Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Self-assembly of highly conductive self-n-doped fullerene ammonium halides and their application in the in situ solution-processable fabrication of working electrodes for alcohol electrooxidation†
H. H. Wang‡
a,
X. Sun‡a,
Z. C. Lina,
Z. F. Panga,
X. Q. Konga,
M. Lei *a and
Y. F. Li
*a and
Y. F. Li b
b
aDepartment of Chemistry, Zhejiang University, Hangzhou 310027, China. E-mail: leiming@zju.edu.cn
bCAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
First published on 6th March 2018
Abstract
Stable and highly conductive self-n-doped fullerene ammonium halides are promising optoelectronic materials. It is necessary to thoroughly understand their structure–function relationship and to develop their applications. Here, the assembly behaviors of the self-n-doped fullerene ammonium halides, as well as the functional areas in the well-developed 2D–3D lamellar structures in their ordered aggregates are systematically characterized using comprehensive methods. In the self-assembly, the solvation effect of DMSO promotes the flexibility of side-chains and drives the formation of fullerene ammonium halides into ordered bilayer structures. The conductivity-active area, which contains tightly packed halide anions sandwiched between fullerenes, provides good electron transfer property. Remarkably, residual DMSO in the side-chain area can induce aqueous Pd precursor into the highly conductive framework. After reduction, Pd nanoparticles are immobilized in the confined spaces within the conductive support. The resulting electrode can be used to electrooxidize ethanol. This study provides a facile solution strategy for the in situ fabrication of electrocatalysts on working electrodes, which can be applied in direct alcohol fuel cells.
Introduction
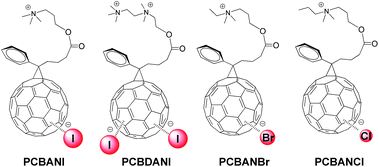
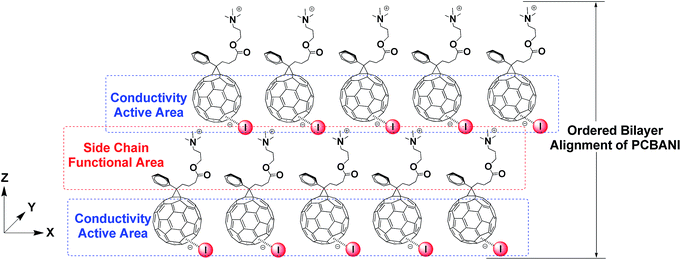
Stable and highly conductive self-n-doped fullerene ammonium halides are promising cathode interfacial layer materials that can facilitate electron transfer and improve the power conversion efficiencies of organic solar cells.1,2 Through systematic studies using molecular models of fullerene ammonium iodide (PCBANI) (Scheme 1), we elucidated the mechanism of a cross-self-n-doping process that involves strong anion–π noncovalent interactions between iodide anions and core fullerenes.3 We found that the doping effect in PCBANI caused the separation of cation–anion pairs and increased polarization. Thus, PCBANIs instinctively aggregated to achieve stability. Moreover, we previously reported that PCBANI self-assembled into a layered stacking supramolecular system through the delicate balance of iodide anion–C60 π, electrostatic, and C60 π–π interactions.4 The proposed ET model, in which the iodide sandwiched in the fullerene core acts as a shuttle to transfer electrons via intramolecular/intermolecular synergistic redox processes, shows that the ordered and tightly packed fullerenes that sandwich iodide can facilitate electron transfer along the network system.3,4We recently performed systematic studies on halide anion–fullerene π interactions in self-n-doped fullerene ammonium halides containing I−, Br−, and Cl− and demonstrated the universality of the halide anion (I−, Br−, and Cl−) n-doping of fullerene (Scheme 1).5 Device experiments revealed that the conductivities of the self-n-doped fullerene ammonium halides successively decreased in the order of PCBANI, PCBANBr and PCBANCl. This indicated that the size of the halide anion is a crucial factor in determining the electron transport property of these materials. In addition, functions of the side-chain needed to be studied. To develop novel functions of these highly conductive fullerenes, it is necessary to thoroughly understand their self-assembly behaviour and detect the functional areas within their supramolecular structures.
In terms of the anode catalysts employed in direct alcohol fuel cells (DAFCs), the alternative palladium (Pd)-based nanocatalysts have attracted much attention because Pd is less expensive and shows greater resistance to CO poisoning than Pt.6,7 In the recent years, considerable efforts have been devoted to the development of Pd-based nanoparticles (NPs), which are dispersed on various supports for electrocatalytic alcohol oxidation.8–11 The supports include conductive polymers,12 carbon nanotubes,13 carbon fibers,14 graphene oxide15 and graphene,16 where polycarbon-based materials are commonly used. Unfortunately, the strong planar π–π stacking of 2D graphene sheets results in their reversible agglomeration17,18 and the drastic loss of electroactive sites during electrode assembly. Consequently, porous 3D graphene19–22 has emerged as an alternative support material for catalyst loading to improve mass transfer and maximize the accessibility of the catalyst surface. By contrast, 0D fullerenes (C60) are less studied supports with relatively low conductivities due to their polycrystallization or short-range order structures.23–26 Notably, the development of single-crystalline, 1D-phototreated C60 nanorods with high aspect ratios and increased electron mobilities has provided new perspectives on the use of fullerenes as an electrocatalyst support.27 Generally, in designing the support for the loading of a catalyst, it is crucial to transfer the complex catalyst system onto the electrode, while maintaining its activity and reproducibility. However, the majority of available cathodic catalysts for DAFCs have been limited by their complicated ex situ fabrication procedures. Therefore, the in situ fabrication of electrocatalysts on an electrode is desirable, economical, and has potential applications for the large-scale manufacture of DAFC electrodes. The introduction of a synergetic functionalized support is of vital importance for such a fabrication strategy.
In the present study, comparative studies on the self-assembly structures of self-n-doped fullerene ammonium halides were performed to understand the relationship between film conductivity and microstructure. Furthermore, a simple and practical method for the in situ fabrication of electrocatalysts on working electrodes was facilitated by the unique self-assembly structure of highly conductive self-n-doped fullerene films. The immobilized Pd NPs catalyst system was designed on the basis of the following considerations: (1) the 2D–3D self-n-doped fullerene framework had relatively high electron conductivity (1 to 2 s m−1). At the same time, strong polar functional groups enhanced the adhesive strength of the support to the electrode preventing its dissociation during operation. (2) The solvation effect of residual DMSO on the side-chain area within the framework facilitated the diffusion of the Pd precursor into the area of the ionic group adjacent to fullerene. (3) Given the advantages of the microstructure effect and weak interactions, the aggregation of ultrafine Pd NPs could be prevented by immobilizing Pd in the confined spaces within packed fullerenes along the side-chain area. In this study, we demonstrated that the highly conductive self-assembled fullerene film can act as a template and support for the immobilization of Pd NPs. Furthermore, it provides a facile solution method for the in situ fabrication of fuel cell electrodes for ethanol electrooxidation.
Results and discussion
Comparative studies on the self-assembly structures
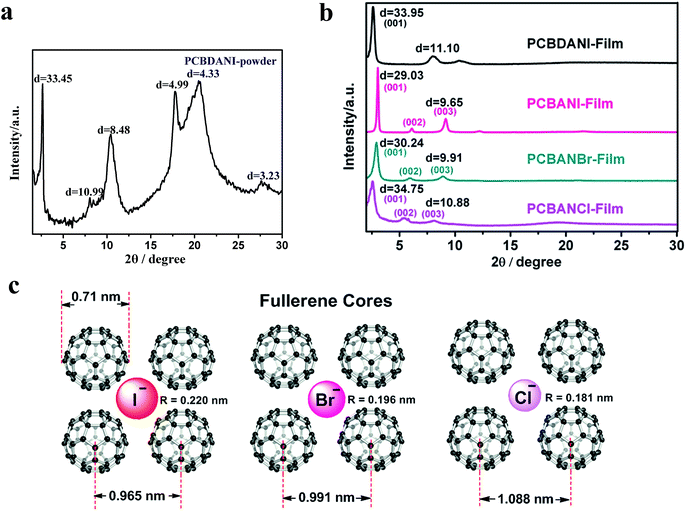 | ||
| Fig. 1 (a) XRD pattern of pristine powder PCBDANI. (b) XRD patterns of self-assembled PCBDANI, PCBANBr, and PCBANCl in comparison with that of PCBANI. (c) Schematic diagram illustrating the structure and size of self-assembled PCBANI, PCBANBr and PCBANCl (side-chains are omitted) based on the Z-view image of the optimized structure of self-assembled PCBANI obtained by computational modeling (the fullerene has a diameter of 0.71 nm (ref. 29)). | ||
Then, we investigated the XRD patterns of PCBANBr and PCBANCl films fabricated through DMSO dispersion. The well-defined XRD patterns of PCBANBr and PCBANCl (Fig. 1b) were attributed to the Bragg reflections from the (001) to (003) planes. Broad peaks at 2θ ≈ 2.5° were assigned to reflections from the (001) plane and had d-spacing values of 3.024 and 3.475 nm for PCBANBr and PCBANCl, respectively; these values corresponded to those of bilayer lengths and were consistent with those of the increased side-chain lengths. Similar to that in PCBANI, the neighboring fullerene core peaks in the XRD spectra of PCBANBr and PCBANCl overlapped with the (003) plane peak and corresponded to 0.991 and 1.088 nm distance, respectively. The increased distance between the fullerene core and the size reduction of halide anions (RI − [0.220 nm] > RBr − [0.196 nm] > RCl − [0.181 nm]) suggested that the assembly into fine structures was governed by the balance between iodide–π and fullerene π–π interactions. We constructed a schematic of the structural sizes of fullerene ammonium halides on the basis of the Z-view image of the optimized self-assembled PCBANI structure obtained by computational modelling (Fig. 1c). It suggested that small anions cannot be tightly sandwiched between fullerenes, resulting in loose self-assembled structures and decreased electron transport properties.
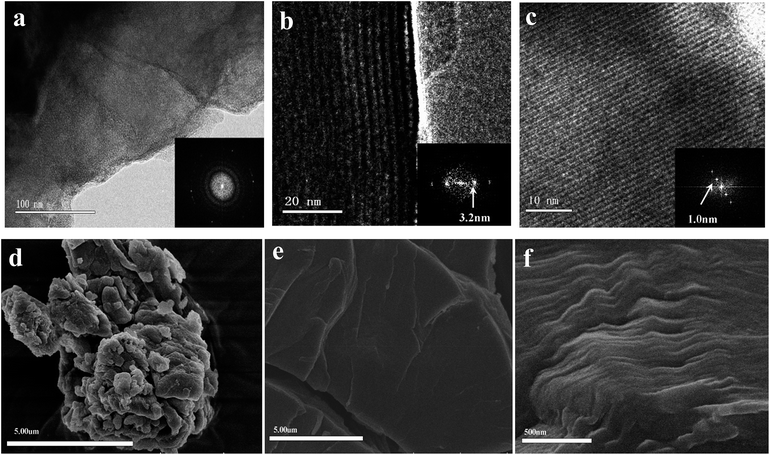 | ||
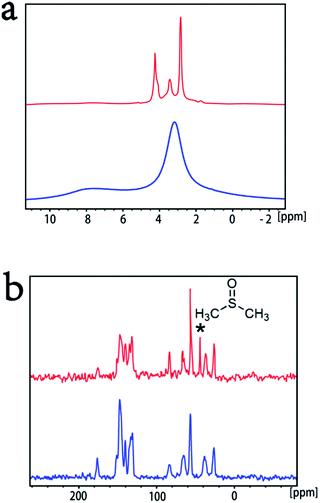
| Fig. 2 HR-TEM image of PCBDANI (a) pristine powder with disordered structure. (b), (c) Cross-sectional images of self-assembled PCBDANI with lamellar structures and the corresponding FFT analysis; the periodicities of the lamellae are 3.2 nm and 1.0 nm, respectively. A large-area image of the lamellae with 3.2 nm periodicity is shown in Fig. S1.† (d) FESEM images of PCBDANI pristine powder with disordered array; (e), (f) self-assembled PCBDANI appears to be solid and tightly packed. | ||
Application in solution-processable fabrication of working electrodes for alcohol electrooxidation
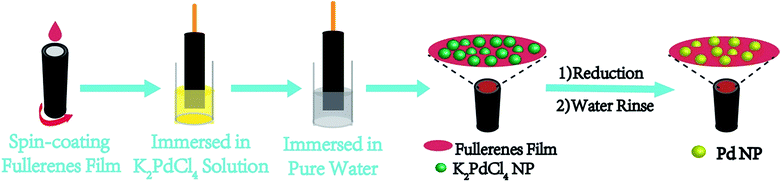 | ||
| Fig. 6 Procedure for in situ fabrication of an immobilized Pd nanoparticle electrocatalyst on a working electrode using PCBANI and PCBDANI as conductive supports. | ||
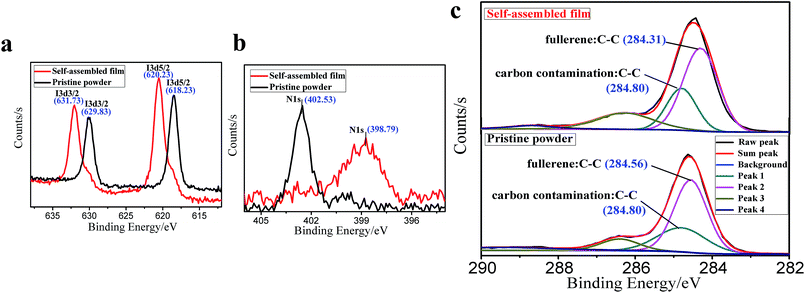
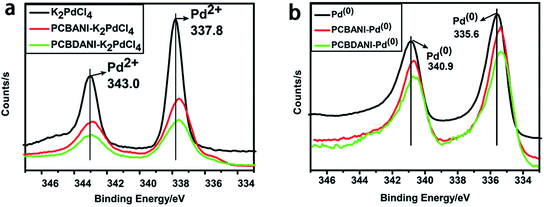
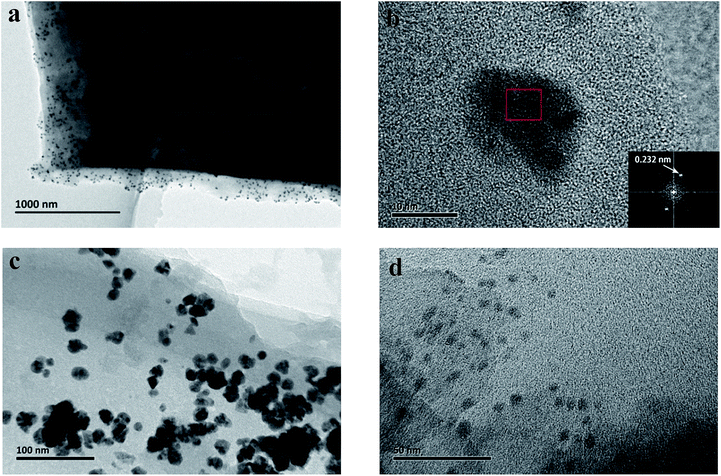
We carefully characterized the immobilized Pd2+ precursor and Pd0 NPs during fabrication to demonstrate our design concept. The XPS measurements shown in Fig. 7 indicated the existence of Pd2+ and Pd0 on the surfaces of PCBANI and PCBDANI films. Slight systematic shifts (∼0.2 eV) in the binding energies of PCBANI and PCBDANI films were compared with those of the control samples. The results suggested that the Pd element and support weakly interacted. As expected, residual DMSO had a delicate role in inducing and locating aqueous-based K2PdCl4 in the framework. Driven by the affinity of water for DMSO, K2PdCl4 diffused and dispersed into an ionic group area and was then immobilized through multiple interactions. After treatment with the hydrazine hydrate solution, Pd NPs with sizes of 5–10 nm were obtained as revealed through HR-TEM (Fig. 8). As shown in Fig. 8b, FFT analysis further revealed the coexistence of a lattice spacing of 0.232 nm in Pd NP. This spacing corresponded to the (111) interplanar distance of a standard Pd crystalline lattice. The dispersibility of Pd NPs on the PCBANI film was better than that on the PCBDANI film. This result can be attributed to the highly ordered microstructure of the self-assembled PCBANI as observed through XRD analysis.
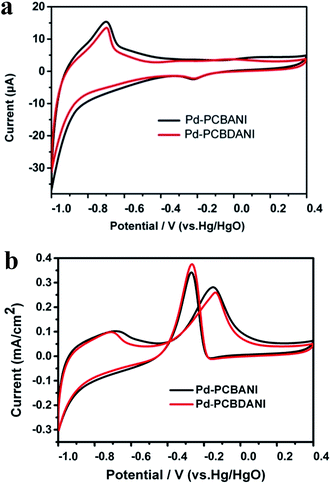 | ||
| Fig. 9 CV results of Pd-PCBANI (black line) and Pd-PCBDANI (red line) coated electrode in blank solution containing 1 M KOH (a) and in 1 M KOH containing 0.5 M ethanol (b). Scan rate: 0.05 V s−1. | ||
Thus, we developed a facile method for the in situ fabrication of electrocatalysts. The interaction between the Pd precursor and conductive support was essential for the design of a highly dispersed composite nanocatalyst. At the same time, preventing the irreversible aggregation of ultrafine Pd NPs was crucial to maintain catalytic activity and durability. Although the catalytic activity of the working electrode is lower than that of the present supported Pd catalyst, it can be improved by optimizing operation parameters and conditions such as film quality, precursor concentration, and treatment time. Moreover, the solution fabrication of new catalyst systems with low Pd usage has potential applications in the in situ fabrication of large-area electrodes for electrocatalysis given its simplicity, improved reproducibility, and low cost.
Experimental
Materials
All chemicals were purchased from Aldrich Chemical Co. and Acros Chemical Co. and were used without further purification unless stated otherwise.Conclusions
In summary, the self-assembly structures of highly conductive self-n-doped fullerene ammonium halides were systematically characterized, and the functional areas in the well-developed 2D–3D lamellar structures in their ordered aggregates were illustrated. The active area, which involves halide anions sandwiched between tightly packed fullerenes, conferred good electron transfer property to the self-n-doped fullerene ammonium halides. Combined with the template functionality of the side-chain, the highly conductive framework could immobilize Pd NPs through interactions between the metal precursor and conductive support. The resulting electrode can be used to electrooxidize ethanol. This study provides an all-solution strategy for the in situ fabrication of electrocatalysts. Moreover, the proposed strategy has vast potential applications in the large-area printing of fuel cell electrodes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support by the National Natural Science Foundation of China (Grant No. 21442005, 21642008).Notes and references
- S. S. Li, M. Lei, M. L. Lv, S. E. Watkins, Z. A. Tan, J. Zhu, J. H. Hou, X. W. Chen and Y. F. Li, Adv. Energy Mater., 2013, 23, 1569 CrossRef.
- W. X. Jiao, D. Ma, M. L. Lv, W. W. Chen, H. Q. Wang, J. Zhu, M. Lei and X. W. Chen, J. Mater. Chem. A, 2014, 2, 14720 CAS.
- W. W. Chen, W. X. Jiao, D. B. Li, X. Sun, X. Guo, M. Lei, Q. Wang and Y. F. Li, Chem. Mater., 2016, 28, 1227 CrossRef CAS.
- X. Sun, W. W. Chen, L. J. Liang, W. Hu, H. H. Wang, Z. F. Pang, Y. X. Ye, X. R. Hu, Q. Wang, X. Q. Kong, Y. Z. Jin and M. Lei, Chem. Mater., 2016, 28, 8726 CrossRef CAS.
- X. Sun, L. Y. Ji, W. W. Chen, X. Guo, H. H. Wang, M. Lei, Q. Wang and Y. F. Li, J. Mater. Chem. A, 2017, 5, 20720 CAS.
- D. Liu, Q. H. Guo, H. Q. Hou, O. Niwa and T. Y. You, ACS Catal., 2014, 4, 1825 CrossRef CAS.
- A. Rabis, P. Rodriguez and T. J. Schmidt, ACS Catal., 2012, 2, 864 CrossRef CAS.
- C. H. Cui and S. H. Yu, Acc. Chem. Res., 2013, 46, 1427 CrossRef CAS PubMed.
- M. A. F. Akhairi and S. K. Kamarudin, Int. J. Hydrogen Energy, 2016, 41, 4214 CrossRef CAS.
- M. Z. F. Kamarudin, S. K. Kamarudin, M. S. Masdar and W. R. W. Daud, Int. J. Hydrogen Energy, 2013, 38, 9438 CrossRef CAS.
- L. Rao, Y. X. Jiang, B. W. Zhang, L. X. You, Z. H. Li and S. G. Sun, Prog. Chem., 2014, 26, 727 CAS.
- A. L. Wang, H. Xu, J. X. Feng, L. X. Ding, Y. X. Tong and G. R. Li, J. Am. Chem. Soc., 2013, 135, 10703 CrossRef CAS PubMed.
- Y. Wei, X. Y. Zhang, Z. Y. Luo, D. Tang, C. X. Chen, T. Zhang and Z. L. Xie, Nano-Micro Lett., 2017, 9, 28 CrossRef.
- B. Pierozynski, Int. J. Electrochem. Sci., 2013, 8, 634 CAS.
- X. M. Chen, G. H. Wu, J. M. Chen, X. Chen, Z. X. Xie and X. R. Wang, J. Am. Chem. Soc., 2011, 133, 3693 CrossRef CAS PubMed.
- R. N. Singh and R. Awasthi, Catal. Sci. Technol., 2011, 1, 778 Search PubMed.
- S. Park, Y. Shao, H. Wan, P. Rieke, V. Viswanathan, S. Towne, L. Saraf, J. Liu, Y. Lin and Y. Wang, Electrochem. Commun., 2011, 13, 258 CrossRef CAS.
- D. S. Yu and L. M. Dai, J. Phys. Chem. Lett., 2010, 1, 467 CrossRef CAS.
- W. Chen, S. Li, C. Chen and L. Yan, Adv. Mater., 2011, 23, 5679 CrossRef CAS PubMed.
- Z. Tang, S. Shen, J. Zhuang and X. Wang, Angew. Chem., Int. Ed., 2010, 49, 4603 CrossRef CAS PubMed.
- C. G. Hu, H. H. Cheng, Y. Zhao, Y. Hu, Y. Liu, L. M. Dai and L. T. Qu, Adv. Mater., 2012, 24, 5493 CrossRef CAS PubMed.
- Z. Y. Zhang, Y. Dong, L. Wang and S. Wang, Chem. Commun., 2015, 51, 8357 RSC.
- Y. Zhang, L. Jiang, H. Li, L. Z. Fan, W. P. Hu, C. R. Wang, Y. F. Li and S. H. Yang, Chem.–Eur. J., 2011, 17, 4921 CrossRef CAS PubMed.
- G. Lee, J. H. Shim, H. Kang, K. M. Nam, H. Song and J. T. Park, Chem. Commun., 2009, 33, 5036 RSC.
- K. Vinodgopal, M. Haria, D. Meisel and P. Kamat, Nano Lett., 2004, 4, 415 CrossRef CAS.
- Q. Zhang, Z. Y. Bai, M. Shi, L. Yang, J. L. Qiao and K. Jiang, Electrochim. Acta, 2015, 177, 113 CrossRef CAS.
- H. R. Barzegar, G. Z. Hu, C. Larsen, X. Jia, L. Edman and T. Wågberg, Carbon, 2014, 73, 34 CrossRef CAS.
- M. J. Hollamby, M. Karny, P. H. H. Bomans, N. A. J. M. Sommerdijk, A. Saeki, S. Seki, H. Minamikawa, I. Grillo, B. R. Pauw, P. Brown, J. Eastoe, H. Möhwald and T. Nakanishi, Nat. Chem., 2014, 6, 690 CrossRef CAS PubMed.
- T. Michinobu, T. Nakanishi, J. P. Hill, M. Funahashi and K. Ariga, J. Am. Chem. Soc., 2006, 128, 10384 CrossRef CAS PubMed.
- M. J. Duer, Solid State NMR Spectroscopy: Principles and Applications, John Wiley & Sons, 2002 Search PubMed.
- A. Marchetti, J. E. Chen, Z. F. Pang, S. H. Li, D. S. Ling, F. Deng and X. Q. Kong, Adv. Mater., 2017, 1605895 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra00100f |
| ‡ H. H. Wang and X. Sun contributed equally to these work. |
| This journal is © The Royal Society of Chemistry 2018 |