 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Apparent power-law behavior of water's isothermal compressibility and correlation length upon supercooling†
Alexander
Späh
a,
Harshad
Pathak
 a,
Kyung Hwan
Kim
a,
Fivos
Perakis
a,
Kyung Hwan
Kim
a,
Fivos
Perakis
 a,
Daniel
Mariedahl
a,
Katrin
Amann-Winkel
a,
Daniel
Mariedahl
a,
Katrin
Amann-Winkel
 a,
Jonas A.
Sellberg
a,
Jonas A.
Sellberg
 b,
Jae Hyuk
Lee
c,
Sangsoo
Kim
c,
Jaehyun
Park
c,
Ki Hyun
Nam
c,
Tetsuo
Katayama
d and
Anders
Nilsson
b,
Jae Hyuk
Lee
c,
Sangsoo
Kim
c,
Jaehyun
Park
c,
Ki Hyun
Nam
c,
Tetsuo
Katayama
d and
Anders
Nilsson
 *a
*a
aDepartment of Physics, AlbaNova University Center, Stockholm University, SE-10691 Stockholm, Sweden. E-mail: andersn@fysik.su.se
bBiomedical and X-Ray Physics, Department of Applied Physics, AlbaNova University Center, KTH Royal Institute of Technology, SE-10691 Stockholm, Sweden
cPohang Accelerator Laboratory, Pohang, Gyeongbuk 37673, Republic of Korea
dJapan Synchrotron Radiation Research Institute, Kouto 1-1-1, Sayo, Hyogo 679-5198, Japan
First published on 14th November 2018
Abstract
The isothermal compressibility and correlation length of supercooled water obtained from small-angle X-ray scattering (SAXS) were analyzed by fits based on an apparent power-law in the temperature range from 280 K down to the temperature of maximum compressibility at 229 K. Although the increase in thermodynamic response functions is not towards a critical point, it is still possible to obtain an apparent power law all the way to the maximum values with best-fit exponents of γ = 0.40 ± 0.01 for the isothermal compressibility and ν = 0.26 ± 0.03 for the correlation length. The ratio between these exponents is close to a value of ≈0.5, as expected for a critical point, indicating the proximity of a potential second critical point. Comparison of γ obtained from experiment with molecular dynamics simulations on the iAMOEBA water model shows that it would be located at pressures in the neighborhood of 1 kbar. The high value and sharpness of the compressibility maximum observed in the experiment are not reproduced by any of the existing classical water models, thus inviting further development of simulation models of water.
Introduction
Water behaves like a normal liquid at high temperatures but when cooled to lower temperatures, it shows anomalies in its thermodynamic properties such as density, heat capacity and compressibility which are enhanced upon decreasing the temperature further.1 At deeply supercooled temperatures, these properties seem to diverge. Speedy and Angell associated this divergence with an underlying thermodynamic singularity and described water's isothermal compressibility (κT) and other properties by a power law.2 Later, many more of water's anomalous quantities, including correlation length (ξ), have been fitted by power laws with a vast majority yielding singularity temperatures (TS) close to 228 K.3–6 However, the reliability of such power-law fits and the existence of a hypothesized singularity had been questioned since homogeneous nucleation of ice precluded measurements close enough to the proposed TS.7–11To date, the liquid–liquid critical point (LLCP) scenario12 is the most supported scenario13–16 among other models,17–19 which potentially explains water's anomalies. In this scenario, the hypothesized singularity is explained as a second critical point between two local and fluctuating configurations in the liquid – a high-density liquid (HDL) and a low-density liquid (LDL). According to the LLCP model, at low temperatures and elevated pressures, a LDL and a HDL exist as pure phases separated by a phase-coexistence line which terminates at the LLCP. Beyond the LLCP, at lower pressures, water is characterized by fluctuations between these HDL and LDL local structures.1,20 The locus of maxima in ξ of these fluctuations defines the Widom line in the pressure–temperature phase diagram, which emanates from the LLCP as an extension of the phase-coexistence line. Near the Widom line, also other lines of thermodynamic response function maxima exist and merge with the Widom line in close proximity to the critical point.21
In a study by Kim et al.13 such maxima in κT and ξ have been found close to 229 K, using small-angle X-ray scattering (SAXS) on evaporative cooled micron-sized water droplets22 in order to achieve temperatures all the way down to the proposed singularity temperature. This study has been criticized23 but all issues could be addressed.24 Interestingly, the temperature of maxima in κT and ξ was found to be very close to the TS of 228 K that had been proposed earlier. This discrepancy between the finite, experimentally observed maxima and the divergence to infinity that would have been anticipated from the power-law extrapolation raises questions about the shape of the maxima in κT and ξ as a function of temperature. In particular, investigation on the behavior of κT and ξ around their maxima together with comparison to simulations may hint to the location of the LLCP in terms of pressure, if existent.
In the present study, we apply the commonly used power-law analysis to κT and ξ, but using the extended temperature range down to 229 K that has been reported recently.13 To stress that there is no critical point at ambient pressure and since power laws can only be fundamentally fitted close to a critical point, we will here instead use an apparent power law in the spirit of Speedy and Angell as a more empirical fit to investigate the steepness of the increase of the thermodynamic response functions. For κT and ξ, we use the notations25
| κT = κT,0ε−γ and ξ = ξ0ε−ν, | (1) |
Results and discussion
A. Apparent power-law analysis of experimental data
Fig. 1 shows κT and ξ of liquid H2O down to ≈250 K2,3,26 from thermodynamic measurements together with data obtained in the experiment by Kim et al.13 with temperatures supercooled down to ≈227 K. We note that the maximum in both κT and ξ is rather sharp which makes it difficult to visualize in the broad plotted temperature range that is shown here (for more details see ref. 13). For temperatures below 250 K the power-law extrapolation given by Speedy and Angell, shown as the black dashed line in Fig. 1(a), deviates from the κT measurements reported by Kim et al.13 The temperature determination of the water droplets reported by Kim et al. is calculated using a ballistic evaporation model, see the ESI of ref. 13. Here we want to give a brief validation of the temperature estimate given by Kim et al. based on homogeneous nucleation rates, and a more detailed discussion on the temperature validation can be found in Section I of the ESI.† If we assume the temperature calibration by Kim et al. to yield too low temperature estimates and instead assume the temperature to match the power-law fit of Speedy and Angell, it would mean that the lowest temperature given by Kim et al.13 is close to 234 K. However, with reported nucleation rates below 1010 cm−3 s−1 close to 234 K,27 we would expect a negligible fraction of the water droplets to crystallize before they are probed by the X-ray pulses. This would be in conflict with the very high crystallization probability that was observed for the lowest temperatures in the experiment by Kim et al. The factor 103 higher homogeneous nucleation rates around 228 K28 are instead in full agreement with the observed nucleation probabilities at the lowest temperatures. We point out that the data reported by Kim et al. is derived only from liquid water droplets where all ice containing events have been sorted out.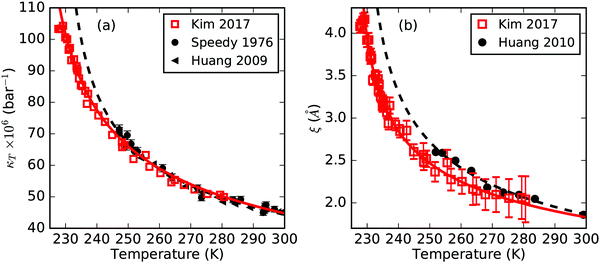 | ||
| Fig. 1 Isothermal compressibility, κT, (a) and correlation length, ξ, (b) obtained from SAXS on supercooled water droplets13 (squares). Error bars for κT are smaller than the symbol size. Literature data from Speedy and Angell2 and Huang et al.3,26 (full symbols) are shown for comparison. Apparent power-law fits with a singularity temperature TS of 228 K, reported by Speedy and Angell and Huang et al., are shown as dashed lines. Solid lines depict the least-squares power-law fits weighted by error bars for all shown data from the temperature of maximum κT or ξ up to 300 K. | ||
The ξ values measured by Huang et al.3 are slightly larger than the current data, as shown in Fig. 1(b). Towards colder temperatures, the enhancement at low-q rapidly grows and the choice of normal and anomalous components becomes increasingly independent, resulting in small error-bars of ξ. Using a slightly extended q-range to higher q-values, we found a slightly lower ξ for the high-temperature data measured at PAL-XFEL than that reported previously.13 However, we note that the trend of increased ξ towards lower temperatures is unaffected. For comparison, the black dashed line in Fig. 1(b) again indicates an apparent power-law fit with the suggested TS of 228 K,3 deviating from the experimental data at low temperatures. Nevertheless, it is still possible to fit κT and ξ by an apparent power-law including the extended temperature range, shown as solid lines.
We note that previous studies on κT of water4,29 first subtracted a normal component, which resembles the behavior of a normal liquid, before the remaining anomalous component was analyzed by an apparent power law. Following this procedure, it was found to yield unreasonably high exponents (see Section II of the ESI†). Therefore, instead of segregating the normal and anomalous components, we decided to limit the temperature range for our apparent power-law analysis to the region where water's anomalous behavior is clearly apparent. For near-ambient pressures, this corresponds to temperatures ranging from the temperature of maximum κT or ξ at 229 K all the way up to the highest measured temperature of 280 K.
Even though the reported data were measured close to ambient pressure and thus far away from the hypothesized LLCP, it is possible to fit an apparent power law within measurement uncertainty up to 300 K, shown as solid lines in Fig. 1. Interestingly we do not see a deceleration in the increase of κT or ξ before they reach their maximum values at 229 K, but instead find an accelerated increase with decreasing temperature according to an apparent power-law behavior all the way up to the maximum.
The best-fit exponents for κT and ξ are γ = 0.40 and ν = 0.26, respectively, and are summarized in Table 1 together with their singularity temperatures TS. The relatively small exponents obtained from the apparent power-law fits are in agreement with a moderate divergent behavior of the thermodynamic properties at ambient pressures and an LLCP at high positive pressure. At the LLCP the exponents are expected to become large and reach values of γ ≈ 1.24 for κT and ν ≈ 0.63 for ξ.30 The general relationship of ν/γ = 1/(2 − η) ≈ 1/2 at a critical point is thus not exactly fulfilled in the current temperature and pressure regime, and instead the ratio ν/γ of the exponents is close to 0.65. We also note that our best-fit results yield different TS values for κT and ξ as reported in Table 1.
| T S (K) | κ T,0 (10−6 bar−1), ξ0 (Å) | γ, ν | |
|---|---|---|---|
| κ T | 219.6 ± 0.6 | 29.9 ± 0.4 | 0.40 ± 0.01 |
| ξ | 225.9 ± 0.8 | 1.36 ± 0.08 | 0.26 ± 0.03 |
It is also important to point out that the general interpretation of the singularity temperature TS in the apparent power-law analysis implies a divergence of the thermodynamic response or correlation functions to infinity and is thus very different from the temperature TW when approaching the Widom line, where the thermodynamic properties experience a finite maximum instead. In this context, TS obtained from the apparent power-law fit is directly connected to the exponent. Smaller exponents correspond to a slower increase with temperature followed by a sudden divergence upon approaching the singularity temperature TS. We would therefore expect a TS that is closer to TW, as is the case for ξ, to yield a smaller exponent compared to the obtained exponent for κT. In fact, only in the vicinity of the LLCP, the approximation of the thermodynamic properties by a power law is accurate and TS and TW coincide. This is further illustrated by the discrepancy of the experimental data compared to the apparent power-law fit when fixing TS to the reported temperature of the maxima in κT or ξ, shown as dashed lines in Fig. 1. The surprisingly close agreement between the experimentally observed temperature of maximum κT at 229 K and the proposed TS of 228 K by Angell and coworkers2 is most likely caused by the slightly too high κT at their lowest temperatures and has already been noted by the authors themselves providing their proposed TS of 228 K with rather large uncertainty.
B. Apparent power-law analysis applied to molecular dynamics simulations
It is of interest to evaluate the exponent of the apparent power-law fits of κT for various pressures and to compare the experimental data to the iAMOEBA water model.31 We note that none of the current MD models shows an accurate description of the experimental κT data. However, we chose this water model since it is known to equilibrate relatively fast even at temperatures below 230 K. In addition, it was found to present the structure of supercooled water favorably in comparison to several other MD models.32Fig. 2(a) shows the simulation data of κT at elevated pressures with increasing divergence close to the LLCP around 1700 bar. Apparent power-law fits are indicated as solid lines. The highest cut-off temperatures that are used for the fits relate approximately to the extent of the anomalous region at various pressures. Thus, closer to the critical point, we chose a narrower temperature range for the fit. The obtained singularity temperatures (TS) and exponents (γ) are shown in Fig. 2(b). We find a linear behavior of TS with pressure as a good approximation and find γ approaching a reasonable value around γ ≈ 1.24 (although with large uncertainty) close to the critical pressure of the iAMOEBA model, which adds further confidence to the chosen temperature ranges.
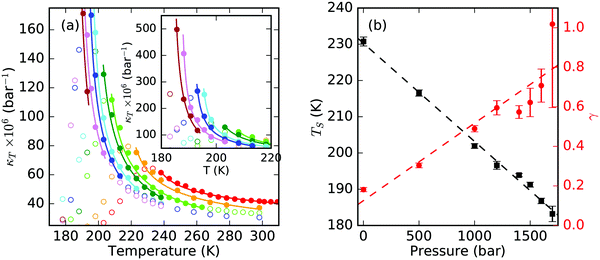 | ||
| Fig. 2 (a) Isothermal compressibility, κT, of iAMOEBA water for pressures of 1, 500, 1000, 1200, 1400, 1500, 1600 and 1700 bar,13 shown in colors from red to brown, respectively. The error bars are not shown for simplification. The inset shows κT at a de-magnified scale for the lower part of the temperature range. Solid lines are apparent power-law fits to the κT data shown as solid points (hollow points are not used for fits). (b) Singularity temperatures (black squares) and exponents (red dots) obtained from apparent power-law fits for κT of the iAMOEBA water model. Dashed lines are a guide to the eye. | ||
The apparent power-law exponent reaches the experimental value of γ = 0.40 around 750 bar, which is approximately 1 kbar away from the LLCP of the iAMOEBA water model. We therefore estimate the LLCP to be in the neighborhood of 1 kbar in real water, assuming the same slope of γ as a function of pressure. A similar pressure of 800 bar was found based on the variation of the κT maximum with pressure.13 Thereby, the location of the critical point in real water is estimated to exist at lower pressures than for the MD model. This indicates that the ratio between hydrogen-bond cooperativity and hydrogen-bond strength is much higher for real water.33
Fig. 3(a) depicts κT of several MD water models, mW,36 SPCE,37 TIP4P/2005,38 E3B339 and iAMOEBA,31 as well as the experimental findings. Although most of the models reproduce κT above the melting temperature reasonably well, they deviate in their prediction upon supercooling. All of them underestimate κT compared to real water and exhibit only rather round maxima with respect to temperature as compared to experiments. The corresponding apparent power-law fits, shown as solid lines, yield very small apparent power-law exponents that are depicted in Fig. 3(b). In contrast, the experimental κT exhibits a strong and accelerated increase upon supercooling. To our knowledge the MB-pol water model40 seems to have the best description of κT but is left out in this discussion, since it is not clear whether the model shows a maximum at deeply supercooled temperatures where it is difficult to equilibrate the simulations. We also note that the WAIL water model41 predicts an extremely high κT maximum that is beyond the experimental value. This is due to the close proximity of the LLCP, which lies at 500 bar for this model. Because of sparse data available for this model, it is also left out of the following discussion.
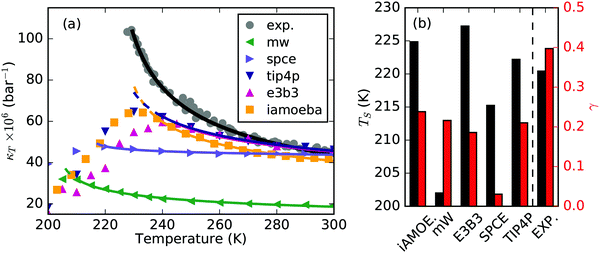 | ||
| Fig. 3 (a) Isothermal compressibility, κT, of various MD water models,32,34,35 mW, SPCE, TIP4P/2005, E3B3 and iAMOEBA, compared to experimental values. Solid lines show the corresponding apparent power law fits in the temperature range from 300 K down to the temperature of maximum κT. (b) Singularity temperatures and exponents obtained from the corresponding apparent power law fits given in (a). | ||
In addition to the underestimated κT, all the depicted MD water models show a very broad maximum with respect to temperature, as compared to the sharp maximum that is observed experimentally. Although the experimental data exhibit an apparent power-law behavior all the way to the maximum, the simulation data start to round off and turn into a broad maximum already at temperatures 5–10 K before it reaches its maximum value. We can partially attribute the broad maximum in the simulations to the large distance in terms of pressure to the location of the LLCP in the models. Also, the choice of particular simulation conditions, i.e. system size, simulation time step, simulation and relaxation times, may partially contribute to a smoothening of the maxima in the simulations. In Fig. 3(a), the rounding-off is best seen for the iAMOEBA and TIP4P/2005 water models, where the dashed lines are continuations of the apparent power law fits.
The sharp maximum that is observed experimentally around the Widom line can be an indication of an increased clustering of hydrogen bonds due to increased intermolecular water–water interaction energy, which has been interpreted in terms of a λ-like transition10 or by the concept of cooperative hydrogen bond connectivity.33 In particular, experimental data at lower temperatures would be necessary for quantitative analysis on the sharpness of the κT maximum. On the other hand, the broad maximum in the simulation data might be related to too small simulation boxes, which limit the growth of the fluctuating structural regions. Also, longer equilibration times might be necessary at these low temperatures to allow for the heterogeneities to develop. However, simulation data for more densely spaced temperature intervals close to the Widom line are necessary to study the shape around the maximum in detail.
Conclusions
We studied the anomalous increase in isothermal compressibility (κT) and correlation length (ξ) of pure water upon supercooling from the data taken from Kim et al.13 We made use of the previously inaccessible low temperature region below 250 K, which allows for apparent power-law analysis without artificial subtraction of a normal component. The results show that the data can be fitted to an apparent power law within measurement uncertainty and for the temperature range from 300 K down to the temperature of maximum κT. From the least-squares apparent power-law fits for ξ and κT, we obtain the ratio of their exponents, ν/γ = 0.65, which is relatively close to the ratio ν/γ ≈ 0.51 that would be expected exactly at a critical point.30 In general, the anomalous increase in κT observed in MD simulations is significantly smaller than that found experimentally. Comparison of γ obtained from experiment and from MD simulations on the iAMOEBA water model indicates an LLCP located at pressures in the neighborhood of 1 kbar. The high value and sharpness of the κT maximum that is observed experimentally are not reproduced by any of the MD simulations, suggesting a slightly different behavior near the Widom line not predicted by the models. Both experiments at even lower temperatures and simulations with larger simulation-box size and much finer temperature resolution would be valuable to further investigate the sharpness of the κT maximum.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work has been supported by the European Research Council (ERC) Advanced Grant under Project No. 667205 and the Swedish Research Council (VR) under Grant No. 2013-8823.References
- A. Nilsson and L. G. M. Pettersson, Nat. Commun., 2015, 6, 8998 CrossRef CAS.
- R. J. Speedy and C. A. Angell, J. Chem. Phys., 1976, 65, 851–858 CrossRef CAS.
- C. Huang, T. M. Weiss, D. Nordlund, K. T. Wikfeldt, L. G. M. Pettersson and A. Nilsson, J. Chem. Phys., 2010, 133, 134504 CrossRef.
- H. Kanno and C. A. Angell, J. Chem. Phys., 1979, 70, 4008–4016 CrossRef CAS.
- C. A. Angell, W. J. Sichina and M. Oguni, J. Phys. Chem., 1982, 86, 998–1002 CrossRef CAS.
- A. Taschin, P. Bartolini, R. Eramo, R. Righini and R. Torre, Nat. Commun., 2013, 4, 2401 CrossRef CAS.
- D. H. Rasmussen and A. P. MacKenzie, J. Chem. Phys., 1973, 59, 5003–5013 CrossRef CAS.
- D. E. Hare and C. M. Sorensen, J. Chem. Phys., 1986, 84, 5085–5089 CrossRef CAS.
- P. Taborek, Phys. Rev. B, 1985, 32, 5902–5906 CrossRef CAS.
- C. A. Angell, J. Shuppert and J. C. Tucker, J. Phys. Chem., 1973, 77, 3092–3099 CrossRef CAS.
- B. J. Mason, Adv. Phys., 1958, 7, 221–234 CrossRef CAS.
- P. H. Poole, F. Sciortino, U. Essmann and H. E. Stanley, Nature, 1992, 360, 324–328 CrossRef CAS.
- K. H. Kim, A. Späh, H. Pathak, F. Perakis, D. Mariedahl, K. Amann-Winkel, J. A. Sellberg, J. H. Lee, S. Kim, J. Park, K. H. Nam, T. Katayama and A. Nilsson, Science, 2017, 358, 1589–1593 CrossRef CAS PubMed.
- P. Gallo, K. Amann-Winkel, C. A. Angell, M. A. Anisimov, F. Caupin, C. Chakravarty, E. Lascaris, T. Loerting, A. Z. Panagiotopoulos, J. Russo, J. A. Sellberg, H. E. Stanley, H. Tanaka, C. Vega, L. Xu and L. G. M. Pettersson, Chem. Rev., 2016, 116, 7463–7500 CrossRef CAS PubMed.
- F. Perakis, K. Amann-Winkel, F. Lehmkühler, M. Sprung, D. Mariedahl, J. A. Sellberg, H. Pathak, A. Späh, F. Cavalca, D. Schlesinger, A. Ricci, A. Jain, B. Massani, F. Aubree, C. J. Benmore, T. Loerting, G. Grübel, L. G. M. Pettersson and A. Nilsson, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 8193–8198 CrossRef CAS.
- S. Woutersen, B. Ensing, M. Hilbers, Z. Zhao and C. A. Angell, Science, 2018, 359, 1127–1131 CrossRef CAS.
- R. J. Speedy, J. Phys. Chem., 1982, 86, 982–991 CrossRef CAS.
- P. H. Poole, F. Sciortino, T. Grande, H. E. Stanley and C. A. Angell, Phys. Rev. Lett., 1994, 73, 1632–1635 CrossRef CAS.
- S. Sastry, P. G. Debenedetti, F. Sciortino and H. E. Stanley, Phys. Rev. E, 1996, 53, 6144–6154 CrossRef CAS.
- J. Russo and H. Tanaka, Nat. Commun., 2014, 5, 3556 CrossRef.
- P. Gallo, D. Corradini and M. Rovere, Nat. Commun., 2014, 5, 5806 CrossRef CAS.
- J. A. Sellberg, C. Huang, T. A. McQueen, N. D. Loh, H. Laksmono, D. Schlesinger, R. G. Sierra, D. Nordlund, C. Y. Hampton, D. Starodub, D. P. DePonte, M. Beye, C. Chen, A. V. Martin, A. Barty, K. T. Wikfeldt, T. M. Weiss, C. Caronna, J. Feldkamp, L. B. Skinner, M. M. Seibert, M. Messerschmidt, G. J. Williams, S. Boutet, L. G. M. Pettersson, M. J. Bogan and A. Nilsson, Nature, 2014, 510, 381–384 CrossRef CAS.
- F. Caupin, V. Holten, C. Qiu, E. Guillerm, M. Wilke, M. Frenz, J. Teixeira and A. K. Soper, Science, 2018, 360, eaat1634 CrossRef.
- K. H. Kim, A. Späh, H. Pathak, F. Perakis, D. Mariedahl, K. Amann-Winkel, J. A. Sellberg, J. H. Lee, S. Kim, J. Park, K. H. Nam, T. Katayama and A. Nilsson, Science, 2018, 360, eaat1729 CrossRef PubMed.
- H. E. Stanley, Introduction to Phase Transitions and Critical Phenomena, Oxford University Press, New York, USA, 1971 Search PubMed.
- C. Huang, K. T. Wikfeldt, T. Tokushima, D. Nordlund, Y. Harada, U. Bergmann, M. Niebuhr, T. M. Weiss, Y. Horikawa, M. Leetmaa, M. P. Ljungberg, O. Takahashi, A. Lenz, L. Ojamäe, A. P. Lyubartsev, S. Shin, L. G. M. Pettersson and A. Nilsson, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 15214–15218 CrossRef CAS PubMed.
- B. J. Murray, S. L. Broadley, T. W. Wilson, S. J. Bull, R. H. Wills, H. K. Christenson and E. J. Murray, Phys. Chem. Chem. Phys., 2010, 12, 10380–10387 RSC.
- H. Laksmono, T. A. McQueen, J. A. Sellberg, N. D. Loh, C. Huang, D. Schlesinger, R. G. Sierra, C. Y. Hampton, D. Nordlund, M. Beye, A. V. Martin, A. Barty, M. M. Seibert, M. Messerschmidt, G. J. Williams, S. Boutet, K. Amann-Winkel, T. Loerting, L. G. M. Pettersson, M. J. Bogan and A. Nilsson, J. Phys. Chem. Lett., 2015, 6, 2826–2832 CrossRef CAS.
- O. Conde, J. Teixeira and P. Papon, J. Chem. Phys., 1982, 76, 3747–3753 CrossRef CAS.
- D. Poland and D. Simmons-Duffin, Nat. Phys., 2016, 12, 535–539 Search PubMed.
- L.-P. Wang, T. Head-Gordon, J. W. Ponder, P. Ren, J. D. Chodera, P. K. Eastman, T. J. Martinez and V. S. Pande, J. Phys. Chem. B, 2013, 117, 9956–9972 CrossRef CAS.
- H. Pathak, J. C. Palmer, D. Schlesinger, K. T. Wikfeldt, J. A. Sellberg, L. G. M. Pettersson and A. Nilsson, J. Chem. Phys., 2016, 145, 134507 CrossRef CAS.
- K. Stokely, M. G. Mazza, H. E. Stanley and G. Franzese, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 1301–1306 CrossRef CAS PubMed.
- V. Holten, D. T. Limmer, V. Molinero and M. A. Anisimov, J. Chem. Phys., 2013, 138, 174501 CrossRef.
- Y. Ni and J. L. Skinner, J. Chem. Phys., 2016, 144, 214501 CrossRef.
- V. Molinero and E. B. Moore, J. Phys. Chem. B, 2009, 113, 4008–4016 CrossRef CAS.
- H. J. C. Berendsen, J. R. Grigera and T. P. Straatsma, J. Phys. Chem., 1987, 91, 6269–6271 CrossRef CAS.
- J. L. F. Abascal and C. Vega, J. Chem. Phys., 2005, 123, 234505 CrossRef CAS PubMed.
- C. J. Tainter, L. Shi and J. L. Skinner, J. Chem. Theory Comput., 2015, 11, 2268–2277 CrossRef CAS.
- S. K. Reddy, S. C. Straight, P. Bajaj, C. Huy Pham, M. Riera, D. R. Moberg, M. A. Morales, C. Knight, A. W. Götz and F. Paesani, J. Chem. Phys., 2016, 145, 194504 CrossRef.
- Y. Li, J. Li and F. Wang, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 12209–12212 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cp05862h |
| This journal is © the Owner Societies 2019 |
