Open Access Article
Open Access ArticleSynergistic and simultaneous biosorption of phenanthrene and iodine from aqueous solutions by soil indigenous bacterial biomass as a low-cost biosorbent
D. Zhang *abc,
S. G. Lu*a,
X. Q. Songb,
J. F. Zhangb,
Z. M. Huob and
H. T. Zhao
*abc,
S. G. Lu*a,
X. Q. Songb,
J. F. Zhangb,
Z. M. Huob and
H. T. Zhao c
c
aCollege of Environmental & Resource Sciences, Zhejiang University, Hangzhou, Zhejiang 310058, China. E-mail: zhangdong@hdu.edu.cn; lusg@zju.edu.cn; Tel: +86-571-86919158
bZhejiang Wulong Chemical Industrial Stock Co. Ltd, Deqing, Zhejiang 313201, China
cCollege of Materials and Environmental Engineering, Hangzhou Dianzi University, Hangzhou, Zhejiang 310018, China
First published on 26th November 2018
Abstract
The removal of phenanthrene and iodine from aqueous solutions in single and binary systems by inactivated soil indigenous bacterial biomass (SIBB), as well as affecting factors, were evaluated. Sorption kinetic and isotherm studies were carried out to investigate the synergistic effects of phenanthrene and iodine. Optimal parameters for the biosorption process included a solution pH of 6.0 and biosorbent dosage of 0.75 g L−1. The ionic strength significantly decreased the biosorption of both phenanthrene and iodine in single conditions, while no obvious influences were found in the binary conditions. A pseudo-second-order model was well fitted to the kinetic biosorption data for both phenanthrene and iodine. The results showed that the presence of co-solute accelerated the biosorption processes and the pseudo-second-order biosorption rates (k2) for phenanthrene and iodine increased from 0.005441 to 0.009825 g mg−1 min−1 and from 0.000114 to 0.000223 g mg−1 min−1, respectively. The SIBB showed strong affinity with both phenanthrene and iodine, with a partition coefficient Kd (Linear model) of 6892.4 L kg−1 for phenanthrene and affinity parameter KL (Langmuir model) of 232![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 L kg−1 for iodine. The presence of co-solute illustrated a synergistic effect on the biosorption of phenanthrene and iodine due to intermolecular forces between phenanthrene and iodine, enhancing the Kd of 34.7% for phenanthrene and KL of 107.0% for iodine, respectively. The results suggested that SIBB was an effective material for the simultaneous biosorption of phenanthrene and iodine from aqueous solutions.
500 L kg−1 for iodine. The presence of co-solute illustrated a synergistic effect on the biosorption of phenanthrene and iodine due to intermolecular forces between phenanthrene and iodine, enhancing the Kd of 34.7% for phenanthrene and KL of 107.0% for iodine, respectively. The results suggested that SIBB was an effective material for the simultaneous biosorption of phenanthrene and iodine from aqueous solutions.
1. Introduction
Contaminants harmful to the health of present and future generations involve nuclear fission products such as iodine radioisotopes. Iodine radioisotopes have been released into our environment due to anthropogenic activities such as the nuclear energy industry, nuclear accidents and wastewater discharge containing radioactive iodine.1,2 Iodine is potentially one of the most mobile radioisotopes3,4 and easily reaches the biosphere5 because of its weak affinity with many geological materials, low-organic-content soils and sediments. Therefore, iodine is potentially acutely and chronically toxic due to bioaccumulation through the food chain and may subsequently affect human health adversely.6,7 It is useful to understand the interactions between iodine and natural or artificial materials of the environment in contact with surface waters and groundwater, as well as co-existing pollutants. Several recent studies reported that aromatic structures in natural organic matter (NOM) played an important role in the sorption of iodine to soil and/or sediment.4,8,9 Aromatic carbon in NOM participated in the oxidation of iodine to some form of intermediates, facilitating the formation of aromatic carbon–iodine bonds rather than aliphatic carbon–iodine bonds.8,10In addition, hydrophobic organic compounds (HOCs) are ubiquitous toxic contaminants,11,12 which can be highly likely to co-exist with iodine in soil and aquifer environments near nuclear power plants. These contaminants are highly transferable and easily to interact with soil particles, biota and microorganisms.4,5 Many HOCs, such as polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs), contain two or more aromatic structures. These co-existing pollutants may exhibit the same, or at least similar, interactions with iodine as NOM do. The formation of aromatic carbon–iodine bonds could affect the transport, fate, and removal of iodine and co-existing HOC pollutants, making it much more complex to evaluate their environmental behaviours and ecological risks. However, little information is available about the interactions of iodine and aromatic containing contaminants, as well as the influence on subsequent sorption and removal of the co-existing pollutants.
To overcome (or at least limit the contribution of) this risk, it would be useful to find efficient scavengers for iodine, as well as HOCs. The use of natural biomaterials (served as adsorbents) is considered promising and the most attractive alternative due to their easy handling, versatility, cost-effectiveness, efficiency, low-selectivity and relative abundance compared with conventional techniques.13–15 Studies have reported that dead or metabolically inactive biomass materials were used as successful biosorbents for the treating or removal of dyes,16–19 heavy metals,20–23 HOCs24–26 and radioactive elements such as uranium27,28 from aqueous solutions. Several mechanisms were also proposed and summarized as follows:29 (1) electrostatic binding for some cationic metals, dyes and radioactive elements due to negative groups in biosorbents;16,27,30 (2) hydrophobic interactions and partitioning for hydrophobic organic compounds;26,31 (3) specific molecular-level interactions such as cation-π interactions;32 and (4) deposition on the cell surface or within the cell wall structures.15 Simultaneous removal of metal–metal20 and metal–organic compounds33 were also reported. However, to our knowledge, limited information is available for the biosorption of iodine, as well as the synergistic influence of aromatic compounds.
Therefore, the main objectives of this study were to evaluate the biosorption capability of a low-cost soil indigenous bacterial biomass (SIBB) for the simultaneous removal of phenanthrene (typical PAHs) and iodine as a model of combined pollutants by (1) examining the biosorption potential of phenanthrene and iodine onto SIBB in single and binary systems, and (2) optimizing the parameters affecting the biosorption process.
2. Materials and methods
2.1 Biosorbent and sorbate preparation
The biosorbent was obtained from physically and thermally inactivated soil indigenous bacterial biomass as described in previous works.31,34 The indigenous bacteria were isolated from soil in an agricultural field of Zhejiang, China, near the Qinshan Nuclear Power Plant and then cultured in Lysogeny Broth. After harvest, bacteria were washed three times with deionized water to remove any remaining culture media. To obtain inactive biomass, indigenous bacteria were dried for 24 h at a temperature of 70 °C following mechanical grinding. The surface properties of SIBB material were measured according to our previous work.34 The specific surface of the biosorbent was determined by a gas sorption analyzer (NOVA-1200, Quantachrome Corp., USA). The biosorbent was further characterized using Fourier transform infrared spectroscopy (FTIR, Shimadzu 8400S, Kyoto, Japan).Stable iodine and phenanthrene were selected as representative iodine species and HOCs and were purchased from Aladdin Reagent Co. (China) with purities > 99.0%. Stock solutions (1000 mg L−1 for phenanthrene in methanol and 200 mg L−1 for iodine in deionized water) were prepared and stored in amber glass bottles at 4 °C. Working solutions in the range of 0–1.0 mg L−1 for phenanthrene and 0–200 mg L−1 for iodine in single and binary systems were prepared by serial dilution. NaOH (0.1 mol L−1) and/or HCl (0.1 mol L−1) were used to adjust the pH of solutions. The inorganic reagents used for the growth of the microorganisms such as tryptone, yeast extract, and sodium chloride, were of analytical grade (Aladdin Reagent Co., China).
2.2 Sorption tests and analytical methods
Batch sorption experiments were carried out in a temperature-controlled shaker at 150 rpm at 25 °C, and tubes were prepared in duplicate, as described in our previous work.31 In the kinetic and isotherm sorption experiments, a certain amount of sorbents, 15 mg for SIBB, were weighted into 22 mL centrifuge tubes with 20 mL single iodine, single phenanthrene and their binary solutions. The concentrations of iodine and phenanthrene covered the range of 0–200 mg L−1 and 0–1.0 mg L−1, respectively. Control treatments without biosorbents were prepared to account for the possible solute loss by handling and other possible ways. Single-factor biosorption experiments were also conducted, and other factors were adjusted to suitable conditions: biosorbent dosage at 0.25, 0.5, 0.75, 1.0, and 1.25 g L−1; ionic strength (CaCl2) at 0, 0.1, 0.2, 0.3, 0.4, and 0.5 mol L−1, pH at 3, 4, 5, 6, 7, 8, 9, and 10; and biosorption time of 60, 90, 120, 180, 240, 480, 720, 960, 1200, and 1440 min in single and binary conditions. The sorption equilibrium time was set up according to the biosorption kinetics experiments. At the equilibrium time, after being centrifuged for 20 min at 4000g, the supernatants were filtrated by 0.22 μm filter membranes (ANPEL Co., Ltd., China).The concentration of phenanthrene in the aqueous phase was determined using HPLC (Agilent 1260) equipped with a 4.6 × 250 mm Eclipse PAH column and a fluorescence detector using a methanol and water mixture (v/v, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as mobile phase at a flow rate of 1.0 mL min−1. The excitation and emission wavelengths of phenanthrene were 244 and 360 nm, respectively. The method quantification limit value for phenanthrene was 3.39 μg L−1, and the relative standard deviation for phenanthrene determination was 0.23%. The determination of iodine followed the method described by Madrakian et al.35 and the concentration was measured using a spectrophotometer (Shimadzu UV-2600) at a wavelength of 460 nm.
5) as mobile phase at a flow rate of 1.0 mL min−1. The excitation and emission wavelengths of phenanthrene were 244 and 360 nm, respectively. The method quantification limit value for phenanthrene was 3.39 μg L−1, and the relative standard deviation for phenanthrene determination was 0.23%. The determination of iodine followed the method described by Madrakian et al.35 and the concentration was measured using a spectrophotometer (Shimadzu UV-2600) at a wavelength of 460 nm.
The removal efficiency of phenanthrene and iodine by SIBB was determined using the following equation:
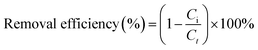 | (1) |
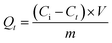 | (2) |
2.3 Kinetic sorption models
To determine the biosorption kinetics, the obtained dynamic experimental data was fitted with several kinetic models, such as the Morris–Weber model, Lagergren model and pseudo-second-order model. The Morris–Weber model36 can be expressed as follows:
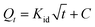 | (3) |
The Lagergren pseudo-first-order model, which was the earliest known equation to explain the adsorption rate based on the adsorption capacity, was also used to stimulate the sorption kinetic data.37 The equation is generally expressed as follows:
lg(Qe − Qt) = lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t Qe − k1t
| (4) |
The non-linear form of the pseudo-second-order model can be written as follows according to Ho:38,39
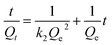 | (5) |
2.4 Isothermal sorption models
The Langmuir, Freundlich, linear, D–R and Temkin models were used to describe the isothermal sorption of phenanthrene and iodine in single and binary systems by the bacterial biomass. The Langmuir isotherm is represented as:
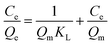 | (6) |
The logarithmic form of the Freundlich model (original form: Qe = KFCe1/n) is used to calculated the Freundlich parameters and is expressed as shown in the following equation:
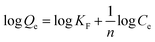 | (7) |
The Linear isotherm model is also used to estimate the sorption of phenanthrene and iodine onto SIBB. The linear model is described as the form of the following equation:
| Qe = KdCe | (8) |
The Dubinin–Radushkevich (D–R) isotherm model is applied to the data to deduce the heterogeneity of the surface energies of adsorption and the characteristic porosity of the biosorbent. The linear form of the D–R model is given as follows:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe = ln Qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xm − βF2 Xm − βF2
| (9) |
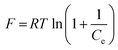 | (10) |
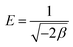 | (11) |
The Temkin isotherm model suggests an equal distribution of binding energies over a number of exchange sites on the surface. The linear form of the Temkin isotherm can be written as:
Qe = B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KT + B KT + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (12) |
3. Results and discussion
3.1 Biosorbent characterization
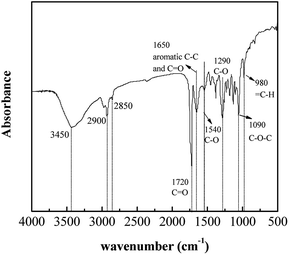
Fig. 1 showed the FTIR spectrum of the biosorbent used in the present study. The peak assignments of bacterial biomass were as follows. At 980 cm−1, the![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretching of unsaturated hydrocarbons was observed. Complementary information could be deduced from the bands between 1200 and 900 cm−1, where the C–O–C and C–O–P functional groups were observed. The region between 1250 and 1200 cm−1 exhibited the
C–H stretching of unsaturated hydrocarbons was observed. Complementary information could be deduced from the bands between 1200 and 900 cm−1, where the C–O–C and C–O–P functional groups were observed. The region between 1250 and 1200 cm−1 exhibited the ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) P
P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double-bond stretching vibrations.26 The bands at 1290 cm−1 and 1540 cm−1 were dominated by C–O stretching of phenolic compounds and the amid II, respectively. The C–C and C
O double-bond stretching vibrations.26 The bands at 1290 cm−1 and 1540 cm−1 were dominated by C–O stretching of phenolic compounds and the amid II, respectively. The C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of aromatic carbons asymmetric stretching frequency could be determined near 1650 cm−1. A characteristic peak at 1720 cm−1 was formed due to the vibrational C
O of aromatic carbons asymmetric stretching frequency could be determined near 1650 cm−1. A characteristic peak at 1720 cm−1 was formed due to the vibrational C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of –COOH stretching frequency. A cluster of bands at the range of 3000–2850 cm−1 referred to saturated aliphatic groups (e.g., –CH3, –CH2–). A broad and strong band at approximately 3450 cm−1 represented the symmetric stretching vibration from water molecules.26 The results confirmed that the bacterial surface was abundant with carboxyl, phosphate, amino groups, saturated and unsaturated aliphatic carbons and aromatic structures.
O of –COOH stretching frequency. A cluster of bands at the range of 3000–2850 cm−1 referred to saturated aliphatic groups (e.g., –CH3, –CH2–). A broad and strong band at approximately 3450 cm−1 represented the symmetric stretching vibration from water molecules.26 The results confirmed that the bacterial surface was abundant with carboxyl, phosphate, amino groups, saturated and unsaturated aliphatic carbons and aromatic structures.
The specific surface area determined by the BET method was 1.43 m2 g−1, which was quite small but comparable with other research.17
3.2 Biosorption of phenanthrene and iodine by SIBB in single and binary systems
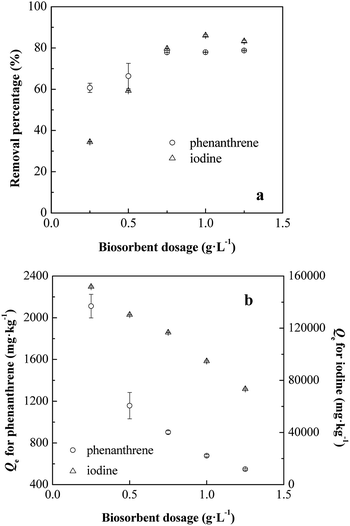
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 to 73
840 to 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 408 mg kg−1, respectively (Fig. 2b). The decreasing capacity with increasing dosage could mainly be related to the number of available binding functional groups and sites on the biosorbent due to aggregation.24 Similar trends had been observed for the biosorption of metals, dyes, uranium and organic compounds onto several biosorbents.17,21,38 In addition, the biosorption processes of phenanthrene and iodine were found to reach their optimum conditions at the dosage of 0.75 g L−1 (Fig. 2a), where the biosorption capacities caught 900 and 11
408 mg kg−1, respectively (Fig. 2b). The decreasing capacity with increasing dosage could mainly be related to the number of available binding functional groups and sites on the biosorbent due to aggregation.24 Similar trends had been observed for the biosorption of metals, dyes, uranium and organic compounds onto several biosorbents.17,21,38 In addition, the biosorption processes of phenanthrene and iodine were found to reach their optimum conditions at the dosage of 0.75 g L−1 (Fig. 2a), where the biosorption capacities caught 900 and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 mg kg−1, respectively.
600 mg kg−1, respectively.
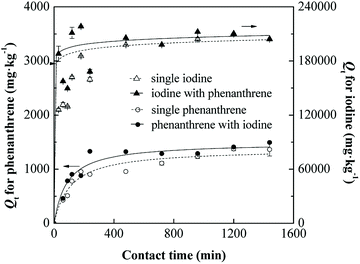
According to Fig. 3, the maximum biosorption amounts of phenanthrene by SIBB were around 1363.8 mg kg−1 and 1494.0 mg kg−1 in single and binary systems and were 204![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020 mg kg−1 and 212
020 mg kg−1 and 212![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 mg kg−1 for iodine in single and binary systems, respectively. The biosorption of HOCs such as phenanthrene onto biomass mainly via hydrophobic interaction due to the presence of aliphatic carbons and aromatic structures, which have been extensively studied.34,41,42 Choung et al. proposed a possible mechanism for the sorption iodine species onto natural organic matters based on XANES spectra.8 The uptake of iodine associated with the formation of intermediate iodine species through oxidation by quinones and ketones of the black carbon. In the present study, as shown in the FTIR spectra (Fig. 1), there are quinones (C
320 mg kg−1 for iodine in single and binary systems, respectively. The biosorption of HOCs such as phenanthrene onto biomass mainly via hydrophobic interaction due to the presence of aliphatic carbons and aromatic structures, which have been extensively studied.34,41,42 Choung et al. proposed a possible mechanism for the sorption iodine species onto natural organic matters based on XANES spectra.8 The uptake of iodine associated with the formation of intermediate iodine species through oxidation by quinones and ketones of the black carbon. In the present study, as shown in the FTIR spectra (Fig. 1), there are quinones (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of aromatic carbon, 1650 cm−1) and ketones (C
O of aromatic carbon, 1650 cm−1) and ketones (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 1720 cm−1) groups present in the SIBB material, which mainly contribute to the sorption of iodine. Therefore, the SIBB material showed relatively large sorption capacities for both phenanthrene and iodine. Furthermore, the results indicated that the co-existence of iodine or phenanthrene enhanced the biosorption of phenanthrene and iodine in the binary conditions. The synergistic effect on phenanthrene and iodine biosorption could be attributed to the specific bonding between the iodine molecules and carbon atoms of polycyclic aromatic hydrocarbons.8
O, 1720 cm−1) groups present in the SIBB material, which mainly contribute to the sorption of iodine. Therefore, the SIBB material showed relatively large sorption capacities for both phenanthrene and iodine. Furthermore, the results indicated that the co-existence of iodine or phenanthrene enhanced the biosorption of phenanthrene and iodine in the binary conditions. The synergistic effect on phenanthrene and iodine biosorption could be attributed to the specific bonding between the iodine molecules and carbon atoms of polycyclic aromatic hydrocarbons.8
3.3 Kinetics modelling
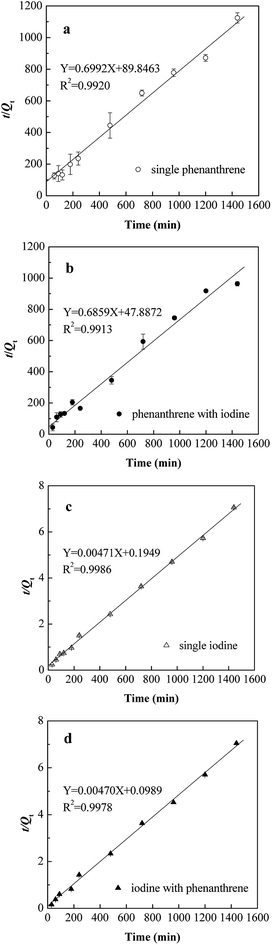
To evaluate the rate of phenanthrene and iodine biosorption in single or binary conditions and to examine the diffusion mechanism involved during the biosorption process, the obtained dynamic experimental data was fitted with several kinetic models, such as the Morris–Weber model, Lagergren model and pseudo-second-order model. The applicability of these kinetic models was determined by the correlation coefficients (R2), as well as the biosorption capacity as suggested by Kausar et al.28The calculated values of Kid, k1, k2, Qe and h are listed in Table 2. As exhibited in Fig. 6, the biosorption of phenanthrene and iodine in both single and binary conditions were superbly suitable for the pseudo-second-order kinetic model in contrast to the Morris–Weber model and pseudo-first-order model (shown in Table 2). The theoretical biosorption capacities calculated from the pseudo-second-order model (1.444 and 1.458 mg g−1 for phenanthrene, and 212.3 and 212.8 mg g−1 for iodine, respectively) were very close to the experimental biosorption capacities (1.364 and 1.490 mg g−1 for phenanthrene, and 204.0 and 212.3 mg g−1 for iodine, respectively). The applicability of pseudo-second-order model to the experimental data suggested that chemisorption was the dominant rate-limiting factor in controlling phenanthrene and iodine biosorption on SIBB.28
| Lagergren model | k1, min−1 | R2 |
|---|---|---|
| Phenanthrene (single) | 0.0008186 | 0.7770 |
| Phenanthrene (binary) | 0.0003880 | 0.2608 |
| Iodine (single) | 0.001170 | 0.8084 |
| Iodine (binary) | 0.000418 | 0.1383 |
| Pseudo-second-order model | k2, g mg−1 min−1 | Qe, mg g−1 | h, mg g−1 min−1 | R2 |
|---|---|---|---|---|
| Type 1 t/Qt = 1/(k2Qe2) + (1/Qe)t t/Qt ∼ t | ||||
| Phenanthrene (S)a | 0.00544 | 1.444 | 0.0111 | 0.9920 |
| Phenanthrene (B)b | 0.00983 | 1.458 | 0.0209 | 0.9913 |
| Iodine (S) | 0.00011 | 212.3 | 5.1308 | 0.9986 |
| Iodine (B) | 0.00022 | 212.8 | 10.111 | 0.9978 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Type 2 1/Qt = 1/Qe + 1/k2Qe2(1/t) 1/Qt ∼ 1/t | ||||
| Phenanthrene (S) | 0.00641 | 1.424 | 0.013 | 0.9432 |
| Phenanthrene (B) | 0.00714 | 1.473 | 0.016 | 0.8967 |
| Iodine (S) | 0.00025 | 194.2 | 9.372 | 0.7234 |
| Iodine (B) | 0.00022 | 211.9 | 9.728 | 0.5128 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Type 3 Qt = Qe − 1/k2Qe(Qt/t) Qt ∼ Qt/t | ||||
| Phenanthrene (S) | 0.00920 | 1.342 | 0.017 | 0.8025 |
| Phenanthrene (B) | 0.00890 | 1.449 | 0.019 | 0.6054 |
| Iodine (S) | 0.00023 | 198.1 | 8.846 | 0.6883 |
| Iodine (B) | 0.00029 | 208.9 | 12.58 | 0.3183 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Type 4 Qt/t = k2Qe2 − k2QeQt Qt/t ∼ Qt | ||||
| Phenanthrene (S) | 0.00696 | 1.422 | 0.014 | 0.8025 |
| Phenanthrene (B) | 0.00468 | 1.670 | 0.013 | 0.6054 |
| Iodine (S) | 0.00015 | 209.2 | 6.432 | 0.6883 |
| Iodine (B) | 0.00008 | 241.5 | 4.630 | 0.3183 |
The biosorption rates of phenanthrene and iodine in single and binary conditions were compared, and results showed that the presence of co-contaminants accelerated the biosorption process. In binary conditions, the pseudo-second-order biosorption rates for phenanthrene and iodine increased from 0.005441 to 0.009825 g mg−1 min−1 and from 0.000114 to 0.000223 g mg−1 min−1, respectively. The initial biosorption rate h (mg g−1 min−1) of the pseudo-second-order model was calculated as follows and listed in Table 2:
| h = k2Qe2 | (13) |
The initial rate might be a better parameter to evaluate the sorption rate considering the importance of an initial fast process in biosorption. Similar to the pseudo-second-order rate (k2), we could find a much larger initial rate (h) for both phenanthrene and iodine in binary conditions. In the binary system, improvements of 88.3% and 97.1% for phenanthrene and iodine, respectively, were achieved compared to single biosorption.
3.4 Isothermal modelling
The applications of the linear, Langmuir, Freundlich, D–R and Temkin models for the biosorption of phenanthrene and iodine in single and binary conditions by inactivated SIBB were explored, and the regression parameters are listed in Table 3. Comparing to Freundlich, D–R and Temkin models, biosorption of phenanthrene on SIBB fits well with the linear equation, while biosorption data of iodine fits well with the Langmuir model.| Langmuir model | KL, L mg−1 | Qm, mg g−1 | RL | R2 |
|---|---|---|---|---|
| Phenanthrene (S) | — | — | — | 0.2153 |
| Phenanthrene (B) | — | — | — | 0.0029 |
| Iodine (S) | 0.2325 | 216.9 | 0.044–0.387 | 0.9953 |
| Iodine (B) | 0.4813 | 199.6 | 0.023–0.234 | 0.9987 |
| Freundlich model | KF, L kg−1 | n | R2 |
|---|---|---|---|
| Phenanthrene (S) | 9034.4 | 0.8949 | 0.9854 |
| Phenanthrene (B) | 7856.0 | 1.0082 | 0.9394 |
| Iodine (S) | 146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 892 892 |
13.550 | 0.8757 |
| Iodine (B) | 162![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630 630 |
25.252 | 0.8530 |
| D–R model | β, mol2 J−2 | Qm, mol g−1 | R2 | E, kJ mol−1 |
|---|---|---|---|---|
| Phenanthrene (S) | 1.19 × 10−8 | 237.56 | 0.9918 | 6.482 |
| Phenanthrene (B) | 1.01 × 10−8 | 143.87 | 0.9327 | 7.036 |
| Iodine (S) | 2.48 × 10−9 | 814.77 | 0.7518 | 14.20 |
| Iodine (B) | 1.32 × 10−9 | 769.40 | 0.7313 | 19.48 |
| Temkin model | b, kJ mol−1 | KT, L kg−1 | R2 |
|---|---|---|---|
| Phenanthrene (S) | 0.6351 | 6297.4 | 0.9302 |
| Phenanthrene (B) | 0.7834 | 8525.4 | 0.8319 |
| Iodine (S) | 0.0448 | 5.93 × 106 | 0.8537 |
| Iodine (B) | 0.0851 | 7.38 × 1011 | 0.8431 |
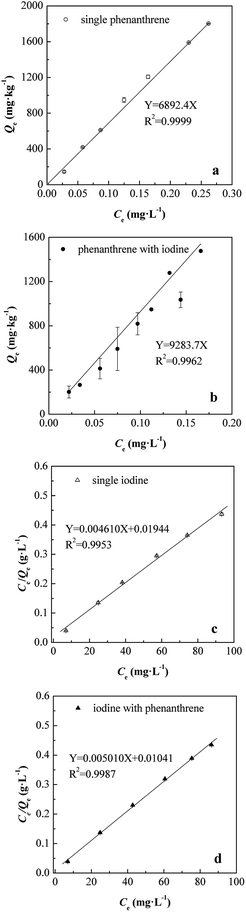
Biosorption of organic pollutants to biomass could involve surface sorption, chemical reactions and partitioning processes. The well-fit with linear model for phenanthrene (Fig. 7a and b) indicated that the primary mechanism of phenanthrene biosorption was partitioning. This was consistent with the previous studies about dead or inactivated bacterial and fungal biomass31,41 and the FTIR results. In the present study, the FTIR spectrum showed that the SIBB was abundant with saturated (3000–2850 cm−1, –CH3, –CH2–) and unsaturated (near 980 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) aliphatic carbon and aromatic structure (1650 cm−1), which provided effective binding sites for phenanthrene. Calculated partition coefficients (Kd) of phenanthrene in single and binary conditions were 6892.4 and 9283.7 L kg−1, respectively. These results showed that SIBB had a high affinity for phenanthrene compared with reported data.31,41 In addition, the presence of iodine highly enhanced the partition coefficient of phenanthrene with an increase of 34.7%. A specific binding would occur between iodine and carbon atoms in aromatic hydrocarbons according to the mechanism illustrated by Choung et al.8 The interaction or binding of phenanthrene with iodine was theoretically possible. As partitioning was the dominant mechanism for biosorption of phenanthrene, the biosorption of iodine on SIBB should not occupy the partition site for phenanthrene. Therefore, the sorbed iodine molecules might provide extra chemical bonding sites for the biosorption of phenanthrene, leading to a significant increase in sorption affinity for phenanthrene of SIBB.
C) aliphatic carbon and aromatic structure (1650 cm−1), which provided effective binding sites for phenanthrene. Calculated partition coefficients (Kd) of phenanthrene in single and binary conditions were 6892.4 and 9283.7 L kg−1, respectively. These results showed that SIBB had a high affinity for phenanthrene compared with reported data.31,41 In addition, the presence of iodine highly enhanced the partition coefficient of phenanthrene with an increase of 34.7%. A specific binding would occur between iodine and carbon atoms in aromatic hydrocarbons according to the mechanism illustrated by Choung et al.8 The interaction or binding of phenanthrene with iodine was theoretically possible. As partitioning was the dominant mechanism for biosorption of phenanthrene, the biosorption of iodine on SIBB should not occupy the partition site for phenanthrene. Therefore, the sorbed iodine molecules might provide extra chemical bonding sites for the biosorption of phenanthrene, leading to a significant increase in sorption affinity for phenanthrene of SIBB.
The partition coefficient (Kd) is a useful parameter to evaluate the distribution of organic compounds between solid and solution phases, and hence, their fate and mobility in the environment. Based on Kd values, the affinity of organic pollutants to biosorbents or solid phases from the aqueous phase can be estimated. The determined log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values of phenanthrene by SIBB were 3.84 and 3.97 for single and bi-solute conditions, respectively. These values were much higher than those of soil–water systems (1.48–2.30), due to the higher carbon content of SIBB.44,45 The log
Kd values of phenanthrene by SIBB were 3.84 and 3.97 for single and bi-solute conditions, respectively. These values were much higher than those of soil–water systems (1.48–2.30), due to the higher carbon content of SIBB.44,45 The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd of phenanthrene valued within a wide range of 2.98 to 4.76 for natural organic matter, microbiological biomass and plant residues, as summarized by Chen et al.46 The log
Kd of phenanthrene valued within a wide range of 2.98 to 4.76 for natural organic matter, microbiological biomass and plant residues, as summarized by Chen et al.46 The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values obtained in this study were higher than live tissues such as wood chips (3.40), plant roots (3.32–3.70), tender tea leaves (3.52–3.54)46 and algae and planktons (3.36–3.89).42 Additionally, these values were lower than the cuticle of plants (4.21–4.73) and humic acid (4.45). Therefore, bacterial biosorption may play an important role in the fate of phenanthrene in soils and surface water.41
Kd values obtained in this study were higher than live tissues such as wood chips (3.40), plant roots (3.32–3.70), tender tea leaves (3.52–3.54)46 and algae and planktons (3.36–3.89).42 Additionally, these values were lower than the cuticle of plants (4.21–4.73) and humic acid (4.45). Therefore, bacterial biosorption may play an important role in the fate of phenanthrene in soils and surface water.41
The sorption mechanism of iodine onto biomass could be proposed to be specific bonding with carbon atoms according to the mechanisms illustrated by Choung et al.8 Accordingly, the aromatic carbon in organic matter participated in the oxidation of iodine to some form of intermediates, facilitating the formation of aromatic carbon–iodine bonds rather than aliphatic carbon–iodine bonds. According to the postulates of the Langmuir model, a good fit would indicate that the monolayer chemical biosorption occurred at a given number of specific homogenous sites due to intermolecular forces.21 Affinity parameter KL values for iodine were 0.2325 and 0.4813 L mg−1 (232![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 and 481
500 and 481![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 L kg−1), as illustrated in Fig. 7c and d. A significant increase of the KL value of 107.0% in the presence of phenanthrene was found compared to the single iodine condition. As mentioned above, the biosorption of phenanthrene followed the partition mechanism and did not occupy the binding sites for iodine. Furthermore, the co-solute (phenanthrene) improved the sorption affinity of SIBB by introducing active binding sites and forming new functional groups that favoured iodine biosorption, which could be attributed to their interaction.8
300 L kg−1), as illustrated in Fig. 7c and d. A significant increase of the KL value of 107.0% in the presence of phenanthrene was found compared to the single iodine condition. As mentioned above, the biosorption of phenanthrene followed the partition mechanism and did not occupy the binding sites for iodine. Furthermore, the co-solute (phenanthrene) improved the sorption affinity of SIBB by introducing active binding sites and forming new functional groups that favoured iodine biosorption, which could be attributed to their interaction.8
The separation factor or equilibrium parameter constant (RL) was an important characteristic of the Langmuir isotherm and was represented as follows:
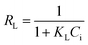 | (14) |
The separation factors (RL) were 0.044–0.387 and 0.023–0.234 following the range of 0–1.0, indicating a favourable biosorption of iodine, as listed in Table 3.21
4. Conclusions
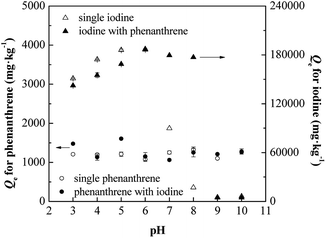
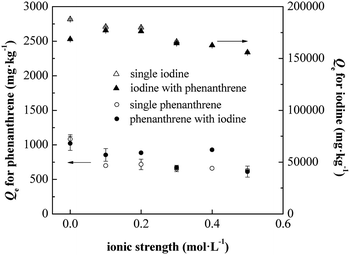
The present work showed that soil indigenous bacterial biomass was successfully applied for the simultaneous biosorption of phenanthrene and iodine from aqueous solutions. Synergistic effects were also found in binary conditions. The pseudo-second-order dynamic model agreed very well for the biosorption of phenanthrene and iodine onto SIBB. Calculated pseudo-second-order rates (k2) illustrated that the co-solute accelerated the biosorption process, with improvements of 80.6% and 95.6% for phenanthrene and iodine, respectively. The SIBB showed strong affinity with both phenanthrene and iodine, with a partition coefficient Kd (Linear model) of 6892.4 L kg−1 for phenanthrene and affinity parameter KL (Langmuir model) of 232![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 L kg−1 for iodine. The presence of co-solute illustrated a synergistic effect on biosorption due to intermolecular forces between phenanthrene and iodine, enhancing the Kd by 34.7% for phenanthrene and KL by 107.0% for iodine, respectively. Affecting factors were also tested to optimize biosorption parameters. Ideal conditions for the biosorption process included a pH of the solution of 6.0 and a 0.75 g L−1 biosorbent dosage within a 480 minutes time contact between the biosorbent and sorbates. The ionic strength significantly affected the biosorption of both phenanthrene and iodine in single condition, while no obvious influences were found in the binary conditions.
500 L kg−1 for iodine. The presence of co-solute illustrated a synergistic effect on biosorption due to intermolecular forces between phenanthrene and iodine, enhancing the Kd by 34.7% for phenanthrene and KL by 107.0% for iodine, respectively. Affecting factors were also tested to optimize biosorption parameters. Ideal conditions for the biosorption process included a pH of the solution of 6.0 and a 0.75 g L−1 biosorbent dosage within a 480 minutes time contact between the biosorbent and sorbates. The ionic strength significantly affected the biosorption of both phenanthrene and iodine in single condition, while no obvious influences were found in the binary conditions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21407037, 21878064, and 41271249), and Project of the Natural Science Foundation of Zhejiang Province (LY19B070009).References
- K. Hirose, 2011 Fukushima Dai-ichi nuclear power plant accident: summary of regional radioactive deposition monitoring results, J. Environ. Radioact., 2012, 111, 13–17 CrossRef CAS PubMed.
- P. S. Rose, R. L. Swanson and J. K. Cochran, Medically-derived 131I in municipal sewage effluent, Water Res., 2012, 46, 5663–5671 CrossRef CAS PubMed.
- G. Lefèvre, J. Bessière, J. J. Ehrhardt and A. Walcarius, Immobilization of iodide on copper(I) sulfide minerals, J. Environ. Radioact., 2003, 70, 73–83 CrossRef.
- C. Xu, S. Zhang, Y. F. Ho, E. J. Miller, K. A. Roberts, H. P. Li, K. A. Schwehr, S. Otosaka, D. I. Kaplan, R. Brinkmeyer, C. M. Yeager and P. H. Santischi, Is soil natural organic matter a sink or source for mobile radioiodine (129I) at the Savannah River Site?, Geochim. Cosmochim. Acta, 2011, 75, 5716–5735 CrossRef CAS.
- L. Liu, W. Liu, X. L. Zhao, D. M. Chen, R. S. Cai, W. Y. Yang, S. Komarneni and D. J. Yang, Selective capture of iodide from solutions by microrosette-like δ-Bi2O3, ACS Appl. Mater. Interfaces, 2014, 6, 16082–16090 CrossRef CAS PubMed.
- V. S. Kazakov, E. P. Demidchik and L. N. Astakhova, Thyroid cancer after Chernobyl, Nature, 1992, 359, 21–22 CrossRef CAS PubMed.
- H. A. Robertson and I. R. Falconer, Accumulation of radioactive iodine in thyroid glands subsequent to nuclear weapon tests and the accident at windscale, Nature, 1959, 184, 1699–1702 CrossRef CAS PubMed.
- S. Choung, W. Um, M. Kim and M. G. Kim, Uptake mechanism for iodine species to black carbon, Environ. Sci. Technol., 2013, 47, 10349–10355 CAS.
- C. Xu, E. J. Miller, S. Zhang, H. P. Li, Y. F. Ho, K. A. Schwehr, D. I. Kaplan, S. Otosaka, K. A. Roberts, R. Brinkmeyer, C. M. Yeager and P. H. Santschi, Sequestration and remobilization of radioiodine (129I) by soil organic matter and possible consequences of the remedial action at Savannah River Site, Environ. Sci. Technol., 2011, 45, 9975–9983 CrossRef CAS PubMed.
- M. L. Schlegel, P. Peiller, F. Mercier-Bion, N. Barre and V. Moulin, Molecular environment of iodine in naturally iodinated humic substances: Insight from X-ray absorption spectroscopy, Geochim. Cosmochim. Acta, 2006, 70, 5536–5551 CrossRef CAS.
- R. Jaffé, Fate of hydrophobic organic pollutants in the aquatic environment: A review, Environ. Pollut., 1991, 69(2–3), 237–257 CrossRef.
- J. K. Mwangi, W. J. Lee, L. C. Wang, P. J. Sung, L. S. Fang, Y. Y. Lee and G. P. Chang-Chien, Persistent organic pollutants in the Antarctic coastal environment and their bioaccumulation in penguins, Environ. Pollut., 2016, 216, 924–934 CrossRef CAS PubMed.
- K. Chojnacka, Biosorption and bioaccumulation – the prospects for practical applications, Environ. Int., 2010, 36, 299–307 CrossRef CAS PubMed.
- Y. Liu and Y. J. Liu, Biosorption isotherms, kinetics and thermodynamics, Sep. Purif. Technol., 2008, 61, 229–242 CrossRef CAS.
- K. Vijayaraghavan and Y. S. Yun, Bacterial biosorbents and biosorption, Biotechnol. Adv., 2008, 26, 266–291 CrossRef CAS PubMed.
- A. A. Azzaz, S. Jellali, R. Souissi, K. Ergaieg and L. Bousselmi, Alkaline-treated sawdust as an effective material for cationic dye removal from textile effluents under dynamic conditions: breakthrough curve prediction and mechanism exploration, Environ. Sci. Pollut. Res., 2017, 24, 18240–18256 CrossRef CAS PubMed.
- M. J. vPuchana-Rosero, E. C. Lima, S. Ortiz-Monsalve, B. Mella, D. Costa, E. Poll and M. Gutterres, Fungal biomass as biosorbent for the removal of Acid Blue 161 dye in aqueous solution, Environ. Sci. Pollut. Res., 2017, 24, 4200–4209 CrossRef PubMed.
- S. Rangabhashiyam, E. Suganya, A. V. Lity and N. Selvaraju, Equilibrium and kinetics studies of hexavalent chromium biosorption on a novel green macroalgae Enteromorpha sp, Res. Chem. Intermed., 2016, 42, 1275–1294 CrossRef CAS.
- N. Saranya, A. Abhishek, V. Sivasubramanian and N. Selvaraju, Hexavalent chromium removal from simulated and real effluents using Artocarpus heterophyllus peel biosorbent – batch and continuous studies, J. Mol. Liq., 2018, 265, 779–1294 CrossRef CAS.
- S. M. Ge and S. C. Ge, Simultaneous Cr(VI) reduction and Zn(II) biosorption by Stenotrophomonas sp. and constitutive expression of related genes, Biotechnol. Lett., 2016, 38, 877–884 CrossRef CAS PubMed.
- M. E. Mahmoud, G. M. E. Zokm, A. E. M. Farag and M. S. Abdelwahab, Assessment of heat-inactivated marine Aspergillus flavus as a novel biosorbent for removal of Cd(II), Hg(II), and Pb(II) from water, Environ. Sci. Pollut. Res., 2017, 24, 18218–18228 CrossRef CAS PubMed.
- M. S. G. Nandagopal, R. Antony, N. Sreekumar and N. Selvaraju, Experimental exploration on degradation of Organe G 16 and azo dye by novel Pseudoalteromonas sp. and its enzyme activity, Arabian J. Sci. Eng., 2015, 40, 1005–1013 CrossRef.
- V. Karthik, K. Saravanan, E. Nakkeeran and N. Selvaraju, Biosorption of Turquoise Blue dye from aqueous solution by dried fungal biomass (Trichoderma harzianum) – kinetic, isotherm and thermodynamic studies, Desalin. Water Treat., 2017, 74, 362–370 CrossRef CAS.
- Z. Aksu and J. Yener, A comparative adsorption/biosorption study of mono-chlorinated phenols onto various sorbents, Waste Manag., 2001, 21, 695–702 CrossRef CAS PubMed.
- L. L. Bai, H. C. Xu, C. H. Wang, J. C. Deng and H. L. Jiang, Extracellular polymeric substances facilitate the biosorption of phenanthrene on cyanobacteria Microcystis aeruginosa, Chemosphere, 2016, 162, 172–180 CrossRef CAS PubMed.
- S. Luo, L. L. Li, A. W. Chen, Q. R. Zeng, H. Xia and J. D. Gu, Biosorption of diethyl phthalate ester by living and nonliving Burkholderia cepacia and the role of its cell surface components, Chemosphere, 2017, 178, 187–196 CrossRef CAS PubMed.
- E. Bağda, M. Tuzen and A. Sarı, Equilibrium, thermodynamic and kinetic investigations for biosorption of uranium with green algae (Cladophora hutchinsiae), J. Environ. Radioact., 2017, 175–176, 7–14 CrossRef PubMed.
- A. Kausar, H. N. Bhatti and G. MacKinnon, Equilibrium, kinetic and thermodynamic studies on the removal of U(VI) by low cost agricultural waste, Colloids Surf., B, 2013, 111, 124–133 CrossRef CAS PubMed.
- D. Zhang and L. Z. Zhu, Controlling microbiological interfacial behaviors of hydrophobic organic compounds by surfactants in biodegradation process, Front. Environ. Sci. Eng., 2014, 8(3), 305–315 CrossRef CAS.
- N. Yee, L. G. Benning, V. R. Phoenix and F. G. Ferris, Characterization of metal-cyanobacteria sorption reactions: A combined macroscopic and infrared spectroscopic investigation, Environ. Sci. Technol., 2004, 38, 775–782 CrossRef CAS PubMed.
- D. Zhang, L. Z. Zhu and F. Li, Influences and mechanisms of surfactants on pyrene biodegradation based on interactions of surfactant with a Klebsiella oxytoca strain, Bioresour. Technol., 2013, 142, 454–461 CrossRef CAS PubMed.
- L. Xiao, X. Qu and D. Q. Zhu, Biosorption of nonpolar hydrophobic organic compounds to Escherichia coli facilitated by metal and proton surface binding, Environ. Sci. Technol., 2007, 41(8), 2750–2755 CrossRef CAS PubMed.
- C. Yang, H. L. Yu, H. Jiang, C. L. Qiao and R. H. Liu, An engineered microorganism can simultaneously detoxify cadmium, chlorpyrifos, and γ-hexachlorocyclohexane, J. Basic Microbiol., 2016, 56, 820–826 CrossRef CAS PubMed.
- D. Zhang, L. Lu, H. T. Zhao, M. Q. Jin, T. Lü and J. Lin, Application of Klebsiella oxytoca biomass in the biosorptive treatment of PAH-bearing wastewater: Effect of PAH hydrophobicity and implications for prediction, Water, 2018, 10, 675 CrossRef.
- T. Madrakian, A. Afkhami, M. A. Zolfigol, M. Ahmadi and N. Koukabi, Application of modified silica coated magnetite nanoparticles for removal of iodine from water samples, Nano-Micro Lett., 2012, 4(1), 57–63 CrossRef CAS.
- W. J. Weber and J. C. Morris, Kinetics of adsorption on carbon from solution, J. Sanit. Eng. Div., Am. Soc. Civ. Eng., 1963, 89, 31–59 Search PubMed.
- H. Javadian, P. Vahedian and M. Toosi, Adsorption characteristics of Ni(II) from aqueous solution and industrial wastewater onto polyaniline/HMS nanocomposite powder, Appl. Surf. Sci., 2013, 284, 13–22 CrossRef CAS.
- Y. S. Ho and G. McKay, Pseudo-second order model for sorption processes, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- Y. S. Ho, Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods, Water Res., 2006, 40, 119–125 CrossRef CAS PubMed.
- V. R. Fanaie, M. Karrabi, M. M. Amin, B. Shahnavaz and A. Fatehizadeh, Biosorption of 4-chlorophenol by dried anaerobic digested sludge: artificial neural network modeling, equilibrium isotherm, and kinetic study, Int. J. Environ. Sci. Technol., 2017, 14, 37–38 CrossRef CAS.
- B. L. Chen, Y. S. Wang and D. F. Hu, Biosorption and biodegradation of polycyclic aromatic hydrocarbons in aqueous solutions by a consortium of white-rot fungi, J. Hazard. Mater., 2010, 179, 845–851 CrossRef CAS PubMed.
- D. N. Zhang, C. Y. Ran, Y. Yang and Y. Ran, Biosorption of phenanthrene by pure algae and field-collected planktons and their fractions, Chemosphere, 2013, 93, 61–68 CrossRef CAS PubMed.
- M. E. Mahmoud, G. M. E. Zokm, A. E. M. Farag and M. S. Abdelwahab, Assessment of heat-inactivated marine Aspergillus flavus as a novel biosorbent for removal of Cd(II), Hg(II), and Pb(II) from water, Environ. Sci. Pollut. Res., 2017, 24, 18218–18288 CrossRef CAS PubMed.
- K. Yang, L. Z. Zhu and B. S. Xing, Enhanced soil washing of phenanthrene by mixed solutions of TX100 and SDBS, Environ. Sci. Technol., 2006, 40, 4274–4280 CrossRef CAS PubMed.
- W. J. Zhou and L. Z. Zhu, Enhanced desorption of phenanthrene from contaminated soil using anionic/nonionic mixed surfactant, Environ. Pollut., 2007, 147, 350–357 CrossRef CAS PubMed.
- B. L. Chen, M. X. Yuan and H. Liu, Removal of polycyclic aromatic hydrocarbons from aqueous solution using plant residue materials as a biosorbent, J. Hazard. Mater., 2011, 188, 436–442 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |