Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An upconversion luminescence and temperature sensor based on Yb3+/Er3+ co-doped GdSr2AlO5†
Gangyi Zhangab,
Qinping Qiangab,
Shanshan Duab and
Yuhua Wang *ab
*ab
aDepartment of Materials Science, School of Physical Science and Technology, Lanzhou University, Lanzhou, 730000, PR China. E-mail: wyh@lzu.edu.cn; Fax: +86 931 8913554; Tel: +86 931 8912772
bKey Laboratory for Special Function Materials and Structural Design of the Ministry of Education, Lanzhou University, Lanzhou 730000, China
First published on 5th March 2018
Abstract
GdSr2AlO5:Yb3+/Er3+ micro-particles were synthesized by a simple solid state method. The structure, morphology, size and upconversion luminescence features have been characterized. These results indicated that GdSr2AlO5 has a contracted tetragonal cell and has irregular block shaped particles with sizes of about 5 μm. During upconversion, green (2H11/2, 4S3/2 → 4I15/2) (527 nm, 549 nm) and red (4F9/2 → 4I15/2) (665 nm) emissions had been observed, both of which occurred via a two-photon population process. In addition, green UC emission characteristics were studied, and it was found that its temperature ranged from 293 K to 473 K and the sensitivity was 0.0054 K−1 at 473 K. This indicated that GdSr2AlO5:Yb3+/Er3+ micro-particles may have potential application in high temperature environments for safety signs.
1. Introduction
Upconversion (UC) luminescence materials are luminescent materials that can convert a near-infrared excitation into a visible emission through lanthanide doping.1 These materials have attracted extensive interest in the past decade because of their unique features, such as high photochemical stability, narrow emission bandwidths, and large anti-Stokes shifts, making them useful in optical communication, lasers, sensors, biomedical analyses and so on.2–4 For efficient UC emission, Yb3+ ions are typically selected as sensitizers together with Er3+, Tm3+ and Ho3+ as activators doped into various kinds of hosts.5,6 Tb3+ and Dy3+ are also activators but they not often been used.7,8 Yb3+ has a larger absorption cross-section than those of rare earth activators when excited by 980 nm laser. What is more, the 2F7/2 → 2F5/2 transition of Yb3+ is exactly resonant with many f–f transitions of Er3+, Ho3+ and Tm3+ which cause to the efficient energy transfer from Yb3+ ions to these ions. Thence, Yb3+ is used extensively as sensitizer as the absorption section is much larger around 980 nm wavelength and can lead efficient energy transfers to Er3+, Ho3+and Tm3+. Because of its beneficial electronic energy level structures. Er3+ ions have been widely studied and a large number of Er3+ doped UC materials have been reported such as NaYF4:Yb,Er, NaYF4:Nd,Yb,Er, NaGdF4:Yb,Er Ba2Y(BO3)2Cl:Yb,Er, and Y2O3:Yb,Er.9–13Temperature-sensitive luminescence materials can provide a non-contact measurement to detect the temperature by probing the dependence of emission intensities on temperature.14 We can make the calibration by analyzing the changes on the relative emission intensities when the temperature of the samples increase. The measurement conditions and time exposure can be simple and convenient by this fluorescence intensity ratio (FIR) technique compared with common temperature monitoring devices.15–17 The premise of using this method is two thermally coupled levels of RE3+ ions. Fortunately the Er3+ ions have two thermally coupled energy levels, 2H11/2 and 4S3/2, with a small energy gap around 770 cm−1 which is just conform to this technique.
Host materials play an important role for the UC luminescence. For purpose of obtaining efficient UC emission, lower phonon energies host materials are required because the value of nonradiative decay rates in these hosts would be minimum.18,19 Thence, rare earth doped fluoride crystals have been studied for a long time due to their low phonon thresholds. However, it is known to all that physical properties of fluoride crystals could not compared with oxide hosts such as chemical stability and mechanical strength. For this reason, it is still important to find new oxide host materials with efficient UC luminescence. Recently, some literatures on the UC luminescence of RE-doped oxide crystals such as Y2O2S, SrWO4, CaWO4 and Zn3Ga2GeO8 have been reported.13,20–22 Many compounds with Gd ions have been reported due to its excellent magnetic properties. And it is possible to use these compounds as multifunctional materials in various fields. There is little difference in radius of the Yb and Er ions compared with the Gd ions. So rare earth ions as luminescent centers are more easily incorporated into the host, resulting in effective emission. Moreover, aluminate phosphors have been studied extensively by virtue of their cheap raw materials, good chemical and physical stability. In a word, the Yb and Er ions can easily doped in the GdSr2AlO5 host and the as prepared GdSr2AlO5 samples are stable enough to work in high temperature and the good magnetic properties would be significant and meaningful for the potential application in biomedical imaging. The structural and optical properties of the Ce3+ doped solid solutions GdSr2AlO5 and Sr3AlO4F are reported by Won Bin Im et al.23 Lately, yellow emitting phosphor GdSr2AlO5:Ce3+24 and afterglow phosphors GdSr2AlO5:RE3+ (RE3+ = Eu3+,Sm3+, Pr3+ and Dy3+)25 have been investigated as well. These facts illustrated that the GdSr2AlO5 host is a favorable optical material, but there is no report on its UC luminescence. So based on these above points, in our work, the GdSr2AlO5 was studied as a new UC host.
In this paper, Yb3+/Er3+ co-doped GdSr2AlO5 were prepared, Yb3+ were added as the sensitizers with Er3+ as the activators due to the efficient energy transfer from Yb3+ to Er3+. Bright UC emissions from the samples can been seen under 980 nm diode laser excitation. Additionally, the possible mechanism of Yb3+ sensitization to Er3+ on tuning of the UC emission properties were discussed and concluded. The thermometry properties based on the green UC emissions of Er3+ were investigated in detail as well.
2. Experimental
2.1. Synthesis
GdSr2AlO5:RE3+ (RE3+ = Yb3+ and Er3+) samples were synthesized by a simple high temperature solid state reaction method. Raw materials Gd2O3 (99.9%), SrCO3 (99.9%), Al(NO3)3·9H2O (99.0%) and RE oxides (Yb2O3 and Er2O3 99.9%) were weighed out according to the stoichiometric ratio. After all the ingredients were fully mixed evenly, the mixtures were placed into a corundum crucible and then put into a SiC rods furnace to be heated to 1510 °C for 4 h. Finally, after calcination, the samples were cooled down to room temperature in the furnace and ground into powders for further use.2.2. Characterization
We use a Bruker D2 PHASER X-ray diffractometer with graphite monochromator using Cu Kα radiation (λ = 1.54184 Å) to determine the structure purity by X-ray powder diffraction (XRD). And the test conditions are 30 kV and 15 mA with a scanning step of 0.02° in the 2θ range from 10° to 80°.The morphologies of the prepared samples were inspected by a thermal field emission scanning electron microscopy (FESEM, TESCAN, MIRA3 XMU). The UC luminescence spectra were measured by using a 980 nm laser diode as the excitation source, with the HORIBA Jobin Yvon Fluorlog-3 Spectro fluorometer system.3. Results and discussion
3.1. Structure and morphology
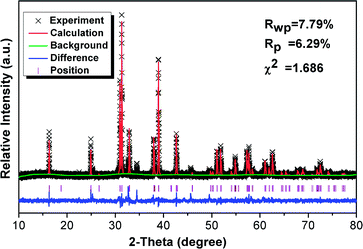
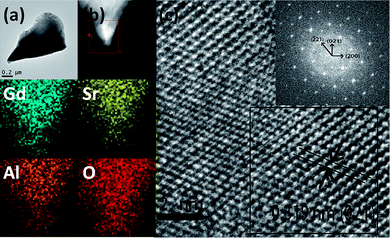
Fig. 1 shows the Rietveld structural refinement of the XRD pattern of the GdSr2AlO5 host obtained using the MS program based on the reported tetragonal EuSr2AlO5 in JCPDS card with no.70-2197. The black crosses, red line and green line are corresponds to the calculated pattern, experimental pattern and background, respectively. The violet short vertical lines show the positions of the Bragg reflections of the calculated pattern. The difference between the experimental and calculated patterns is plotted by the blue line at the bottom. The sample can be found to crystallize in the tetragonal space group I 4/m c m (no. 140). The refinement finally converged to χ2 = 1.686, Rwp = 7.79% and Rp = 6.29%, indicating that our prepared samples is single phase because all the observed peaks suit the reflection conditions. Furthermore, the lattice constants of GdSr2AlO5:6%Yb3+/1%Er3+ are a = b = 6.70508 (ref. 12) Å, c = 10.90337 (ref. 22) Å, α = γ = β = 90°.Fig. 2 presents the TEM, elemental mapping, HRTEM and FFT images of GdSr2AlO5 host. The mapping images indicate a homogeneous distribution of Gd, Sr, Al and O. The HRTEM image exhibits a clear lattice fringe with lattice interplanar spacings of 3.19 Å, corresponding to the (021) plane of GdSr2AlO5. The HRTEM and FFT images demonstrate that the as prepared sample has a tetragonal crystal structure, which is the same as the XRD result.
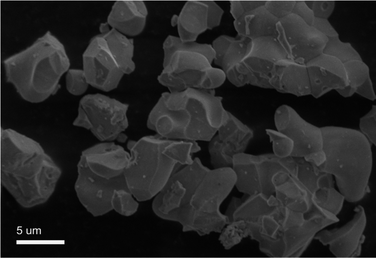
As a typical sample the SEM image of the GdSr2AlO5:6%Yb3+/1%Er3+ is shown in Fig. 3. It can been find that some of these particles were reunited and the grains have irregular blocky particle shapes with sizes around 5 μm. And the SEM image of the GdSr2AlO5 host is show in Fig. S1,† which is almost the same as Fig. 3.
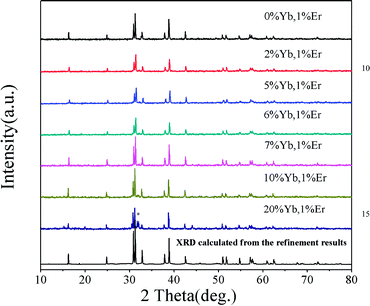
Fig. 4 shows the XRD patterns of various RE ion-doped GdSr2AlO5 samples as well as the experimental and calculated XRD patterns of the GdSr2AlO5 host according to the refinement results. It is clear that the XRD profiles are well fitted with the calculated XRD pattern, and all these diffraction peaks of these phosphors can be accurately assigned to the GdSr2AlO5 host. It can be seen that when the Yb3+ content increased from 0 to 10%, the phase is still the same as GdSr2AlO5 host. So the introduction of lanthanide ions (Yb3+ and Er3+) would not change the phase structure of GdSr2AlO5 except when the Yb3+ doping concentration is 20%. The change of the phase structure means new phase appears with the substantial increase of Yb3+. This phenomenon implies the successful incorporation of Yb ions into the GdSr2AlO5 host.
Furthermore, the experimental values of Gd:Sr:Al:Yb:Er in the as-synthesized 1, 2, 3, 4, 5 and 6 samples are determined by ICP-AES (Table 1), which are all close to the theoretical values.
| Samples | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| Gd | Theoretical content | 0.99 | 0.97 | 0.94 | 0.93 | 0.92 | 0.89 |
| Experimental | 0.91167 | 0.90009 | 0.88310 | 0.96490 | 0.95768 | 0.93287 | |
| Sr | Theoretical content | 2 | 2 | 2 | 2 | 2 | 2 |
| Experimental | 2 | 2 | 2 | 2 | 2 | 2 | |
| Al | Theoretical content | 1 | 1 | 1 | 1 | 1 | 1 |
| Experimental | 1.01010 | 0.93194 | 0.95639 | 1.05427 | 1.07315 | 1.07779 | |
| Yb | Theoretical content | 0 | 0.02 | 0.05 | 0.06 | 0.07 | 0.10 |
| Experimental | 0 | 0.01796 | 0.05127 | 0.06522 | 0.07615 | 0.10998 | |
| Er | Theoretical content | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Experimental | 0.00866 | 0.00947 | 0.01067 | 0.01109 | 0.01010 | 0.01001 |
3.2. UC emission properties
UC luminescence spectroscopy was used to investigate the UC properties of the GdSr2AlO5: Yb, Er under 980 nm laser excitation. It can been seen from Fig. 5 that two UC emissions lied in 527 nm and 549 nm, which were assigned to 2H11/2 → 4I15/2 (Er3+) and 4S3/2 → 4I15/2 (Er3+) transitions, respectively.26 Meanwhile, the red UC emission line centered at 665 nm is observed, which comes from the transition of 4F9/2 → 4I15/2 (Er3+). As the Yb3+ doping concentration increased from 0 to 10%, the UC emission first rapidly increased and then decreased, when the Yb3+ doping concentration reach 6% we get the best emission.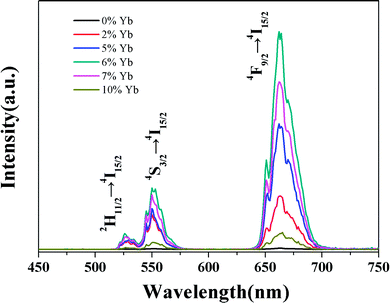 | ||
| Fig. 5 UC spectra of GdSr2AlO5:Yb3+,1%Er3+ crystals with different Yb3+ doping conditions under the excitation of 980 nm laser. | ||
Generally speaking, the absorption can be improved by increasing the doping concentration of the lanthanide ions in the material. But non-radiative multi-phonon relaxation can occur, and the process of cross-relaxation severely limits the range of useful dopant concentrations. Non-radiative multi-phonon relaxation rate between energy levels is another important factor that dictates the population of intermediate and emitting levels and subsequently determines the efficiency of the upconversion process. Therefore, when Yb3+ was co-doped into the lattice in high concentrations, the upconversion luminescence intensity decreased. It can been find that an elevated amount of Yb3+ content is likely to enhance the luminescence efficiency of UC as the Yb3+ ion has a sufficient absorption cross-section matched to common 980 nm laser excitation. Thereby, successive energy transfer processes from Yb3+ to Er3+ play an important role in promoting the Er3+ ion on the ground state to the excited state.27
Additional, we added the upconversion luminescence spectra of Yb and Er doped samples in order to prove the existence of the energy transfer between Yb and Er. From Fig. S2† we can find that the intensity for the upconversion luminescence of Yb, Er co-doped sample is much higher than the singly doped samples. This can be the evidence of the energy transfer between Yb and Er.
These changes of UC luminescence intensity also means the successful incorporation of Yb3+ and Er3+ into the GdSr2AlO5 host. To get a better understanding of the UC luminescence mechanism, the power-dependent UC luminescence properties were studied. In any unsaturated UC process, it is generally considered that the visible emission intensity (Iup) increases regularly according to the pumping power (P) (seen Fig. 6). From the UC luminescence spectra of the prepared Yb3+/Er3+ co-doped GdSr2AlO5 under the excitation of a 980 nm diode laser at different pumping powers (100–500 mW cm−2), we can easily find the intensity continued to increase as the pumping power increased.
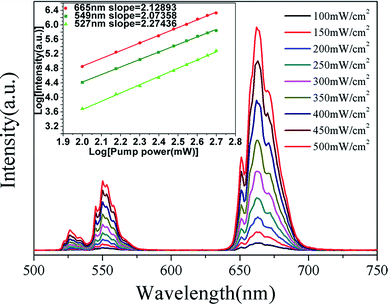 | ||
| Fig. 6 UC spectra of GdSr2AlO5:6%Yb3+/1%Er3+ and (inset)Ln–Ln plot of emission intensity of different excited states versus excitation power of GdSr2AlO5:6%Yb3+/1%Er3+. | ||
The relationship between the intensity of UC luminescence Iup and the pump power P can be written as:28–30
| Iup ∝ PN | (1) |
Fig. 7 shows simplified energy level chart of the Yb3+, Er3+ as well as the proposed UC mechanisms to produce the multicolor radiation. Since the Yb ions concentration are much higher than Er ions, the most probable UC process is via energy transfer from the Yb ions to Er ions. When excited with laser light at 980 nm, the Yb ions are excited from the 2F7/2 level to the 2F5/2 level, and then transfer the energies to the nearby Er ions. In Yb3+/Er3+ co-doped GdSr2AlO5, firstly the Er3+ is excited from the 4I15/2 ground state to the 4I11/2 level and subsequently to the 4F7/2 level, and the nonradiative relaxations then populate the states of 2H11/2 and 4S3/2. Er3+ in 4I13/2 level are promoted to 4F9/2 level by the same energy transfer. Finally, the green emissions at 527 nm and 549 nm are generated by the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions of the Er3+, and the red emission centered at 665 nm is due to the 4F9/2 → 4I15/2 Er3+ transition of the Er3+.
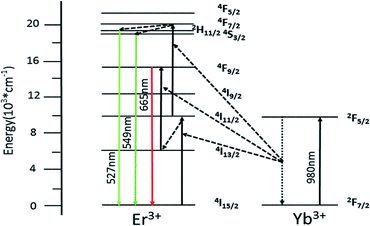 | ||
| Fig. 7 Implified energy level diagram of Yb3+ and Er3+ and the possible UC mechanism of GdSr2AlO5:6%Yb3+/1%Er3+. | ||
3.3. Temperature sensing properties
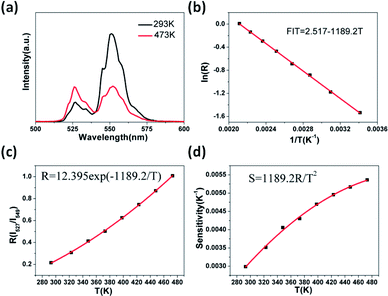
Fig. 8a shows the green UC emission spectra of Er3+/Yb3+ co-doped GdSr2AlO5 in the wavelength range of 500–600 nm at the measured temperatures of 293 K and 473 K. When the temperature increases, we can find the positions of the two green UC emission bands did not change. However, the R(I527/I549)of the two emissions bands varies at different temperature. When the temperature is 293 K, the emission intensity of the 2H11/2 → 4I15/2 band (527 nm) is lower than 4S3/2 → 4I15/2 (549 nm) but inverts at a higher temperature (473 K). The relative populations of the two thermally coupled levels, 2H11/2 and 4S3/2, follows a Boltzmann law, which leads to the variations in the intensities of the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions of the Er3+ ions at an raising temperature.The emission intensity ratio R of the two thermally coupled Er3+ energy levels can be defined as:31–33
R = I527/I549 = C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−ΔE/kT) exp(−ΔE/kT)
| (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R as a function of 1/T is perfectly fitted to a linear equation with the slope of −1189.2 and use the eqn (2) the C can be calculated. Thus, the expression of R could be determined as R = 12.395
R as a function of 1/T is perfectly fitted to a linear equation with the slope of −1189.2 and use the eqn (2) the C can be calculated. Thus, the expression of R could be determined as R = 12.395![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−1189.2/T) (see Fig. 8c). Furthermore, understanding the rate at which the measured temperature-sensitive parameter varies for a certain change in temperature is significance for temperature sensing applications. Hence the sensor sensitivity can be written as:
exp(−1189.2/T) (see Fig. 8c). Furthermore, understanding the rate at which the measured temperature-sensitive parameter varies for a certain change in temperature is significance for temperature sensing applications. Hence the sensor sensitivity can be written as:| Sensitivity (S) = dR/dT = R(−ΔE/kT2) | (3) |
Here the symbols have their usual meanings. The sensor sensitivity was calculated by this equation and it was plot ted in Fig. 8d. Impressively, the sensitivity increases gradually as the temperature rise from 293 K to 473 K, and reached the peak at about 0.0054 K−1at 473 K. The UC emission intensity is quite stable up to the measured temperature at 473 K, which suggests it may be suitable at higher temperatures as well. However as a lack of available devices the research at higher temperature could not be furthered done. Therefore, this study reveals that the GdSr2AlO5 host is a good material for UC-based temperature sensors.
Besides the dependence of the R(Ired/Igreen)ratio on temperature, the effect of temperature on the ratio of red and green emissions (R/G) were also observed. Fig. 9a shows the relationship between temperature and the R/G ratio. Evidently, the R/G ratio tends to decreases with the increase of the temperature. So the emission color of the sample could be changed by controlling the temperature. In order to insight the relationship between emission color of the sample and temperature more clearly, the CIE 1931 coordinates of the prepared GdSr2AlO5:6%Yb3+,1%Er3+ micro-particles in the range of temperatures 293–473 K were calculated from the recorded UC luminescence spectra and the corresponding results are shown in the CIE 1931 coordinates diagram in Fig. 9b. It can be seen that the UC emission color can be changed from yellow to green as the rise of temperature, this means a decrease tendency of the emission intensity ratio of red and green emissions can been observed with the increase of temperature. This is the same as the result shows in Fig. 9a.
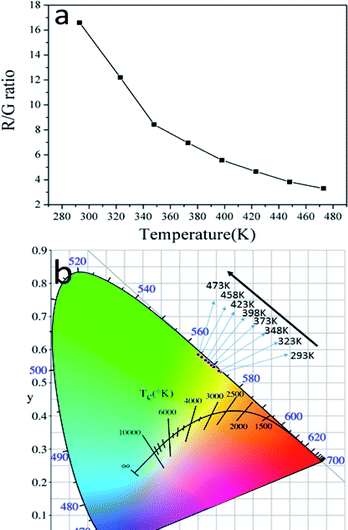 | ||
| Fig. 9 (a) The dependence of R/G ratio on temperature and (b) CIE coordinates GdSr2AlO5:6%Yb3+/1%Er3+ micro-particles in the temperature range of 293–473 K. | ||
It is known to all that the nonradiative transition rate of RE3+ ions for multiphonon relaxation can be written by the following equation:35,36
| WNR = WNR(0)exp−(ΔE/kT) | (4) |
Thereby, both the green and red emission intensities will decrease greatly at higher temperature in normal situation. However, owing to the 2H11/2 and 4S3/2 levels are thermally coupled, much more electrons will be excited to the 2H11/2 level from the 4S3/2 level at high temperature. The occurrence of this phenomenon would block the de-excited process of the 4S3/2 level extremely via the nonradiative transition of 2H11/2, 4S3/2 → 4F9/2. Thence, the decrease of the total emission intensity of green color would be limited, this lead to the greatly decrease of the ratio of red and green emissions when the temperature arises. The temperature-dependent characteristic of the as-prepared GdSr2AlO5:Yb3+, Er3+ micro-particles means that this host could be also adopted as safety sign in high temperature environment as well as optical thermograph.
4. Conclusions
Micro-sized GdSr2AlO5:Yb3+/Er3+ particles with different doping concentrations of Yb3+ are designed and prepared by a high-temperature solid state reaction method. The UC luminescence properties of Yb3+/Er3+ co-doped GdSr2AlO5 upon 980 nm excitation have been systematically investigated. The UC emission spectra of GdSr2AlO5: Yb3+/Er3+ mainly contain two parts, the green emission peaks at 527 nm and 549 nm assigned to the (2H11/2, 4S3/2) → 4I15/2 transitions of the Er3+, and the red emission peak centered at 665 nm attributed to the Er3+ 4F9/2 → 4I15/2 transition, as a result, visible emission can be observed by the naked eye under 980 nm laser excitation. The intensity of the emission can be controlled by varying the Yb3+ concentrations. As a function of temperature ranging from 293 K to 473 K the green UC emission was tested and it has a sensitivity of 0.0054 K−1 at 473 K. Besides the UC emission color can be adjusted from the yellow to green, which could be regarded as another virtue of temperature-dependent UC emission color. In conclusion, the UC luminescence property and temperature dependence of GdSr2AlO5: Yb3+/Er3+ are reported for the first time and these characteristics indicate that the oxide host material GdSr2AlO5 has potential applications in UC-based optical temperature sensors as well as high temperature safety signal.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is supported by the International Sci. & Tech. Cooperation Foundation of Gansu Provincial, China (Grant No. 1504WKCA088), the Gansu Province Development and Reform Commission (NDRC, No. 2013-1336) and the National & Local Joint Engineering Laboratory for Optical Conversion Materials and Technology.Notes and references
- F. Auzel, Chem. Rev., 2004, 104, 139 CrossRef CAS PubMed.
- J. Zhou, Q. Liu, W. Feng and Y. Sun, Chem. Rev., 2015, 115, 395–465 CrossRef CAS PubMed.
- E. M. Chan, Chem. Soc. Rev., 2015, 44, 1653–1679 RSC.
- G. Y. Chen, H. Ågren, T. Y. Ohulchanskyya and P. N. Prasad, Chem. Soc. Rev., 2015, 44, 1680–1713 RSC.
- J. N. Shan, X. Qin, N. Yao and Y. G. Ju, Nanotechnology, 2007, 18, 445607 CrossRef.
- G. S. Yi and G. M. Chow, J. Mater. Chem., 2005, 15, 4460–4464 RSC.
- L. Feng, L. Bian, W. Ren, X. Y. Zhang and H. L. Li, Mater. Res. Bull., 2017, 89, 263–266 CrossRef CAS.
- Y. M. Yang, L. L. Liu, S. Z. Cai, F. Y. Jiao, C. Mi, X. Y. Su, J. Zhang, F. Yu, X. D. Li and Z. Q. Li, J. Lumin., 2014, 146, 284–287 CrossRef CAS.
- T. Rinkel, A. N. Raj, S. Dühnen and M. Haase, Angew. Chem., Int. Ed., 2016, 55, 1164–1167 CrossRef CAS PubMed.
- Y. D. Wang, Z. W. Yang, Y. J. Ma, Z. Z. Chai, J. B. Qiu and Z. G. Song, J. Mater. Chem. C, 2017, 5, 8535–8544 RSC.
- C. Liu, Z. Gao, J. Zeng, Y. Hou, F. Fang, Y. Li, R. Qiao, L. Shen, H. Lei, W. Yang and M. Gao, ACS Nano, 2013, 7, 7227–7240 CrossRef CAS PubMed.
- A. J. Huang, Z. W. Yang, C. Y. Yu, J. B. Qiu and Z. G. Song, J. Am. Ceram. Soc., 2017, 100, 4994–4998 CrossRef CAS.
- Y. Tian, Y. Fu, M. Xing and X. Luo, J. Nanomater., 2015, 573253 Search PubMed.
- Y. Tian, B. Tian, C. Cui, P. Huang, L. Wang and B. Chen, RSC Adv., 2015, 5, 14123–14128 RSC.
- W. Xu, Z. G. Zhang and W. W. Cao, Opt. Lett., 2012, 37, 4865–4867 CrossRef CAS PubMed.
- D. Jaque and F. Vetrone, Nanoscale, 2012, 4, 4301–4326 RSC.
- C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millán, V. S. Amaral, F. Palacio and L. D. Carlos, Nanoscale, 2012, 4, 4799–4829 RSC.
- F. Wang and X. Q. Liu, Chem. Soc. Rev., 2009, 38, 976–989 RSC.
- D. Q. Chen, Y. L. Yu, F. Huang, P. Huang, A. P. Yang, Z. X. Wang and Y. S. Wang, Chem. Commun., 2011, 47, 11083–11085 RSC.
- K. Song and H. Chen, Superlattices Microstruct., 2015, 85, 842–848 CrossRef CAS.
- W. Xu, H. Zhao, Y. X. Li, L. J. Zheng, Z. G. Zhang and W. W. Cao, Sens. Actuators, B, 2013, 188, 1096–1100 CrossRef CAS.
- F. Liu, Y. J. Liang and Z. W. Pan, Phys. Rev. Lett., 2014, 113, 177401 CrossRef PubMed.
- W. B. Im, Y. Fourré, S. Brinkley, J. Sonoda, S. P. DenBaars and R. Seshadri, Opt. Express, 2009, 17, 22673–22679 CrossRef CAS PubMed.
- J. Y. Park, J. H. Lee, G. S. R. Raju, B. K. Moon, J. H. Jeong, B. C. Choi and J. H. Kim, Ceram. Int., 2014, 40, 5693–5698 CrossRef CAS.
- G. Li, Y. H. Wang, W. Zeng, W. B. Chen, S. C. Han and H. J. Guo, RSC Adv., 2015, 5, 20884–20889 RSC.
- F. V. D. Rijke, H. Zijlmans, S. Li, T. Vail, A. K. Rapp, R. S. Niedbala and H. J. Tanke, Nat. Biotechnol., 2001, 19, 273 CrossRef PubMed.
- F. Vetrone, J. C. Boyer, J. A. C. Speghini and M. Bettinelli, J. Appl. Phys., 2004, 96, 661–667 CrossRef CAS.
- Y. Tian, R. N. Hua, N. S. Yu, W. Zhang, L. Y. Na and B. J. Chen, J. Alloys Compd., 2011, 509, 9924–9929 CrossRef CAS.
- M. Guan, H. Zheng, L. F. Mei, M. S. Molokeev, J. Xie, T. Yang, X. W. Wu, S. F. Huang and Z. H. Huang, J. Am. Ceram. Soc., 2015, 98, 1182–1187 CrossRef CAS.
- Z. G. Xia, J. Li, Y. Luo and L. B. Liao, J. Am. Ceram. Soc., 2012, 95, 3229–3234 CrossRef CAS.
- C. L. H. Fischer, G. S. Harms and O. S. Wolfbeis, Angew. Chem., Int. Ed., 2011, 50, 4546–4551 CrossRef PubMed.
- C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millán, V. S. Amaral, F. Palaciob and L. D. Carlos, New J. Chem., 2011, 35, 1177–1183 RSC.
- S. A. Wade, S. F. Collins and G. W. Baxter, J. Appl. Phys., 2003, 94, 4743–4756 CrossRef CAS.
- A. K. Singh, S. K. Singh, B. K. Gupta, R. Prakash and S. B. Rai, Dalton Trans., 2013, 42, 1065–1072 RSC.
- Y. Q. Lei, H. W. Song, L. M. Yang, L. X. Yu, Z. X. Liu, G. H. Pan, X. Bai and L. B. Fan, J. Chem. Phys., 2005, 123, 174710 CrossRef PubMed.
- Y. Y. Tian, Y. Tian, P. Huang, L. Wang, Q. F. Shi and C. Cui, Chem. Eng. J., 2016, 297, 26–34 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra13759a |
| This journal is © The Royal Society of Chemistry 2018 |