No. 51671029). Aoni Xu gratefully acknowledges the China Scholarship Council for supporting her stay at the University of California at Berkeley.References
- H. Lee and K. Nobe, Kinetics and mechanisms of Cu electrodissolution in chloride media, J. Electrochem. Soc., 1986, 133(10), 2035–2043 CrossRef CAS.
- E. D'Elia, O. E. Barcia, O. R. Mattos, N. Pébère and B. Tribollet, High-rate copper dissolution in hydrochloric acid solution, J. Electrochem. Soc., 1996, 143(3), 961–967 CrossRef.
- M. A. Amin, K. Khaled, Q. Mohsen and H. Arida, A study of the inhibition of iron corrosion in HCl solutions by some amino acids, Corros. Sci., 2010, 52(5), 1684–1695 CrossRef CAS.
- M. Edwards, J. F. Ferguson and S. H. Reiber, The pitting corrosion of copper, J. - Am. Water Works Assoc., 1994, 86, 74–90 CrossRef CAS.
- G. Kear, B. Barker and F. Walsh, Electrochemical corrosion of unalloyed copper in chloride media––a critical review, Corros. Sci., 2004, 46, 109–135 CrossRef CAS.
- S. Sathiyanarayanan, M. Sahre and W. Kautek, In-situ grazing incidence X-ray diffractometry observation of pitting corrosion of copper in chloride solutions, Corros. Sci., 1999, 41, 1899–1909 CrossRef CAS.
- C. Deslouis, B. Tribollet, G. Mengoli and M. M. Musiani, Electrochemical behaviour of copper in neutral aerated chloride solution. I. Steady-state investigation, J. Appl. Electrochem., 1988, 18, 374–383 CrossRef CAS.
- H. Strandberg and L. G. Johansson, Some aspects of the atmospheric corrosion of copper in the presence of sodium chloride, J. Electrochem. Soc., 1998, 145(4), 1093–1100 CrossRef CAS.
- X. Wei, C. Dong, P. Yi, A. Xu, Z. Chen and X. Li, Electrochemical measurements and atomistic simulations of Cl−-induced passivity breakdown on a Cu2O film, Corros. Sci., 2018, 136, 119–128 CrossRef CAS.
- L. Lin, C. Chao and D. Macdonald, A point defect model for anodic passive films II. Chemical breakdown and pit initiation, J. Electrochem. Soc., 1981, 128, 1194–1198 CrossRef CAS.
- T. Dull, W. Frieze, D. Gidley, B. Scherer, D. Ellerbrock and D. Macdonald, Positron DBS studies of the corrosive breakdown of the passive film on titanium, in Materials Science Forum, Trans Tech Publ, 1997, vol. 255, pp. 671–673 Search PubMed.
- S. Ahn, H. Kwon and D. D. Macdonald, Role of chloride ion in passivity breakdown on iron and nickel, J. Electrochem. Soc., 2005, 152, B482–B490 CrossRef.
- I. T. Fonseca, N. Lima, J. A. Rodrigues, M. I. S. Pereira, J. C. Salvador and M. G. Ferreira, Passivity breakdown of Al 2024-T3 alloy in chloride solutions: a test of the point defect model, Electrochem. Commun., 2002, 4, 353–357 CrossRef CAS.
- P. Zu, Z. Tang, G. K. Wong, M. Kawasaki, A. Ohtomo, H. Koinuma and Y. Segawa, Ultraviolet spontaneous and stimulated emissions from ZnO microcrystallite thin films at room temperature, Solid State Commun., 1997, 103, 459–463 CrossRef CAS.
- M. Abdel-Rahman, M. Abdallah and E. A. Badawi, Study of trapping rate and defect density in AlSi 11.35 Mg 0.23 by positron annihilation technique, Surf. Rev. Lett., 2004, 11, 427–432 CrossRef CAS.
- Y. Bai, Y. Ling, W. Lai, S. Xing and W. Ma, Electrochemical properties of the passive film on bulk Zr–Fe–Cr intermetallic fabricated by spark plasma sintering, Appl. Surf. Sci., 2016, 388, 212–222 CrossRef CAS.
- A. Fattah-Alhosseini, F. Soltani, F. Shirsalimi, B. Ezadi and N. Attarzadeh, The semiconducting properties of passive films formed on AISI 316 L and AISI 321 stainless steels: a test of the point defect model (PDM), Corros. Sci., 2011, 53, 3186–3192 CrossRef CAS.
- L. Jinlong, L. Tongxiang, W. Chen and D. Limin, Comparison of corrosion properties of passive films formed on coarse grained and ultrafine grained AISI 2205 duplex stainless steels, J. Electroanal. Chem., 2015, 757, 263–269 CrossRef.
- L. Jinlong, L. Tongxiang, W. Chen and G. Ting, The passive film characteristics of several plastic deformation 2099 Al–Li alloy, J. Alloys Compd., 2016, 662, 143–149 CrossRef.
- D. D. Macdonald, The passive state in our reactive metals-based civilization, Arabian J. Sci. Eng., 2012, 37, 1143–1185 CrossRef.
- G. Paul, Y. Nawa, H. Sato, T. Sakurai and K. Akimoto, Defects in Cu2O studied by deep level transient spectroscopy, Appl. Phys. Lett., 2006, 88, 141901 CrossRef.
- H. Raebiger, S. Lany and A. Zunger, Origins of the p-type nature and cation deficiency in Cu2O and related materials, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 045209 CrossRef.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, Erratum: “hybrid functionals based on a screened Coulomb potential” [J. Chem. Phys., 118, 8207 (2003)], J. Chem. Phys., 2006, 124, 219906 CrossRef.
- A. Xu, C. Dong, X. Wei, X. Li and D. D. Macdonald, DFT and photoelectrochemical studies of point defects in passive films on copper, J. Electroanal. Chem., 2019, 834, 216–222 CrossRef CAS.
- W. Jia, Z. Cao, L. Wang, J. Fu, X. Chi, W. Gao and L.-W. Wang, The analysis of a plane wave pseudopotential density functional theory code on a GPU machine, Comput. Phys. Commun., 2013, 184, 9–18 CrossRef CAS.
- W. Jia, J. Fu, Z. Cao, L. Wang, X. Chi, W. Gao and L.-W. Wang, Fast plane wave density functional theory molecular dynamics calculations on multi-GPU machines, J. Comput. Phys., 2013, 251, 102–115 CrossRef.
- J. Tauc, Absorption edge and internal electric fields in amorphous semiconductors, Mater. Res. Bull., 1970, 5, 721–729 CrossRef CAS.
- U. Stimming, Photoelectrochemical studies of passive films, Electrochim. Acta, 1986, 31, 415–429 CrossRef CAS.
- S. Lee, E. Cho, S. Ahn and H. Kwon, Photo-electrochemical analysis of the passive film on zircaloy-4, Electrochim. Acta, 2001, 46, 2605–2611 CrossRef CAS.
- F. Caballero-Briones, A. Palacios-Padros, O. Calzadilla, I. d. P. Moreira and F. Sanz, Disruption of the chemical environment and electronic structure in p-type Cu2O films by alkaline doping, J. Phys. Chem. C, 2012, 116, 13524–13535 CrossRef CAS.
- J. Ghijsen, L. v. Tjeng, J. Van Elp, H. Eskes, J. Westerink, G. Sawatzky and M. Czyzyk, Electronic structure of Cu2O and CuO, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 38, 11322 CrossRef CAS.
- H.-H. Strehblow, V. Maurice and P. Marcus, Initial and later stages of anodic oxide formation on Cu, chemical aspects, structure and electronic properties, Electrochim. Acta, 2001, 46, 3755–3766 CrossRef CAS.
- A. Fattah-Alhosseini, O. Imantalab and F. R. Attarzadeh, Electrochemical Behavior of Nano-grained Pure Copper in Dilute Alkaline Solution with Chloride Ion Trace, J. Mater. Eng. Perform., 2016, 25, 4478–4483 CrossRef CAS.
- J. Kunze, V. Maurice, L. H. Klein, H.-H. Strehblow and P. Marcus, In situ STM study of the duplex passive films formed on Cu (1 1 1) and Cu (0 0 1) in 0.1 M NaOH, Corros. Sci., 2004, 46, 245–264 CrossRef CAS.
- M. Nolan and S. D. Elliott, The p-type conduction mechanism in Cu2O: a first principles study, Phys. Chem. Chem. Phys., 2006, 8, 5350–5358 RSC.
- A. Soon, X.-Y. Cui, B. Delley, S.-H. Wei and C. Stampfl, Native defect-induced multifarious magnetism in nonstoichiometric cuprous oxide: first-principles study of bulk and surface properties of Cu2− δ O, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 035205 CrossRef.
| This journal is © The Royal Society of Chemistry 2019 |