Open Access Article
Open Access ArticleEffect of oxygen on power frequency breakdown voltage and decomposition characteristics of the C5F10O/N2/O2 gas mixture
Yue Zhang a,
Xiaoxing Zhang
a,
Xiaoxing Zhang *ba,
Yi Li
*ba,
Yi Li *a,
Yalong Li
*a,
Yalong Li a,
Qi Chen
a,
Qi Chen a,
Guozhi Zhang
a,
Guozhi Zhang b,
Song Xiao
b,
Song Xiao a and
Ju Tang
a and
Ju Tang a
a
aSchool of Electrical Engineering and Automation, Wuhan University, Wuhan 430072, China. E-mail: xiaoxing.zhang@outlook.com; liyi_whuee@163.com
bSchool of Electrical and Electronic Engineering, Hubei University of Technology, Wuhan 430072, China
First published on 17th June 2019
Abstract
Sulfur hexafluoride (SF6) is widely used in the power industry because of its excellent insulation and arc extinguishing performance; however, as the global environment is deteriorating, the need to replace SF6 is becoming significantly critical. In recent years, C5F10O has received extensive attention as a potential alternative to SF6. In this study, a part of N2 in C5F10O/N2 was replaced by O2, and the breakdown voltages of C5F10O/N2/O2 at different oxygen concentrations under a slightly uneven electric field were tested. The dispersion of breakdown voltage and the discharge decomposition components of C5F10O/N2/O2 with different oxygen concentrations were analysed. It was found that as the oxygen concentration increased, the breakdown voltage of C5F10O/N2/O2 with 15 kPa C5F10O at 0.2 MPa increased, and the dispersion of the breakdown voltage became worse. When 0.5% O2 or more O2 was added to the C5F10O/N2 gas mixture, the carbon precipitates on the electrode surface disappeared. As the oxygen concentration continued to increase, another characteristic component, CF2O, could be detected, whereas C2F4 and C3F6 disappeared. It is believed that O2 can inhibit the formation of C2F6, C3F8, C4F10, and C3F7H. Therefore, it is recommended to use oxygen as the second buffer gas for the engineering applications of C5F10O. Moreover, the ratio of C5F10O to O2 is recommended to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
1 Introduction
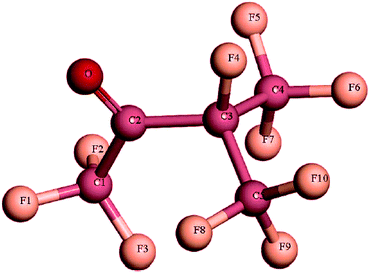
The gas insulated switchgear (GIS) has become an indispensable part of the power system due to its advantages such as small size and high operational reliability.1–5 Sulfur hexafluoride (SF6) is widely used in the GIS as a gas insulating medium with high dielectric strength and excellent arc extinguishing performance.6–8 However, the global warming potential (GWP) value of SF6 is as high as 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, and its atmospheric lifetime is about 3200 years, SF6 is one of the most greenhouse gases.9–11 Therefore, the demand to limit the application of SF6 in the power industry is urgent.12–14 In this regard, researchers have paid significant attention to find alternative gases for SF6 from the perspective of insulation strength, environmental characteristics, decomposition properties as well as safety. Over the past three years, fluorocarbon macromolecules, such as c-C4F8, CF3I, C4F7N, C5F10O, and C6F12O, have attracted attention.15–25 Among these molecules, C5F10O has shown significant advantages in terms of insulation strength, low GWP and reliable biosafety. The molecular structure of C5F10O is shown in Fig. 1; C5F10O is a non-toxic, colourless, and odourless substance.
500, and its atmospheric lifetime is about 3200 years, SF6 is one of the most greenhouse gases.9–11 Therefore, the demand to limit the application of SF6 in the power industry is urgent.12–14 In this regard, researchers have paid significant attention to find alternative gases for SF6 from the perspective of insulation strength, environmental characteristics, decomposition properties as well as safety. Over the past three years, fluorocarbon macromolecules, such as c-C4F8, CF3I, C4F7N, C5F10O, and C6F12O, have attracted attention.15–25 Among these molecules, C5F10O has shown significant advantages in terms of insulation strength, low GWP and reliable biosafety. The molecular structure of C5F10O is shown in Fig. 1; C5F10O is a non-toxic, colourless, and odourless substance.
Its chemical properties are stable; according to the data provided by 3M™, the initial decomposition temperature of C5F10O is up to 600 °C, whereas the temperature of the hottest spot in the equipment is usually less than 200 °C.26 In addition, C5F10O has the GWP of only 1, and its atmospheric lifetime is only 14 days. Moreover, the ozone depletion potential (ODP) value of C5F10O is zero; this means that C5F10O does not consume the ozone layer of the atmosphere and fully meets the requirements of the “Montreal Protocol on Substances that Deplete the Ozone Layer”.26 The insulation strength of pure C5F10O is twice that of SF6; however, the liquefaction temperature of pure C5F10O under normal pressure is high (i.e. 26.9 °C); therefore, it is usually mixed with a buffer gas that has very low liquefaction temperature during its research and practical application.27,28
Recently, several studies have been carried on C5F10O gas mixtures. Saxegaard et al. have demonstrated that the use of C5F10O/air with the liquefaction temperature of −25 °C can increase the rated voltage of an air switchgear from 12 kV to 24 kV.29 In addition, it has been reported that the AirPlus™ ring network unit of ABB in the Netherlands meets the insulation expectations well and has no impact on the equipment life in the first year.30 Wang et al. tested the power-frequency (AC) breakdown characteristics of C5F10O/CO2 under a non-uniform electric field. They believe that the insulation performance of the gas mixture can be effectively improved by increasing the content of C5F10O.31 Aints et al. found that an increase in the content of C5F10O in C5F10O/air would reduce the effective ionization coefficient.32 Chachereau et al. have found that there is a significant synergy between C5F12O and N2 or CO2, and the C5F12O gas mixtures can reach the insulation strength requirement of SF6 by increasing the gas pressure.33
In addition, it is important to study the decomposition characteristics of the C5F10O gas mixtures. Our team calculated the decomposition mechanism of C5F10O using density functional theory (DFT). It has been found that the activity of carbonyl in the C5F10O molecule (as shown in Fig. 1) is strong. And, C1–C2 and C2–C3 in the C5F10O molecule easily break to form CF3CO˙ and C3F7˙ or C3F7CO˙ and CF3˙.34,35 Wang et al. have also explored the decomposition pathway of C5F10O and pointed out that the C2–C3 and C3–C4 (or C3–C5) bonds in the C5F10O molecule are more likely to break.18 The main decomposition components for the AirPlus™ switchgear after arc extinguishing are CO2, CO, and HF, among others, and it has been considered that the toxicity of the C5F10O gas mixture is mainly determined by CO.36 Hammer et al. detected the decomposition components of C5F10O/N2 under a dielectric barrier discharge. Several by-products such as CO, C2F6, and C3F8 were detected.37
Previous studies have shown that the addition of O2 to a C5F10O/CO2 gas mixture can improve the insulation strength of the C5F10O gas mixture.38 However, at present, only few studies have been reported on the influence of O2 on the insulation and decomposition properties of a C5F10O gas mixture. In this study, C5F10O was mixed with N2, and O2 was added as the second buffer gas. By adjusting the oxygen concentration, the breakdown voltage and decomposition components of a C5F10O/N2/O2 ternary gas mixture after AC breakdown tests were investigated. The influence of oxygen on the insulation and decomposition properties of the C5F10O/N2/O2 gas mixture was explored first. Relevant results can provide reference for the engineering application of a C5F10O gas mixture.
2 Test equipment, conditions and methods
2.1 Test equipment and conditions
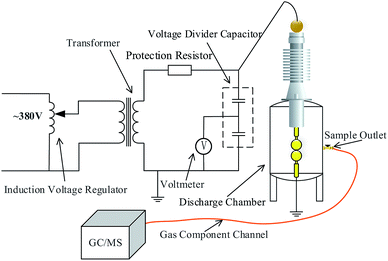
Fig. 2 shows the gas insulation performance and composition analysis platform. The output voltage of the high-voltage test transformer (100 kV/0.5 A) was adjusted by an induction voltage regulator. The AC voltage applied on the electrodes was measured by a voltage divider capacitor. Moreover, protective resistance (10 kΩ) was used to limit the short-circuit current during gap breakdown to prevent damage to the test transformer. Gas chromatography and mass spectrometry (GC/MS) were used for the qualitative analysis of the decomposition components.A slightly uneven electric field is the most common electric field in the SF6 equipment. In the experiments to explore the insulation and decomposition characteristics of a potential SF6 substitute gas, sphere–sphere electrodes with the gap distance of 2 mm were used to simulate the slightly uneven electric field (the non-uniform coefficient of the electric field was calculated to be 1.02 by COMSOL Multiphysics with Emax = 2.04 × 107 V m−1 and Eav = 2 × 107 V m−1). The sphere–sphere electrodes are made of brass, and their radius is 25 mm.
Although the insulation strength of the C5F10O gas mixtures can be increased by increasing the content of C5F10O in the gas mixture, high liquefaction temperature of C5F10O under normal pressure will severely limit the application of C5F10O in high-voltage gas insulation equipment.29 Exploration of the application of C5F10O gas mixtures in medium-voltage or low-voltage gas insulated equipment (GIE) has become the focus of research in the industry. For this reason, all the experiments in this study were carried out under the condition of the absolute pressure of 0.2 MPa.
Considering the low liquefaction temperature of nitrogen and oxygen (−140.7 °C and −118.57 °C, respectively), the liquefaction temperature of a C5F10O gas mixture under normal pressure is determined by the partial pressure of C5F10O in the gas mixture.39 In this study, the partial pressure of C5F10O was set to 15 kPa, and the corresponding liquefaction temperature of the gas mixtures was −15 °C. In addition, because the pressure remains unchanged, the addition of oxygen will reduce the nitrogen concentration accordingly.
To investigate the effect of oxygen concentration on the breakdown voltage and decomposition characteristics of C5F10O/N2/O2, herein, four groups of gas mixtures with the oxygen concentrations of 0%, 0.5%, 7.5% and 19.33% were tested. The corresponding partial pressure of oxygen was 1 kPa when the oxygen concentration was 0.5%. The oxygen concentration of 7.5% corresponded to the oxygen partial pressure of 15 kPa (i.e. C5F10O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 was equal to 1
O2 was equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and when the oxygen concentration was 19.33%, the partial pressure of oxygen was 38.66 kPa (actually, it was a gas mixture of C5F10O and air).
1), and when the oxygen concentration was 19.33%, the partial pressure of oxygen was 38.66 kPa (actually, it was a gas mixture of C5F10O and air).
2.2 Test methods
The gas chamber was vacuum-pumped and then filled with buffer gas until the absolute pressure was greater than 0.2 MPa. Then, the abovementioned steps were repeated three times to ensure that each test was unaffected by previous tests and other impurities in the chamber. Because of the low saturated vapor pressure of C5F10O at normal temperature, it should be filled into the chamber first followed by oxygen and nitrogen. It was necessary to stand the gas mixture for 24 hours to make it mix evenly. The power frequency voltage was applied to the discharge chamber by a step-up method with a gap of at least 5 minutes between every two breakdowns. A total of 60 breakdown tests were conducted for each group of gas mixture. The average value of the breakdown voltage of the first ten breakdowns was taken as the breakdown voltage of C5F10O/N2/O2 under each oxygen concentration condition.The C5F10O/N2/O2 samples after 60 times breakdown were obtained and tested by GC/MS. Moreover, the qualitative analysis of the gas was carried out by comparing the scanned results of the standard gas chromatography with the standard chromatographic database of the National Institute of Standards and Technology (Nist14.0).
3 Test results and analysis
3.1 Effect of oxygen concentration on the breakdown voltage of C5F10O/N2/O2
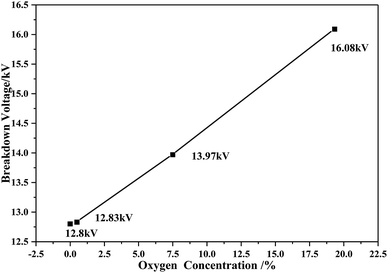
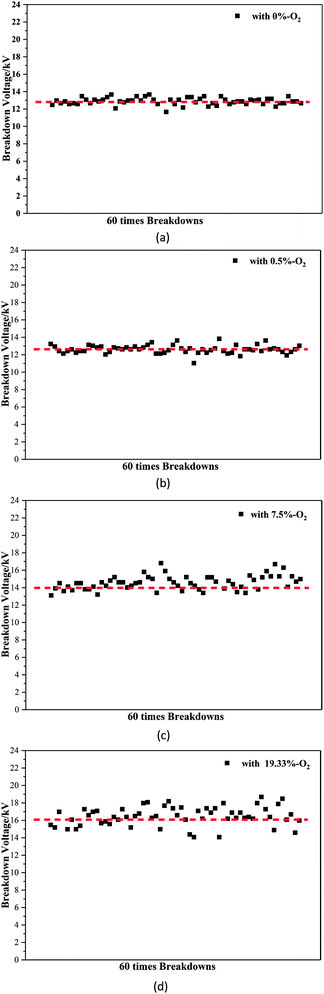
As shown in Fig. 3, the breakdown voltage of C5F10O/N2/O2 increases with the increasing oxygen concentration. The electronegativity of oxygen is greater than that of nitrogen, and its ability to attract electrons is stronger; thus, oxygen is more likely to hinder the development of discharge. Therefore, in the case of constant pressure and C5F10O concentration, the dielectric strength of the C5F10O gas mixture will increase with the increasing oxygen content.Fig. 4 shows the breakdown voltage values of C5F10O/N2/O2 for 60 breakdown tests under different oxygen concentration conditions. Due to the random nature of discharge, the standard deviation is defined to characterize the dispersion of the breakdown voltage of C5F10O/N2/O2 at different oxygen concentrations. The formula for the calculation is as follows:
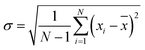 | (1) |
![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) is the average value, and σ is the standard deviation of the breakdown voltage of the experimental set.
is the average value, and σ is the standard deviation of the breakdown voltage of the experimental set.
When the oxygen concentration was 0%, the breakdown voltages of C5F10O/N2/O2 for 60 times breakdown were around 12.8 kV with σ = 0.40. For gas mixture with 0.5% oxygen, a few data points deviated from 12.83 kV, and σ = 0.46; with an increase in the oxygen concentration to 7.5%, the amount of the breakdown voltage data deviating from 13.97 kV increased, and σ = 0.83. When the oxygen concentration gradually increased to 19.33%, most of the breakdown voltage values deviated from 16.08 kV, and σ = 1.08.
Overall, the breakdown voltage of C5F10O/N2/O2 increased as the oxygen concentration increased; however, most of the breakdown voltage data deviated from the average breakdown voltage values, and σ became larger; this indicated that the dispersion of gas insulation strength gradually became worse.
3.2 Effect of oxygen concentration on the decomposition characteristics of C5F10O/N2/O2
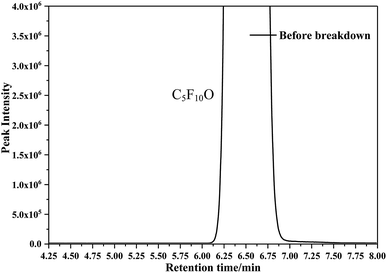
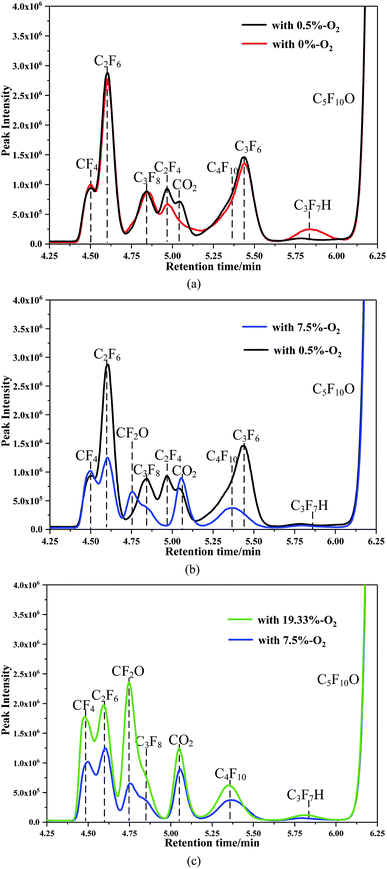
Fig. 5 and 6 show the detection results of the C5F10O/N2/O2 components before and after breakdown obtained by GC/MS in the SCAN mode. As shown in Fig. 5, there are no other impurities in the gas.Fig. 6 shows the component detection results of the C5F10O gas mixture with different oxygen concentrations after 60 times breakdown. Since no substance is detected before 4.4 min and after 7 min, to facilitate the description of the discharge decomposition components of the C5F10O gas mixture, only the GC/MS scan results from 4.25 to 6.25 min are shown in Fig. 6. Considering that the breakdown voltage is positively related to the energy generated during breakdown, we have plotted the scanning results of the discharge decomposition components of the C5F10O gas mixture at different oxygen concentrations in the same figure in turn. Therefore, the yield of each component can be compared intuitively by the peak area of each component.
As shown in Fig. 6a, when the oxygen concentration was 0%, the characteristic peaks of CF4, C2F6, C3F8, C2F4, C4F10, C3F6 and C3F7H were found. Moreover, the peak area of C2F6 is largest. The peak area of CF4 is significantly less than that of C2F6. However, when 0.5% oxygen was added to the gas mixture, the content of C3F7H was reduced relative to the case when the oxygen concentration was 0%. The content of other components is close to that of the C5F10O gas mixture without oxygen.
As shown in Fig. 6b, when the oxygen concentration is increased from 0.5% to 7.5%, the content of C2F6 is greatly reduced, and the peak area of CF4 is close to that of C2F6. Moreover, the contents of C3F8 and C4F10 are greatly reduced. In addition, C2F4 and C3F6 are not detected in the gas mixture with 7.5% oxygen, and a small amount of CF2O is detected at 4.757 min. The content of CO2 increased when compared with that of the gas mixture with 0.5% oxygen. The contents of most of the decomposition components of the C5F10O gas mixture with 7.5% oxygen are lower than those of the gas mixture with 0.5% oxygen, except for CF2O and CO2. Thus, the addition of oxygen inhibits the formation of macromolecules such as C2F6, C3F8, C4F10, and C3F7H.
As shown in Fig. 6c, when the oxygen content increases from 7.5% to 19.33%, the peak area of CF4 is closer to that of C2F6. The peak areas of CF4, C2F6, CF2O, C3F8, CO2, C4F10 and C3F7H increased to different extents. Among them, the peak area of CF2O is significantly increased. Note that oxygen cannot be considered to promote the production of C2F6, C3F8, C4F10, etc. As the oxygen concentration increases, the breakdown voltage increases as well; thus, the greater energy generated during the discharge breakdown causes more C5F10O to decompose; this results in an increase in the decomposition of the C5F10O gas mixture.
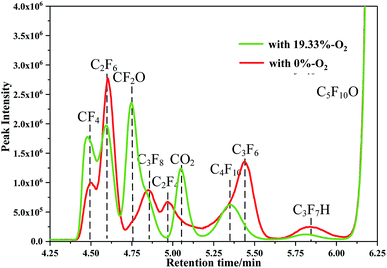
To more clearly illustrate the inhibitory effect of oxygen on the formation of C2F6, C3F8, C4F10 and C3F7H, the results of the decomposition components of the C5F10O gas mixtures with the oxygen concentrations of 0% and 19.33% were compared, as shown in Fig. 7.
As shown in Fig. 6c, when the oxygen concentration increased from 7.5% to 19.33%, the breakdown voltage increased by 2.11 kV; this resulted in a significant increase in the contents of the decomposition components of the C5F10O gas mixture. However, as shown in Fig. 7, the breakdown voltage of C5F10O/N2 increased from 12.8 kV to 16.08 kV (an increase by 3.28 kV) when the oxygen concentration was increased from 0% to 19.33%. However, the contents of C2F6, C3F8, C4F10 and C5F12 for the C5F10O gas mixture with 19.33% oxygen were not more than those of the C5F10O gas mixture without oxygen addition.
The electrode surfaces with and without oxygen were also compared. As shown in Fig. 8, it was found that a small amount of black substance was adhered to the surface for the gas mixture without oxygen addition after 60 times of breakdown (Fig. 8 left). When the oxygen concentration was 0.5% or more, only discharge ablation traces could be observed (Fig. 8 right). Therefore, it can be believed that oxygen can inhibit the precipitation of carbon black when C5F10O decomposes. This may be because the generated carbon particles in the discharge can react with oxygen to produce carbon dioxide in the presence of a high-energy electric field.
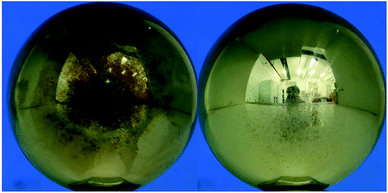 | ||
| Fig. 8 (Left) Electrode surface after 60 times breakdown of C5F10O/N2 (right) electrode surface after 60 times breakdown of C5F10O/N2/O2. | ||
Moreover, when the oxygen concentration was 0.5%, C2F4 and C3F6 still existed, and CF2O was not detected. When the oxygen concentration was increased to 7.5% or more, C2F4 and C3F6 were not detected in the decomposition components, whereas CF2O began to appear, and its content increased with an increase in oxygen concentration.
Under certain conditions, O2 will react with C2F4 and C3F6. Several studies have reported the reaction between O atoms and C2F4 or C3F6. In the literature,40 a mercury lamp was used to irradiate N2O to produce O atoms, which could react with C2F4 or C3F6 and produce CF2O. Moreover, in literature,41 it has been mentioned that the carbon–carbon double bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) of C2F4 and C3F6 weakens after ionization. Another study42 has pointed out that the O atoms can attack C
C) of C2F4 and C3F6 weakens after ionization. Another study42 has pointed out that the O atoms can attack C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in C3F6 leading to the generation of CF2O and CF3CF:. Moreover, CF3CF: will rapidly undergo spin relaxation and then form C2F4. Due to the difference in the experimental conditions, these studies can only provide reference for the mechanism of CF2O production in our experiments. Based on these studies as well as our team's computational study on the mechanism of CF2O production when studying the decomposition characteristics of CF3I,43 the following pathways can be obtained.
C in C3F6 leading to the generation of CF2O and CF3CF:. Moreover, CF3CF: will rapidly undergo spin relaxation and then form C2F4. Due to the difference in the experimental conditions, these studies can only provide reference for the mechanism of CF2O production in our experiments. Based on these studies as well as our team's computational study on the mechanism of CF2O production when studying the decomposition characteristics of CF3I,43 the following pathways can be obtained.
| O2 + e → O˙ + O− | (2) |
| C3F6 + O → CF3CF: + CF2O | (3) |
| CF3CF: → C2F4 | (4) |
| C2F4 → 2CF2: | (5) |
| CF2:+ O2 → CF2O + O˙ | (6) |
4 Discussion
The uniformity of the electric field is vulnerable to the conductive particles in the insulating gap and causes local aggrandizement of the electric field.26 Although under our experimental conditions, the carbon black adhered on the electrodes had less effect on the dispersion of the breakdown voltage, with an increase in the operating voltage and time, the amount of carbon black increased, and when it accumulated to a certain extent, the insulation safety of the equipment was seriously threatened.Note that CF2O is highly toxic, and its LC50 (Lethal Concentration 50, 4 h) value is only 270 mg m−3 (inhaled by rats). According to the Globally Harmonized System of Classification and Labelling of Chemicals toxicity classification standard, the acute inhalation toxicity of CF2O is level 1. It has a strong stimulating effect on respiratory mucosa and can cause chemical pneumonia, pulmonary edema, and acute poisoning. Inevitably, the equipment contains a small amount of water, and CF2O itself is highly corrosive and easily reacts with water to release highly toxic corrosive gases such as HF, thereby affecting the operating life of the equipment. However, note that although CF2O is highly toxic, CF2O is irritating as well, and the occurrence of gas leakage is easily detected in time. In addition, CF2O is soluble in water and ethanol; thus, the waste gas can be treated harmlessly. Therefore, the toxicity of the decomposition products of a C5F10O mixture will not affect its application in the power industry to a large extent.
Although the breakdown voltage of C5F10O/N2/O2 increases with an increase in oxygen concentration and there is no inflection point value in the curve of breakdown voltage and oxygen concentration, this does not mean that it is reasonable to increase the oxygen concentration or even replace all N2 to increase the insulation strength of C5F10O/N2/O2. In fact, when the oxygen concentration is 7.5% (C5F10O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 is 1
O2 is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the breakdown voltage of C5F10O/N2/O2 reaches 53.20% that of SF6 under the same conditions (the power frequency breakdown voltage of SF6 tested herein is 26.26 kV). It is possible to achieve the insulation requirements of the SF6 equipment by increasing the pressure of the C5F10O gas mixture.44 In addition, a partial discharge will inevitably occur during the long-term operation of the equipment, which will lead to the decomposition of the insulation medium. If C5F10O/N2/O2 with higher insulation strength is pursued by further increasing the oxygen concentration, the service life of the equipment and the safety of the field personnel will be greatly threatened. For this reason, it is recommended to use oxygen as the second buffer gas for C5F10O; however, it is not recommended to increase the dielectric strength of C5F10O/N2/O2 by greatly increasing the oxygen concentration. In the practical application of C5F10O/N2/O2, the 1
1), the breakdown voltage of C5F10O/N2/O2 reaches 53.20% that of SF6 under the same conditions (the power frequency breakdown voltage of SF6 tested herein is 26.26 kV). It is possible to achieve the insulation requirements of the SF6 equipment by increasing the pressure of the C5F10O gas mixture.44 In addition, a partial discharge will inevitably occur during the long-term operation of the equipment, which will lead to the decomposition of the insulation medium. If C5F10O/N2/O2 with higher insulation strength is pursued by further increasing the oxygen concentration, the service life of the equipment and the safety of the field personnel will be greatly threatened. For this reason, it is recommended to use oxygen as the second buffer gas for C5F10O; however, it is not recommended to increase the dielectric strength of C5F10O/N2/O2 by greatly increasing the oxygen concentration. In the practical application of C5F10O/N2/O2, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of C5F10O
1 ratio of C5F10O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 can be considered.
O2 can be considered.
5 Conclusion
In this study, the breakdown voltages of C5F10O/N2/O2 at different oxygen concentrations were tested by a gas insulation performance test and gas composition analysis platform. The dispersion of the C5F10O/N2/O2 discharge breakdown voltage was analyzed, and the decomposition components were compared. The following conclusions can be obtained:(1) When the absolute pressure is 0.2 MPa and the partial pressure of C5F10O is 15 kPa, the breakdown voltage of C5F10O/N2/O2 and its dispersion increase with an increase in oxygen concentration under a slightly uneven electric field.
(2) The carbon precipitates on the electrode surfaces disappear after multiple discharge breakdowns when 0.5% or more O2 is added to the gas mixture.
(3) With an increase in the oxygen concentration, the peak area of CF4 in the C5F10O/N2/O2 gas mixture gradually approaches that of C2F6. When the oxygen content reaches 7.5%, C2F4 and C3F6 disappear due to reaction with oxygen to form CF2O. The addition of oxygen inhibits the formation of C2F6, C3F8, C4F10 and C3F7H to a certain extent.
(4) CF2O generated after the C5F10O/N2/O2 discharge breakdown is highly corrosive and extremely toxic, which is harmful to the equipment and personnel. Therefore, it is not preferable to increase the dielectric strength of C5F10O/N2/O2 by further increasing the oxygen concentration. The recommended C5F10O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 ratio is 1
O2 ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The current work was supported by the National Natural Science Foundation of China (No. 51707137, 51877157).References
- D. Chen, X. Zhang and J. Tang, et al., Using Single-Layer HfS2 as Prospective Sensing Device Toward Typical Partial Discharge Gas in SF6-Based Gas-Insulated Switchgear, IEEE Trans. Electron Devices, 2019, 66(1), 689–695 CAS.
- J. Chu, X. Wang and D. Wang, et al., Highly selective detection of sulfur hexafluoride decomposition components H2S and SOF2, employing sensors based on tin oxide modified reduced graphene oxide, Carbon, 2018, S0008622318303968 Search PubMed.
- H. Cui, X. Zhang and G. Zhang, et al., Pd-doped MoS2 monolayer: a promising candidate for DGA in transformer oil based on DFT method, Appl. Surf. Sci., 2019, 470, 1035–1042 CrossRef CAS.
- D. Wang, X. Wang and A. Yang, et al., A first principles theoretical study of the adsorption of SF6, decomposition gases on a cassiterite (110) surface, Mater. Chem. Phys., 2018, 212, 453–460 CrossRef CAS.
- D. Chen, X. Zhang and J. Tang, et al., Pristine and Cu decorated hexagonal InN monolayer, a promising candidate to detect and scavenge SF6 decompositions based on first-principle study, J. Hazard. Mater., 2019, 363, 346–357 CrossRef CAS PubMed.
- A. V. Tatarinov, I. V. Bilera and S. V. Avtaeva, et al., Dielectric Barrier Discharge Processing of trans-CF3 CH=CHF and CF3C(O)CF(CF3) 2, Their Mixtures with Air, N2, CO2 and Analysis of Their Decomposition Products, Plasma Chem. Plasma Process., 2015, 35(5), 845–862 CrossRef CAS.
- X. Zhang, Z. Cui and Y. Li, et al., Abatement of SF6 in the presence of NH3 by dielectric barrier discharge plasma, J. Hazard. Mater., 2018, 360, 341–348 CrossRef CAS PubMed.
- P. Simka and N. Ranjan, Dielectric strength of C5 perfluoroketone,//Proceedings of the 19th International Symposium on High Voltage Engineering, Pilsen, Czech Republic, 2015, pp. 23–28 Search PubMed.
- X. Zhang, Y. Li and D. Chen, et al., Dissociative adsorption of environment-friendly insulating medium C3F7CN on Cu (111) and Al (111) surface: a theoretical evaluation, Appl. Surf. Sci., 2018, 434, 549–560 CrossRef CAS.
- Y. Deng, D. Xiao and J. Chen, Insulation performance of CF3I-N2 gas mixtures as alternative for SF6 in GIS/C-GIS, High Voltage Eng., 2013, 39(9), 2288–2293 CAS.
- D. Chen, X. Zhang and J. Tang, et al., Noble metal (Pt or Au)-doped monolayer MoS2 as a promising adsorbent and gas-sensing material to SO2, SOF2 and SO2F2: a DFT study, Appl. Phys. A: Mater. Sci. Process., 2018, 124(2), 194 CrossRef.
- L. G. Christophorou and R. J. Van Brunt, SF6/N2 mixtures: basic and HV insulation properties, IEEE Trans. Dielectr. Electr. Insul., 1995, 2(5), 952–1003 CrossRef CAS.
- H. Cui, X. Zhang and D. Chen, et al., Adsorption mechanism of SF6, decomposed species on pyridine-like PtN3, embedded CNT: a DFT study, Appl. Surf. Sci., 2018, S0169433218309280 Search PubMed.
- J. Xiong, X. Li and J. Wu, et al., Calculations of total electron-impact ionization cross sections for Fluoroketone C5F10O and Fluoronitrile C4F7N using modified Deutsch–Märk formula, J. Phys. D: Appl. Phys., 2017, 50(44), 445206 CrossRef.
- Y. Li, X. Zhang and D. Chen, et al., Theoretical study on the interaction between C5-PFK and Al (1 1 1), Ag (1 1 1): a comparative study, Appl. Surf. Sci., 2019, 464, 586–596 CrossRef CAS.
- C. Zhang, H. Shi and L. Cheng, et al., First principles based computational scheme for designing new SF6 replacements, IEEE Trans. Dielectr. Electr. Insul., 2016, 23(5), 2572–2578 CAS.
- Y. Li, X. Zhang and S. Tian, et al., Insight into the decomposition mechanism of C6F12O-CO2 gas mixture, Chem. Eng. J., 2019, 360, 929–940 CrossRef CAS.
- H. Katagiri, H. Kasuya and H. Mizoguchi, et al., Investigation of the performance of CF3I Gas as a Possible Substitute for SF6, IEEE Trans. Dielectr. Electr. Insul., 2008, 15(5), 1424–1429 CAS.
- Y. K. Deng and D. M. Xiao, Analysis of the insulation characteristics of c-C4F8 and N2 gas mixtures by Boltzmann equation method, Eur. Phys. J.: Appl. Phys., 2012, 57(2), 20801 CrossRef.
- R. Ullah, A. Rashid and A. Rashid, et al., Dielectric characteristic of dichlorodifluoromethane (R12) gas and mixture with N2/air as an alternative to SF6 gas, High Voltage, 2017, 2(3), 205–210 CrossRef.
- D. Chen, X. Zhang and J. Tang, et al., High selective SO2 gas sensor based on monolayer β-AsSb to detect SF6 decompositions, IEEE Sens. J., 2018, 1 Search PubMed.
- Y. Li, X. Zhang and Q. Chen, et al., Study on the thermal interaction mechanism between C4F7N-N2 and copper, aluminium, Corros. Sci., 2019, 153, 32–46 CrossRef CAS.
- Y. Deng and D. Xiao, Analysis of the insulation characteristics of CF3I gas mixtures with Ar, Xe, He, N2, and CO2 using Boltzmann equation method, Jpn. J. Appl. Phys., 2014, 53(9), 096201 CrossRef.
- D. Chen, J. Tang and X. Zhang, et al., Detecting decompositions of sulfur hexafluoride using MoS2 monolayer as gas sensor, IEEE Sens. J., 2018, 1 Search PubMed.
- Y. Li, X. Zhang and J. Zhang, et al., Assessment on the toxicity and application risk of C4F7N: a new SF6 alternative gas, J. Hazard. Mater., 2019, 368, 653–660 CrossRef CAS PubMed.
- L. Niemeyer, A systematic search for insulation gases and their environmental evaluation,//Gaseous Dielectrics VIII. Springer, Boston, MA, 1998, pp. 459–464 Search PubMed.
- Y. Li, X. Zhang and S. Xiao, et al., Decomposition properties of C4F7N/N2 gas mixture: an environmentally friendly gas to replace SF6, Ind. Eng. Chem. Res., 2018, 57(14), 5173–5182 CrossRef CAS.
- Y. Fu, X. Wang and X. Li, et al., Theoretical study of the decomposition pathways and products of C5-perfluorinated ketone (C5 PFK), AIP Adv., 2016, 6(8), 085305 CrossRef.
- M. Saxegaard, M. Kristoffersen and P. Stoller, et al., Dielectric properties of gases suitable for secondary medium voltage switchgear, CIRED Paper, 2015, p. 926 Search PubMed.
- M. Kristoffersen, T. Endre and M. Saxegaard, et al., Ring main units with eco-efficient gas mixtures: field experience, CIRED-Open Access Proceedings Journal, 2017, 2017(1), 412–415 CrossRef.
- F. Wang, X. Fu and G. Han, et al., Insulation performance of C5F10O/CO2 gas mixture, Gaodianya Jishu, 2017, 43(3), 715–720 Search PubMed.
- M. Aints, I. Jõgi and M. Laan, et al., Effective ionization coefficient of C5 perfluorinated ketone and its mixtures with air, J. Phys. D: Appl. Phys., 2018, 51(13), 135205 CrossRef.
- A. Chachereau, A. Hösl and C. M. Franck, Electrical insulation properties of the perfluoroketone C5F10O, J. Phys. D: Appl. Phys., 2018, 51(33), 335204 CrossRef.
- X. Zhang, Y. Li and S. Xiao, et al., Decomposition mechanism of C5F10O: an environmentally friendly insulation medium, Environ. Sci. Technol., 2017, 51(17), 10127–10136 CrossRef CAS PubMed.
- X. Zhang, Y. Li and S. Tian, et al., Decomposition mechanism of the C5-PFK/CO2 gas mixture as an alternative gas for SF6, Chem. Eng. J., 2018, 336, 38–46 CrossRef CAS.
- M. Hyrenbach, T. A. Paul and J. Owens, Environmental and safety aspects of airplus insulated GIS, CIRED-Open Access Proceedings Journal, 2017, 2017(1), 132–135 CrossRef.
- Z. Guo, X. Li and B. Li, et al., Dielectric properties of C5-PFK mixtures as a possible SF6 substitute for MV power equipment, IEEE Trans. Dielectr. Electr. Insul., 2019, 26(1), 129–136 Search PubMed.
- P. C. Stoller, C. B. Doiron and D. Tehlar, et al., Mixtures of CO2 and C5F10O perfluoroketone for high voltage applications, IEEE Trans. Dielectr. Electr. Insul., 2017, 24(5), 2712–2721 CAS.
- L. Zhong, M. Rong and X. Wang, et al., Compositions, thermodynamic properties, and transport coefficients of high-temperature C5F10O mixed with CO2 and O2 as substitutes for SF6 to reduce global warming potential, AIP Adv., 2017, 7(7), 075003 CrossRef.
- K. A. Kobe, The properties of gases and liquids, Phys. Today, 1959, 12(4), 38–40 Search PubMed.
- G. K. Jarvis, K. J. Boyle and C. A. Mayhew, et al., Threshold Photoelectron−Photoion Coincidence Spectroscopy of Perfluorocarbons. 2. Unsaturated and Cyclic Perfluorocarbons C2F4, C3F6, 2-C4F8, and c-C4F8, J. Phys. Chem. A, 1998, 102(19), 3230–3237 CrossRef CAS.
- D. Saunders and J. Heicklen, Some Reactions of Oxygen Atoms. I. C2F4, C3F6, C2H2, C2H4, C3H6, 1-C4H8, C2H6, c-C3H6, and C3H81a, J. Phys. Chem., 1966, 70(6), 1950–1958 CrossRef CAS.
- S. Xiao, Y. Li and X. Zhang, et al., Formation mechanism of CF3I discharge components and effect of oxygen on decomposition, J. Phys. D: Appl. Phys., 2017, 50(15), 155601 CrossRef.
- X. Li, D. Yun and H. Zhao, et al., Insulation Performance and Application of Environment-friendly Gases Mixtures of C4F7N and C5F10O with CO2, High Voltage Eng., 2017, 43(3), 708–714 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |